Abstract
Background
Post-stroke hemiparesis is usually considered a unilateral motor control deficit of the paretic leg, while the non-paretic leg is assumed to compensate for paretic leg impairments and have minimal to no deficits. While the non-paretic leg EMG patterns are clearly altered, how the non-paretic leg acts to compensate remains to be established.
Methods
Kinesiological data were recorded from sixty individuals with chronic hemiparesis (age: 60.9, S.D. =12.6 years, 21 females, 28 right hemiparetic, time since stroke: 4.5 years, S.D. 3.9 years), divided into three speed-based groups, and twenty similarly aged healthy individuals (age: 65.1, S.D. =10.4 years, 15 females). All walked on an instrumented split-belt treadmill at their self-selected speed and control subjects also walked at slower speeds matching those of the persons with hemiparesis. We determined the differences in magnitude and timing of non-paretic EMG activity relative to healthy control subjects in four pre-defined regions of stance phase of the gait cycle.
Findings
Integrated EMG activity and EMG timing in the non-paretic leg were different in many muscles. Multiple compensatory patterns identified included: increased EMG output when the muscle was typically active in controls and novel compensatory EMG patterns that appeared to provide greater propulsion or support with little evidence of impaired motor performance.
Interpretation
Most novel compensations were made possible by altered kinematics of the paretic and non-paretic leg (i.e., early stance plantarflexor activity provided propulsion due to the decreased advancement of the non-paretic foot) while others (late single limb stance knee extensor and late stance hamstring activity) appeared to be available mechanisms for increasing propulsion.
Keywords: Electromyography, Stroke, Walking, Muscle
Introduction
Non-paretic leg performance post-stroke is different from that of a healthy leg, with the differences presumably representing compensation (Kim and Eng 2004; Olney et al. 1994; Olney et al. 1991; Parvataneni et al. 2007), although there may be some differences that indicate impairment (Bagnato et al. 2009). While non-paretic leg compensatory mechanisms may assist a person in attaining steady-state walking even with a poorly coordinated paretic leg, these compensations might inhibit expression of a more appropriate biomechanical pattern of the paretic leg. This interference could be either a mechanical phenomenon (e.g. one leg doing more than half of the task requirements does not allow expression of a normal pattern) or it could be a neural phenomenon (e.g. the altered sensorimotor state of the non-paretic leg might interfere with normal bilateral pattern formation). For instance, pedaling studies in individuals with hemiparesis post-stroke have demonstrated a strong negative influence of the non-paretic leg participation on the paretic leg performance, thereby further deteriorating the performance of paretic leg pedaling (Kautz and Patten 2005; Kautz et al. 2006). The implication is that the altered sensorimotor state of the non-paretic leg, even if it is just to perform compensation, may interfere with expression of a normal motor pattern by the paretic leg. If this finding can be extrapolated to walking, the performance of the non-paretic leg should also potentially be a clinical concern as compensations may lead to sub-optimal rehabilitation outcomes for the paretic leg.
The first step toward understanding the potential interference of non-paretic leg mechanical output on the paretic leg motor pattern is to specifically define how the output of the non-paretic leg is altered compared to a healthy leg at the same speed. Although there is ample evidence to suggest that changes in non-paretic leg output are a substantial determinant of the altered walking pattern post-stroke, there has been little quantitative investigation of how the non-paretic leg performs during walking. We hypothesize that the mechanical output of the non-paretic leg is increased by doing more than merely increasing the activity of a healthy leg. Explicitly, there are specific functions usually performed by the paretic leg (such as propulsion) that are more affected than others, and thus the muscles in the non-paretic leg that can contribute to propulsion (Neptune et al. 2004) will likely have to compensate more than other muscles.
For the purpose of this study, we will define compensation as a change in the motor output of the non-paretic leg to perform a function usually performed by a healthy contralateral leg (presumably because it is not being performed by the paretic leg). We hypothesize that we will identify two types of compensation: 1) expected compensations, which are increases in output during normally active periods in a control leg to increase the contribution to a muscle’s usual biomechanical function, and 2) novel compensations, which are activities during a normally inactive period in a control leg that results in either a new function being performed or the same function being performed at a different time. Of particular interest, it may be that a novel compensation may only be possible because the kinematics of hemiparetic walking differ from those of control walking and the change in the kinematic state of the leg can allow a muscle to perform a function it normally could not perform during the same period of the gait cycle in a control leg. For example, if the non-paretic leg is placed behind the paretic leg in a step-to gait pattern, the plantarflexor activity could produce propulsion very early in the non-paretic leg gait cycle as the non-paretic foot will spend more time behind the center of mass of the body. If, on the other hand, muscle activity occurs during a normally inactive period when there appears to be no constructive biomechanical function being performed, that might be suggestive of impairment.
