Abstract
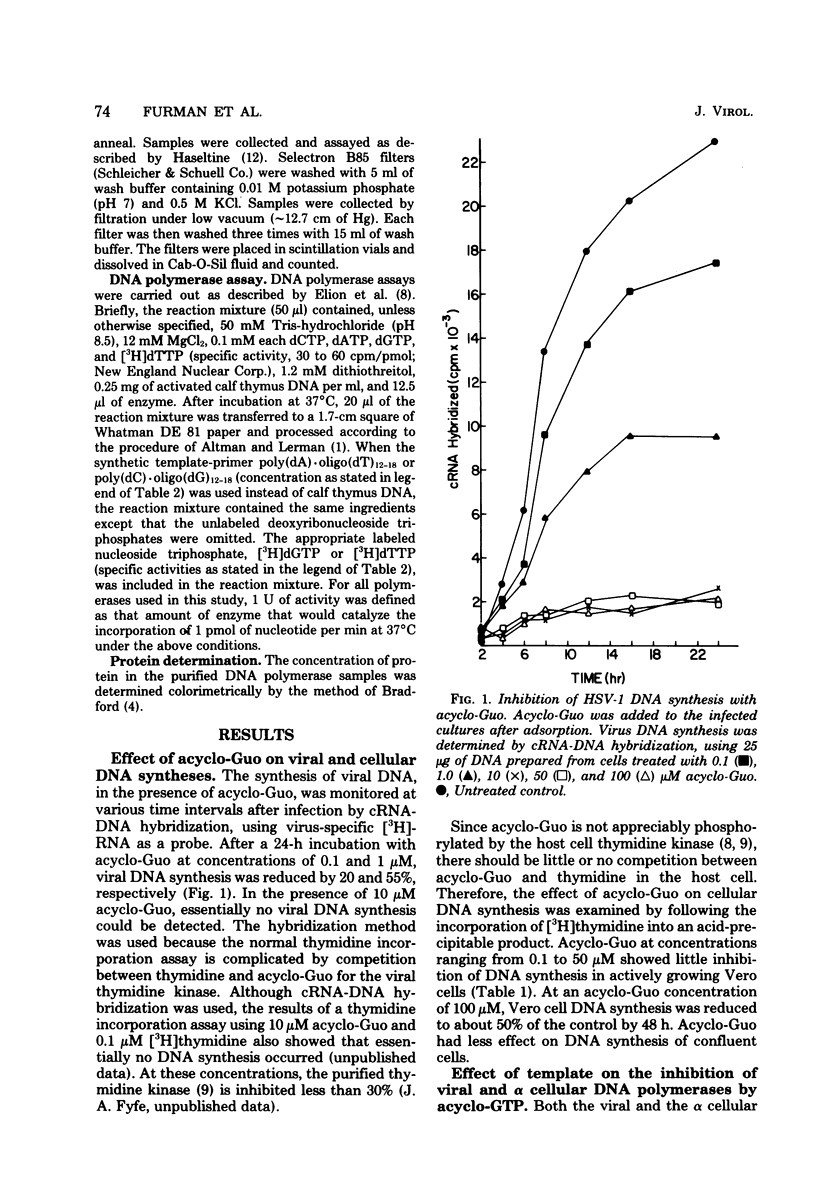
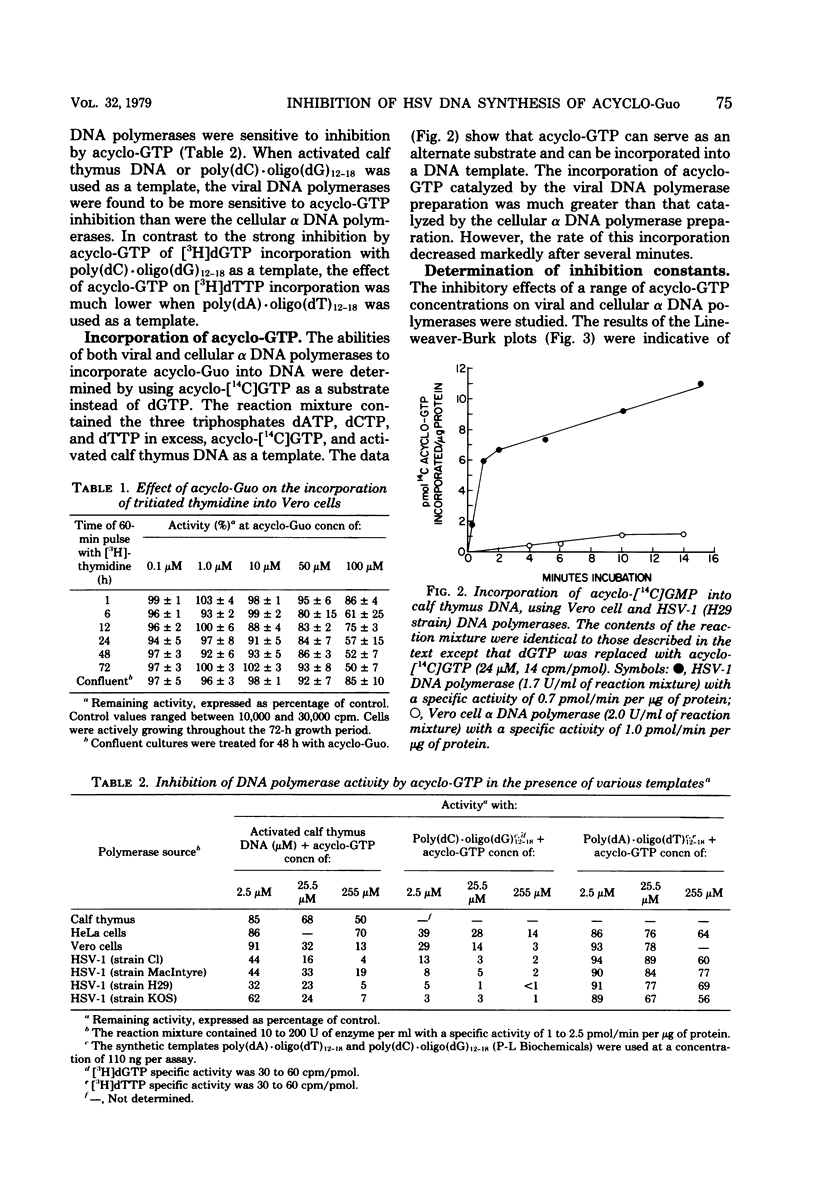
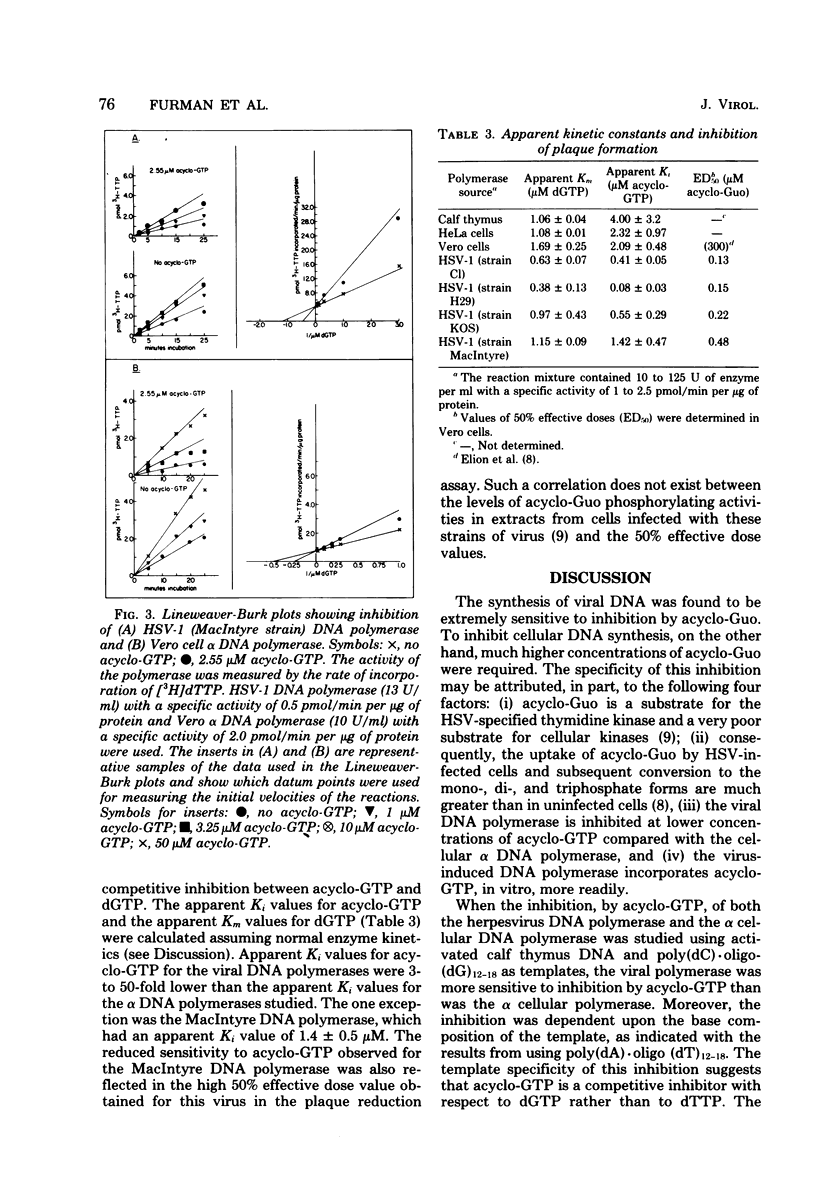
The effect of the nucleoside analog 9-(2-hydroxyethoxymethyl)guanine (acycloguanosine) on herpes simplex virus type 1 DNA synthesis was examined. Acycloguanosine inhibited herpesvirus DNA synthesis in virus-infected cells. The synthesis of host cell DNA was only partially inhibited in actively growing cells at acycloguanosine concentrations several hundred-fold greater than the 50% effective dose for herpes simplex virus type 1. Studies using partially purified enzymes revealed that the triphosphate of this compound inhibited the virus-induced DNA polymerases (DNA nucleotidyltransferases) to a greater degree than the DNA polymerase of the host cell, that the inhibition was dependent upon the base composition of the template, and that the triphosphate was a better substrate for the virus-induced polymerases than for the alpha cellular DNA polymerases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Lerman L. S. Kinetics and intermediates in the intracellular synthesis of bacteriophage T4 deoxyribonucleic acid. J Mol Biol. 1970 Jun 14;50(2):235–261. doi: 10.1016/0022-2836(70)90190-7. [DOI] [PubMed] [Google Scholar]
