Abstract
Dietary restriction, or reduced food intake without malnutrition, increases life span, health span, and acute stress resistance in model organisms from yeast to nonhuman primates. Although dietary restriction is beneficial for human health, this treatment is not widely used in the clinic. Here, we show that short-term, ad libitum feeding of diets lacking essential nutrients increased resistance to surgical stress in a mouse model of ischemia reperfusion injury. Dietary preconditioning by 6 to 14 days of total protein deprivation, or removal of the single essential amino acid tryptophan, protected against renal and hepatic ischemic injury, resulting in reduced inflammation and preserved organ function. Pharmacological treatment with halofuginone, which activated the amino acid starvation response within 3 days by mimicking proline deprivation, was also beneficial. Both dietary and pharmacological interventions required the amino acid sensor and eIF2α (eukaryotic translation initiation factor 2α) kinase Gcn2 (general control nonderepressible 2), implicating the amino acid starvation response and translational control in stress protection. Thus, short-term dietary or pharmacological interventions that modulate amino acid sensing can confer stress resistance in models of surgical ischemia reperfusion injury.
INTRODUCTION
Ischemia reperfusion (IR) injury is an acute, multifactorial stress initiated by a temporary stoppage of blood flow that results in tissue damage, up-regulation of proinflammatory cytokines, chemokines, and cell surface adhesion molecules, and recruitment of innate immune cells (1). IR injury is a major cause of mortality and morbidity from stroke and heart attack. Numerous planned vascular procedures such as coronary artery bypass graft and carotid endarterectomy carry a risk of perioperative stroke or myocardial infarction. These ischemic events can be caused by atherosclerotic plaque rupture or microembolization of plaque material during the surgery (2). Although the incidence of stroke associated with cardiovascular surgery (0.8 to 9.7% depending on the procedure) is higher than with noncardiovascular surgery (0.08 to 0.7%) (3), perioperative mortality in patients suffering ischemic stroke is high in both (22% and 18 to 26%, respectively) (4). In 2007, there were about 7 million cardiovascular and 18 million noncardiovascular operations in the United States alone (5). Despite the considerable morbidity and mortality associated with these large numbers, effective preventative strategies are currently lacking.
Dietary restriction (DR), defined as reduced food intake without malnutrition,isbestknownforextending life span in model organisms from yeast to nonhuman primates (6–8). DR also improves health span and increases resistance to multiple forms of acute stress including paraquat toxicity (9)and IR injury(10–15) in experimental mammals. The pleiotropic effects of DR on a broad range of molecular and physiological processes likely underlies its ability to protect against multiple forms of acute stress, including stress associated with IR injury, a multifactorial insult characterized by energy depletion, loss of membrane potential, and cell death from ischemia, followed by sterile inflammation upon reperfusion. Humans on DR show metabolic fitness responses similar to those seen in laboratory mammals (16–18). Nonetheless, there are currently few, if any, clinical applications of DR in part as a result of the perception that reduced total calorie intake is required for benefit and the difficulties associated with long-term, self-imposed food restriction.
The nutritional basis of DR benefits, including the relative role of reduced calories and nutrients, is poorly characterized, particularly in mammals. In fruit flies, restriction of the protein source (yeast or casein) extends life span more than an isocaloric restriction of carbohydrate (sucrose) (19, 20). Addition of essential amino acids (EAAs) abrogates the life-span benefits of DR, but addition of EAA lacking methionine (or to a lesser degree tryptophan) does not (21, 22). Reduced insulin/insulin-like growth factor (IGF) signaling has been implicated in the benefits of DR on life-span extension and stress resistance (21), but the underlying genetic requirements for a beneficial response to DR remain poorly defined (23). In rodents, methionine or tryptophan restriction extends life span without enforced DR (24–26). Methionine restriction in mice results in a phenotype highly reminiscent of DR, including delayed aging, reduced serum Igf1, and increased resistance to acetaminophen toxicity (27). It is not known whether the benefits of methionine or tryptophan restriction are shared by other EAAs, nor have the upstream sensors, which may be best suited to pharmacological manipulation, been identified.