Methods
Participants
Sixty individuals (age: 60.9, S.D. =12.6 years, 21 females, 28 right hemiparetic, time since stroke= 4.54, S.D. = 3.9 years, weight: 88.12, S.D. = 22.45 kg, height: 1.68, S.D. = 0.25 m) with chronic hemiparesis and twenty similarly aged healthy individuals (age: 65.1, S.D. =10.4 years, 15 females, weight: 73.97 kg, S.D. = 15.66, height: 1.47, S.D. = 0.47) were tested. The inclusion criteria for the study were: subjects should have hemiparesis secondary to a single onset unilateral stroke; should be able to ambulate independently with or without an assistive device over 10 m on a level surface; have the ability to walk on a regular basis at least at home; do not have any significant lower extremity joint pain and any major sensory deficits; do not have any significant lower limb contractures or significant cardiovascular or respiratory symptoms contraindicative to walking. Criteria for the exclusion of participants from the study were: any orthopedic or neurologic conditions in addition to stroke, any significant musculoskeletal problems that would limit hip and knee extension or ankle plantar flexion to neutral, or inability to provide informed consent. All participants in the study signed a written informed consent and the study was approved by Institutional Review Board of University of Florida.
Procedures
Subject preparation
Reflective markers and rigid body clusters were positioned on specified bony prominences and limb segments respectively, to acquire 3-D motion data. Marker positions were based on the Vicon Plug-In Gait marker set (modified Helen Hayes set). In addition, disposable bipolar Ag-AgCl surface electrodes (Vermed, Inc, bellows Falls, VT) were used to record muscle activity from eight lower extremity muscles bilaterally (TA: Tiabialis Anterior, SO: Soleus, MG: Medial Gastrocnemius, VM: VastusMedialis, RF: Rectus Femoris, BF: Biceps Femoris LH: Lateral Hamstrings, MH: Medial Hamstrings and GM: Gluteus Medius) (Leis et al. 2000). Adequate skin preparations were performed (electrode placement site was shaved and wiped with alcohol) to facilitate maximum skin conduction. A reference electrode was placed on the electrically neutral patella.
Data Collection
The subjects walked on a split-belt instrumented treadmill (Techmachine, Andrezieux Boutheon, France) for 30 seconds (3 trials) at their self-selected walking speed. The treadmill was started at a slower speed and the speed was adjusted until the subjects attained their self-selected comfortable treadmill walking speed. Subjects wore a safety harness mounted to the ceiling while walking on the treadmill. The harness did not offload any body weight, only provided support in case of loss of balance. A physical therapist was also present for close supervision and providing assistance if required. Trials with assistance were not included in the final analysis. For the purpose of data collection, all subjects walked without ankle-foot orthoses or walking aid and wore closed toe tennis shoes. However, if necessary subjects wore an ankle brace (DJO, Vista, California), which provided medial-lateral stability to the ankle while allowing sagittal plane movement. Rest breaks were provided as needed to the subjects between the walking trials.
Additionally, healthy control subjects walked at their self-selected speed and three randomly presented additional walking speeds: 0.3m/s, 0.6m/s and 0.9m/s. These speeds were selected a priori to represent a likely range within which persons with stroke would elect to walk.
Data Recording and Processing
A twelve camera VICON motion-capture system (Vicon Motion Systems, Los Angeles, CA), instrumented treadmill and telemetric EMG system (Konigsberg Instruments, Pasadena, CA) were used to capture the kinematic, kinetic and EMG data as the subjects walked on the split-belt instrumented treadmill.
Ground reaction forces were acquired from each foot separately at a sampling rate of 2000Hz, and low-pass filter using a fourth-order zero-lag Butterworth filter with a 20 Hz cut-off frequency. A 13 segment musculoskeletal model was created using Visual 3D (V3D) (C-Motion, Inc., Germantown, MD). Visual 3D models were used to conduct inverse dynamics analyses for calculation of inter-segmental joint kinetics. In addition, the EMG signals were collected as analog signals at 2000Hz, amplified, digitized and stored. The EMG signals were then filtered with a 40Hz high pass filter, demeaned, rectified and then low pass filtered (25Hz). Filtering was done with 4th order Butterworth filters in Matlab (MathWorks Inc, Natick, MA).