- Atkinson M. R., Deutscher M. P., Kornberg A., Russell A. F., Moffatt J. G. Enzymatic synthesis of deoxyribonucleic acid. XXXIV. Termination of chain growth by a 2',3'-dideoxyribonucleotide. Biochemistry. 1969 Dec;8(12):4897–4904. doi: 10.1021/bi00840a037. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Domin B. A., Sharma R. A., Bobek M. Antiviral action and cellular toxicity of four thymidine analogues: 5-ethyl-,5-vinyl-, 5-propyl-, and 5-allyl-2'- deoxyuridine. Antimicrob Agents Chemother. 1976 Jul;10(1):119–122. doi: 10.1128/aac.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Goz B., Neenan J. P., Ward D. C., Prusoff W. H. Selective inhibition of herpes simplex virus by 5-amino-2,5-dideoxy-5-iodouridine. J Virol. 1975 May;15(5):1284–1285. doi: 10.1128/jvi.15.5.1284-1285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Bauer D. J. Relative potencies of anti-herpes compounds. Ann N Y Acad Sci. 1977 Mar 4;284:49–59. doi: 10.1111/j.1749-6632.1977.tb21936.x. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Gentry G. A., Aswell J. F. Inhibition of herpes simplex virus replication by araT. Virology. 1975 May;65(1):294–296. doi: 10.1016/0042-6822(75)90034-3. [DOI] [PubMed] [Google Scholar]