Data were processed with a K-means cluster algorithm to determine whether each muscle is “on” or “off” for each point in the gait cycle (Den Otter et al. 2007). K-means cluster analysis is a statistical technique that groups similar data points in a single cluster. In the context of EMG analysis it differentiates data as “on” and “off” by finding similarities between the data points of the rectified and filtered EMG signal (Den Otter et al. 2007). The individual data points are assigned to K-cluster by MATLAB such that the distance from the centroid is minimized. Therefore, the most similar data points are grouped together. The cluster with the lowest mean value are assumed to correspond to inactivity and all other clusters correspond to muscle activity (Den Otter et al. 2007).
Calculations of Study Variables
Same variables were recorded and calculated for healthy individuals and individuals with hemiparesis during walking. All the variables were calculated by averaging across multiple steps of each trial. Since there was no statistically significant difference between the performance of the left and right side of the healthy control subjects, the mean value of the two sides was used for all the analyses. The stance phase was divided into four functional regions for analysis: first double support (region 1), the first (region 2) and second (region 3) halves of ipsilateral single support, and the second double support (region 4).
EMG Variables
EMG magnitude
Integrated EMG was used to quantify the magnitude of EMG activity over the stance phase and for each region of the stance phase as defined above. It was calculated by numerically integrating absolute EMG signals (in μV) with respect to time.
EMG timing
Percent duration of activity of a muscle in stance phase/region is the duration of “on” activity divided by the total activity in the stance phase/region multiplied by 100.
Propulsive Impulse
Propulsive impulse was defined as the positive impulse of the anterior-posterior ground reaction force and was calculated during each region of the stance phase.
Statistical Analyses
Independent sample t-tests were performed to compare the magnitude and timing of muscle activity in different regions of the stance phase to that of healthy control subjects walking at matched speeds. Furthermore, we evaluated the propulsive impulse during the regions of changed EMG (defined by stance phase events). We classified subjects with hemiparesis into three groups based on their speed: slow speed group (0–<0.4m/s, average speed= 0.22m/s) was compared to control subjects at 0.3m/s, the moderate speed group (0.4m/s – 0.8m/s, average speed= 0.47m/s) was compared to control subjects at 0.6m/s and the fast speed group (0.8m/s–1.0m/s, average speed= 0.86m/s) was compared to control subjects walking at 0.9m/s. Significance for all tests was set at alpha < 0.05 and we indicate any significant difference between the non-paretic leg and healthy control subjects. All statistics were run using SPSS version 17.0 (SPSS, Inc. Chicago IL).
Results
Magnitude of EMG Activity
Integrated EMG activity over the gait cycle
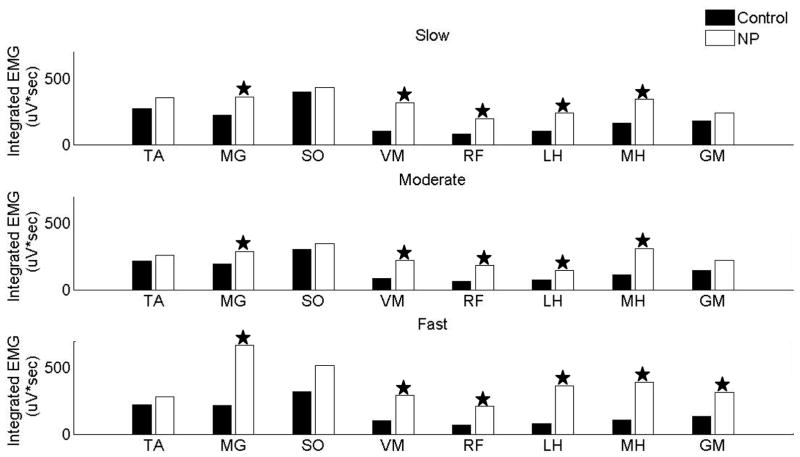
The dominant feature of the magnitude of integrated EMG activity in the non-paretic leg was that it was increased in the majority of muscles. Figure 1 presents the magnitude of integrated EMG activity in the non-paretic leg during stance phase as compared to the healthy control subjects walking at the matched speeds. Five of the eight recorded muscles in slow and moderate speed group (MG, VM, RF, LH and MH) had significantly increased integrated EMG activity in the non-paretic leg, while fast speed group had significantly increased activity in MG, VM, RF, LH, MH and GM.