- Goz B., Prusoff W. H. Pharmacology of viruses. Annu Rev Pharmacol. 1970;10:143–170. doi: 10.1146/annurev.pa.10.040170.001043. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A. In vitro transcription of Escherichia coli ribosomal RNA genes. Nature. 1972 Feb 11;235(5337):329–333. doi: 10.1038/235329a0. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Detection of Epstein-Barr viral genome in nonproductive cells. Nat New Biol. 1971 Sep 22;233(38):103–106. doi: 10.1038/newbio233103a0. [DOI] [PubMed] [Google Scholar]
- North T. W., Cohen S. S. Erythro-9-(2-hydroxy-3-nonyl)adenine as a specific inhibitor of herpes simplex virus replication in the presence and absence of adenosine analogues. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4684–4688. doi: 10.1073/pnas.75.10.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Schildkraut I., Cooper G. M., Greer S. Selective inhibition of the replication of herpes simplex virus by 5-halogenated analogues of deoxycytidine. Mol Pharmacol. 1975 Mar;11(2):153–158. [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]
- Yoshikawa M., Kato T., Takenishi T. A novel method for phosphorylation of nucleosides to 5'-nucleotides. Tetrahedron Lett. 1967 Dec;50:5065–5068. doi: 10.1016/s0040-4039(01)89915-9. [DOI] [PubMed] [Google Scholar]