Figure 1.
Integrated EMG activity in different regions of the stance phase in slow speed group
The individuals with hemiparesis in the slow speed group revealed significantly increased integrated EMG activity in quadriceps (RF and VM) muscles throughout stance phase in the non-paretic leg as compared to healthy control subjects walking at matched speeds, while all muscles had significantly increased activity in region 1 of stance phase (figure 2a).
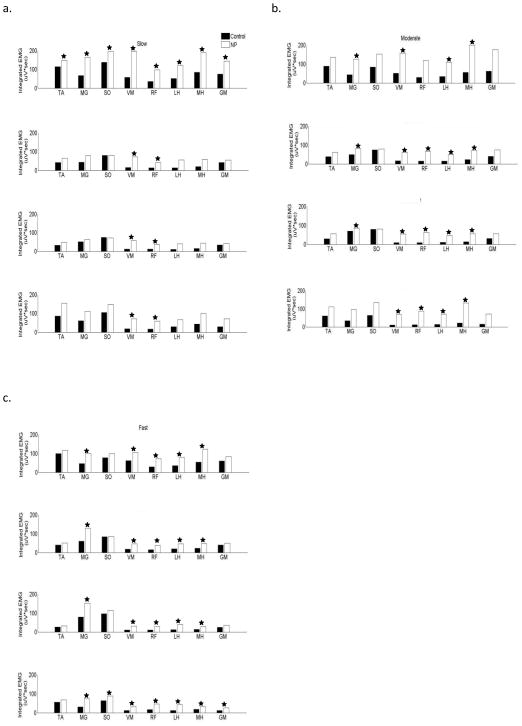
Figure 2.
Integrated EMG activity in different regions of the stance phase in moderate speed group
The integrated EMG activity of upper leg muscles (RF, VM, MH, LH) was mostly increased while the EMG in distal muscles (SO, MG, TA) was mostly similar in the non-paretic leg as compared to healthy control subjects walking at matched speeds. The quadriceps and hamstring muscles had significantly increased magnitude throughout the stance phase of the gait cycle (except RF in region 1). TA and SO did not reveal a significant difference in any region, while the magnitude of MG was increased in regions1, 2 and 3 (figure 2b).
Integrated EMG activity in different regions of the stance phase in fast speed group
The upper leg muscles (RF, VM, MH, and LH) had significantly increased magnitude in the stance phase in the non-paretic leg as compared to healthy control subjects walking at matched speeds. Furthermore, MG also had significantly increased activity in the stance phase (SO only region 4). All muscles (except TA) had increased activity in region 4 (figure 2c).
Timing of EMG Activity
Percentage duration of muscle activity for the entire gait cycle
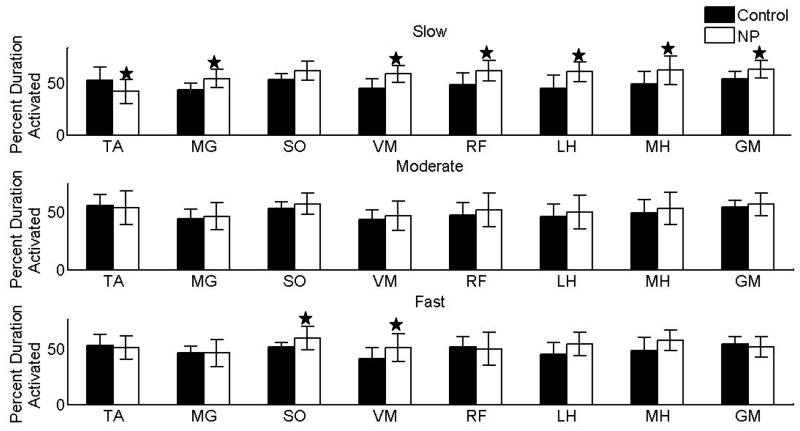
Only the subjects in the slow group showed significant changes (all muscles increased, SO activity reduced) in the percent duration of muscle activity for the entire gait cycle. Unlike the magnitude measures in which there were many changes, the individuals in moderate and fast speed groups had no significant difference in the percentage duration of activity as compared to healthy control subjects (except SO and VM) (figure 3).
Figure 3.
Percent duration of muscle activity in different regions of stance phase
Individuals in the slow group revealed the most differences in muscle activity as compared to healthy control subjects walking at matched speeds, specifically the majority of muscles revealed significant differences in region 1. LH revealed significantly increased activity throughout stance phase (figure 4a). On the other hand, the individuals walking at the moderate speed revealed significant differences in the duration of activity in SO and LH throughout the stance phase (figure 4b). Participants walking at faster speeds did not differ from the healthy control subjects walking at the matched speeds except for SO and LH in region 4 and MH in the region 2 (figure 4c).
Figure 4.
Propulsive Impulse
Individuals in the slow speed group revealed significantly increased magnitude of propulsive impulse in the non-paretic leg in regions 1 and 2 of the stance phase as compared to healthy control subjects walking at matched speeds. However, the non-paretic leg propulsive impulses in regions 3 and 4 were unchanged when compared to those of healthy control subjects at matched speed (Table 1).
Table 1.
Positive propulsive impulse (in BW* seconds) generated by the non-paretic leg and healthy controls walking at self-selected speed and matched speeds during different regions of the stance phase
| Speed Groups | Region | Non-paretic | Controls at matched speeds |
|---|---|---|---|
| Slow Speed (0-<0.4m/s) | Region 1 | 0.005 | 0.002* |
| Region 2 | 0.003 | 0.0003* | |
| Region 3 | 0.003 | 0.005 | |
| Region 4 | 0.012 | 0.012 | |
| Moderate Speed (0.4 <0.8m/s) | Region 1 | 0.002 | 0.0015 |
| Region 2 | 0.002 | 0* | |
| Region 3 | 0.005 | 0.004 | |
| Region 4 | 0.012 | 0.015 | |
| Fast Speed (>0.8m/s) | Region 1 | 0.001 | 0.001 |
| Region 2 | 0.002 | 0* | |
| Region 3 | 0.007 | 0.005 | |
| Region 4 | 0.015 | 0.017 |
represents the significant differences.
Individuals in the moderate speed group revealed significantly increased magnitude of propulsive impulse in region 2 as compared to healthy control subjects walking at matched speed, while there were no differences in the other regions (Table 1).
Individuals in the fast speed group had significantly greater propulsive impulse in the non-paretic leg in region 2 as compared to healthy control subjects walking at matched speed, while there was no difference in the other regions (Table 1).
Discussion
Nearly all of the increases in integrated EMG magnitude and duration in the different phases of stance phase appear to be compensatory in nature and these compensations appear to serve the purpose of augmenting the reduced performance of biomechanical functions typically performed by a contralateral leg. Specifically, reduced propulsion generation by the paretic leg is being offset by additional propulsion generated by the non-paretic leg. This is evident by the statistically significant differences in propulsive impulse occurring in regions that were demonstrated to produce propulsion in previous computer simulation research (Turns et al. 2007). Compensations were either “expected”, because the activity was increased in the muscles and regions known to be the primary propulsion bursts for normal walking, or “novel”, because the activity was in specific regions of the stance phase during which a control leg is not typically active when walking at a matched speed. This implies that individuals walking post-stroke may employ several different compensatory mechanisms in order to attain a steady-state walking pattern and that the non-paretic leg is not unaffected as has been supported by previous research (Desrosiers et al. 1996; Kim et al. 2003).
Because compensatory activity likely involves multiple different mechanisms and different muscles (which are not independent) to influence the impulse measures, we did not correct for multiple comparisons. Since the results of the statistical analyses were mostly in agreement with our initial hypotheses that the muscle activity will be increased in the non-paretic leg based on evidence in the literature, as opposed to many more random appearing changes in different directions, we feel that this choice was justified. Nonetheless, we present the p-values to allow the reader to assess the strength of evidence for the changes observed in the coordination of the non-paretic leg.
Another limitation is that absolute magnitude of EMG (in μV) recorded via surface electrodes is influenced by several intrinsic (thickness of sub-cutaneous fat, distribution of motor units etc.) and extrinsic factors (location and area of the electrode and distance between them), and thus is limited in its ability to provide strong evidence for changes in the magnitude of muscle activity between subjects (Farina 2006; Burdette 1990). Nevertheless, we believe that despite the limitations, integrated absolute EMG provides evidence for increased activity that is confirmatory of the increased propulsion generation typically measured in the non-paretic leg (Bowden et al. 2006; Kim and Eng 2004; Olney et al. 1991) and it is also useful when interpreted in the context of the increased duration of activity observed for many muscles.
Reduced propulsion and thus reduced walking speed are one of the primary concerns after stroke. The non-paretic leg typically generates greater propulsive force (Bowden et al. 2006) to compensate for the weak and discoordinated paretic leg. It appears several compensatory mechanisms are involved.
Increased hamstring activity during early stance, presumably is responsible for increasing propulsion. The results of our analysis reveal that the magnitude and duration (except in the fast speed group) of LH and MH are significantly increased in region 1 of stance phase of the non-paretic leg (presumably contributing to the work done by hip extensors/hamstrings muscles (H1)). The increased activity of the MH and LH muscles likely facilitates an increase in the magnitude of the positive H1 power burst to increase propulsion. The, increased hamstring activity might compensate for reduced propulsion by promoting forward acceleration of the trunk (Neptune et al. 2004) at a time when the paretic leg is in pre-swing and not contributing adequately to propulsion. This is further supported by a significantly increased magnitude of propulsive impulse in region 1of the stance phase of slow and moderate speed groups.
Increased plantarflexor activity earlier in the stance phase was likely a novel compensation to produce increased propulsion that might possibly be facilitated by the altered asymmetric kinematics. The increased plantarflexor activity in this region of the stance phase may facilitate propulsion if the non-paretic leg is placed behind the body center of mass or only slightly beyond (as happens in a step–to-gait pattern (Balasubramanian et al. 2010)). Studies have suggested that a shorter non-paretic step results in reduced paretic propulsion (because in addition to their impaired power generation the paretic plantarflexors are put in a biomechanically disadvantaged position for generating propulsion (Balasubramanian et al. 2010). Therefore, the increased activity of SO and MG in this region may compensate for the reduced propulsion generation. The increased propulsive impulse of the non-paretic leg as compared to healthy controls walking at their self-selected or matched speeds further suggests that the increase in the plantarflexor activity in early stance facilitated increased propulsion by the non-paretic leg. Thus, the individuals walking at slow speeds were in a kinematically advantageous position where they were able to plantarflex the ankle in this region, which is normally characterized by dorsiflexion.
Another potentially novel compensation by the non-paretic leg is increased VM and RF activity in region 2 of the non-paretic stance phase in the slow and moderate speed group individuals walking post-stroke. According to a recent simulation study, increased VM activity in the stance phase of the non-paretic leg might facilitate propulsion of the swinging paretic leg (Hall 2010), as further supported by increased propulsive impulse of the non-paretic leg in our analysis. They also reported increased contribution of RF and VM in the non-paretic leg of a group of slow walkers (limited community ambulatory) as compared to group of fast walkers (community ambulatory) (Hall 2010), consistent with the increased VM activity in the slow and moderate speed group and not in the fast speed group of our analyses. Furthermore, the increased VM and RF activity in the stance phase of the non-paretic leg also generates greater force to maintain vertical support, while the total body weight is being supported by one leg. According to previous simulation work, SO is a primary muscle to provide limb extension to support body weight during regions 2 and 3 (Neptune et al. 2004). However, our analysis shows that SO activity was reduced in the slow and moderate speed group, perhaps because less contribution from the SO muscle is needed once VM and RF increase their activity (Higginson et al. 2006).
An expected compensation observed was an increased plantarflexor output during region 4. The magnitude and duration of the plantarflexors were increased in region 4 of slow and moderate speed individuals, in order to increase the magnitude of work done by the ankle plantarflexors (A2). A2 is typically the most important source of positive work generation in the gait cycle (Winter 1991), and thus helps to generate greater propulsive force to attain a faster walking speed. This greater activation of the plantarflexors on the non-paretic side is expected to generate greater force to produce increased propulsion to maintain steady-state walking. In addition to increased plantarflexor activity, there is an additional burst of TA activity during region 4 of stance phase. In theory, the increased magnitude of TA activity in this region may interfere with generation of propulsion during this phase (Turns et al. 2007). We note that the propulsion in this region was not significantly increased, which could be due to other muscle activity offsetting the increased plantarflexor activity, decreased braking by either leg or the slightly slower average speed of the hemiparetic subjects for each group (when compared to the control group).
An increased burst in the RF muscle during region 4 (both duration and timing) was exhibited in the non-paretic leg of individuals in the slow and moderate speed groups. Excessive RF activity has been cited as a contributor to reduced knee flexion (Gage et al. 1987; Perry 1987). Furthermore, RF tends to decrease the peak knee flexion velocity (Goldberg et al. 2004; Goldberg et al. 2006) and over activity during region 4 and swing is often implicated in stiff knee gait (Goldberg et al. 2004). Additionally, Hernandez et al. (2010) in their electrical stimulation study reported that RF stimulation before the toe-off largely reduced the peak knee flexion during swing as opposed to the stimulation during the swing phase. Reduced propulsion due to increased activity of RF in the non-paretic leg is further supported by reduced propulsive impulse generation during region 4 of stance phase, specifically in moderate and fast speed groups.
Implications of Analysis
Many of the expected or novel compensatory mechanisms by the non-paretic leg are aimed at providing greater propulsion. We consider it to be an expected compensation when it is accomplished by an increase in normally existing sources of muscle work. For instance, increased hamstring activity in region 1 of stance phase provides greater forward propulsion of the trunk (Neptune et al. 2004). We consider it to be a novel compensatory pattern of the non-paretic leg when a muscle is active during a region it is not usually active. For example, increased activity of the VM and RF in regions 2 and 3 of stance phase provides increased support to the non-paretic leg (Higginson et al. 2006) while increasing the propulsion of the swinging paretic leg (Hall 2010).
On the other hand, the non-paretic leg exhibits some increases in muscle activity that may not have a positive influence on walking and might suggest impairments. For example, increased TA activity in the pre-swing phase (region 4) of the stance phase, that likely interferes with generation of propulsion by the non-paretic leg (Turns et al. 2007) and the increased burst of RF during region 4 of stance phase, possibly negatively influences knee angle and knee flexion velocity during the swing phase (Goldberg et al. 2004; Goldberg et al. 2006; Hernandez et al.2010). Further analysis will need to be done to investigate this possibility.
Clinical Relevance
The results of our study demonstrate how substantially altered the coordination of the non-paretic leg is after stroke. Therefore, this emphasizes bilateral involvement and that stroke is not a unilateral motor control problem, that leaves an unaffected limb. A better understanding of how the non-paretic leg is compensating for the paretic leg is important to provide effective rehabilitation and will potentially offer new avenues for clinicians to alter the hemiparetic gait pattern. For example, an increased MH activity in late stance likely increases extension of the non-paretic leg, which in turn provides mechanical advantage to the paretic leg to swing through without greater recruitment of muscle activity. However, if the excessive extension of the non-paretic leg is prevented during therapy, it would potentially facilitate greater use of the paretic leg plantarflexors and hip flexors to swing the leg in order to attain a functional walking state. Thus, to improve walking after stroke, it is important not only to investigate and understand the mechanics of paretic leg output, but also understand the non-paretic leg performance to gain important clinical insights.
Supplementary Material
Acknowledgments
The authors would like to thank Helen Emery, Dr. Mark Bowden, Kelly Rooney, Francis Bergschneider, Dr. Cameron Nott and Ryan Knight for help with the data collection and processing. This work was funded by NIH grant RO1 HD46820 and the Rehabilitation Research & Development Service of the VA. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, NICHD, VA or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagnato S, Boccagni C, Boniforti F, Trinchera A, Guercio G, Letizia G, Galardi G. Motor dysfunction of the “non-affected” lower limb: a kinematic comparative study between hemiparetic stroke and total knee prosthesized patients. Neurol Sci. 2009;30:107–113. doi: 10.1007/s10072-009-0031-0. [DOI] [PubMed] [Google Scholar]
- Balasubramanian CK, Neptune RR, Kautz SA. Foot placement in a body reference frame during walking and its relationship to hemiparetic walking performance. Clin Biomech. 2010;25:483–490. doi: 10.1016/j.clinbiomech.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37:872–876. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- Burdette BH, Gale EN. Reliability of surface electromyography of the masseteric and anterior temporal areas. Arch Oral Biol. 1990;35:747–751. doi: 10.1016/0003-9969(90)90098-u. [DOI] [PubMed] [Google Scholar]
- Den Otter AR, Geurts AC, Mulder T, Duysens J. Abnormalities in the temporal patterning of lower extremity muscle activity in hemiparetic gait. Gait Posture. 2007;25:342–352. doi: 10.1016/j.gaitpost.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the “unaffected” upper extremity of elderly stroke patients. Stroke. 1996;27:1564–1570. doi: 10.1161/01.str.27.9.1564. [DOI] [PubMed] [Google Scholar]
- Farina D. Interpretation of the surface electromyogram in dynamic contractions. Exerc Sport Sci Rev. 2006;34:121–127. doi: 10.1249/00003677-200607000-00006. [DOI] [PubMed] [Google Scholar]
- Gage JR, Perry J, Hicks RR, Koop S, Werntz JR. Rectus femoris transfer to improve knee function of children with cerebral palsy. Dev Med Child Neurol. 1987;29:159–166. doi: 10.1111/j.1469-8749.1987.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Anderson FC, Pandy MG, Delp SL. Muscles that influence knee flexion velocity in double support: implications for stiff-knee gait. J Biomech. 2004;37:1189–1196. doi: 10.1016/j.jbiomech.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Ounpuu S, Arnold AS, Gage JR, Delp SL. Kinematic and kinetic factors that correlate with improved knee flexion following treatment for stiff-knee gait. J Biomech. 2006;39:689–698. doi: 10.1016/j.jbiomech.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Hall LH. Understanding changes in post-stroke walking ability through simulation and experimentalanalyses. Austin: University of Texas; 2010. [Google Scholar]
- Hernandez A, Lenz A, Thelen D. Electrical Stimulation of the Rectus Femoris During Pre-Swing Diminishes Hip and Knee Flexion During the Swing Phase of Normal Gait. IEEE Trans Neural Syst Rehabil Eng. 2010;18:523–530. doi: 10.1109/TNSRE.2010.2053150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginson JS, Zajac FE, Neptune RR, Kautz SA, Delp SL. Muscle contributions to support during gait in an individual with post-stroke hemiparesis. J Biomech. 2006;39:1769–1777. doi: 10.1016/j.jbiomech.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Kautz SA, Patten C. Interlimb influences on paretic leg function in poststroke hemiparesis. J Neurophysiol. 2005;93:2460–2473. doi: 10.1152/jn.00963.2004. [DOI] [PubMed] [Google Scholar]
- Kautz SA, Patten C, Neptune RR. Does unilateral pedaling activate a rhythmic locomotor pattern in the nonpedaling leg in post-stroke hemiparesis? J Neurophysiol. 2006;95:3154–3163. doi: 10.1152/jn.00951.2005. [DOI] [PubMed] [Google Scholar]
- Kim CM, Eng JJ. Magnitude and pattern of 3D kinematic and kinetic gait profiles in persons with stroke: relationship to walking speed. Gait Posture. 2004;20:140–146. doi: 10.1016/j.gaitpost.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Pohl PS, Luchies CW, Stylianou AP, Won Y. Ipsilateral deficits of targeted movements after stroke. Arch Phys Med Rehabil. 2003;84:719–724. doi: 10.1016/s0003-9993(02)04973-0. [DOI] [PubMed] [Google Scholar]
- Leis AA, Trapani VC. Atlas of Electromyography. New York: Oxford University Press; 2000. [Google Scholar]
- Neptune RR, Zajac FE, Kautz SA. Muscle force redistributes segmental power for body progression during walking. Gait Posture. 2004;19:194–205. doi: 10.1016/S0966-6362(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Griffin MP, Monga TN, McBride ID. Work and power in gait of stroke patients. Arch Phys Med Rehabil. 1991;72:309–314. [PubMed] [Google Scholar]
- Olney SJ, Griffin MP, McBride ID. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: a regression approach. Phys Ther. 1994;74:872–85. doi: 10.1093/ptj/74.9.872. [DOI] [PubMed] [Google Scholar]
- Parvataneni K, Olney SJ, Brouwer B. Changes in muscle group work associated with changes in gait speed of persons with stroke. Clinical Biomechanics. 2007;22:813–820. doi: 10.1016/j.clinbiomech.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Perry J. Distal rectus femoris transfer. Dev Med Child Neurol. 1987;29:153–158. doi: 10.1111/j.1469-8749.1987.tb02130.x. [DOI] [PubMed] [Google Scholar]
- Turns LJ, Neptune RR, Kautz SA. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch Phys Med Rehabil. 2007;88:1127–1135. doi: 10.1016/j.apmr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA. Waterloo Biomechanics. 1991. The Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.