Amino acids are sensed in mammals by at least two distinct signal transduction pathways, involving either mTOR (mammalian target of rapamycin) or GCN2 (general control nonderepressible 2). The mTOR signal transduction pathway integrates nutrient, energy, and growth factor availability to promote anabolic processes such as fatty acid synthesis and to suppress catabolic processes such as autophagy. Although the molecular mechanism underlying amino acid recognition by the mTOR pathway is not known, mTOR signaling is strongly dependent onthepresenceofspecificaminoacids, primarily leucine but also argi-nine and methionine (28–30). In contrast, intracellular amino acid depletion is sensed directly by GCN2 kinase, which binds uncharged transfer RNAs (tRNAs) and can thus in theory detect deficiencies in any EAA or NEAA (nonessential amino acid) (31). Uncharged tRNA-bound GCN2 phosphorylates the eukaryotic translation initiation factor 2α (eIF2α)(32, 33)and inhibits 43S preinitiation complex formation, thereby repressing general translation initiation. At the same time, specific mRNAs with initiating codons downstream of noncoding micro-open reading frames (ORFs), which are normally translated with low efficiency, are derepressed when translation initiation is inefficient and the micro-ORFs upstream of the full-length ORF are skipped (34). Transcription factors such as ATF4 (activating transcription factor 4) that drive the transcriptional response to amino acid starvation contain such micro-ORFs and are translationally derepressed upon eIF2a phosphorylation (34, 35).
GCN2 is one of a family of four eIF2a kinases (32, 33) that are triggered by diverse stressors including heme depletion [heme-regulated inhibitor kinase (HRI)], viral double-stranded RNA (dsRNA) [protein kinase activated by dsRNA (PKR)], and endoplasmic reticulum (ER) stress [PKR-like ER kinase (PERK)] and that activate a coordinated shift in translation and transcription known as the integrated stress response (ISR) (36). Reduced eIF2a activity and increased ATF4 levels have been implicated in oxidative stress resistance in mammalian tissue culture models (35, 37), suggesting that oxidative stress resistance may be a common output of the ISR independent of which upstream eIF2a kinase is activated. This could occur in part through dimerization of ATF4 with NF-E2-related factor 2 (NRF2) to regulate transcription of antioxidant response element (ARE)-containing genes such as heme oxygenase-1(HO-1)(38–40). Cross talk between mTOR and GCN2 pathways has been reported in both yeast and mammals (41, 42). Reduced mTOR signaling through pharmacological or genetic means increaseslife spaninmodel mammalian organisms(43, 44).
Halofuginone (HF) is a halogenated derivative of febrifugine (45) originally described as an antimalarial agent and still in wide use as an antiprotozoal agent in poultry and cattle. In mammalian cells, HF has a number of effects including inhibition of interleukin-17 (IL-17)- producing helper T cell (TH17) differentiation through activation of the amino acid starvation response (AASR) (46). HF also inhibits collagen type I gene expression in fibroblasts (47, 48) and reduces cellular proliferation (49). In animal models, HF is effective against the inflammatory disorders experimental autoimmune encephalomyelitis (46)and colitis (50), fibrosis in various organs (51, 52), angiogenesis (53), and tumor metastasis (54, 55). In humans, it is used against scleroderma and is in clinical trials against solid tumors (56). However, the relevant targets of HF and which of its activities require activation of the AASR remain unknown.
Short-term dietary interventions that we collectively refer to as “dietary preconditioning,” including 2 weeks of 30% reduced daily food intake or up to 3 days of water-only fasting, protect against renal and hepatic IR in rodent models (15). Here, we examined the possibility that dietary protein/amino acid deficiency and pharmacological activation of the nutrient deprivation sensor Gcn2 can induce surgical stress resistance independent of reduced calorie or food intake.
RESULTS
Rapid onset of protection against renal ischemic injury by isolated protein deficiency
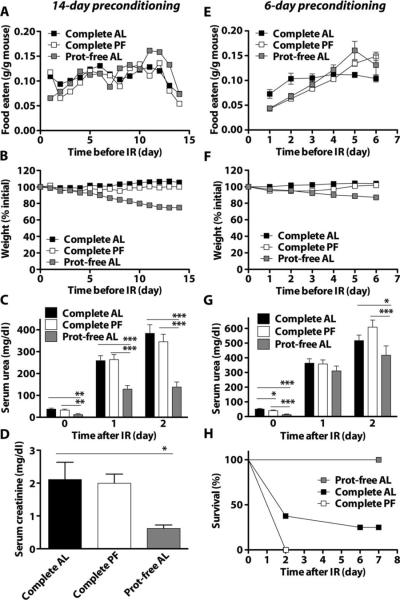
To elucidate the contribution of isolated protein deficiency to dietary preconditioning, we assigned the mice to one of three diets for 14 days: a complete diet, a protein-free diet, or a complete diet but restricted (or pair-fed) to the amount of food eaten by the protein-free group. Food intake corrected for body weight was not significantly different (Fig. 1A and fig. S1A), although mice on the protein-free diet lost weight steadily (Fig. 1B) and had reduced blood sugar (fig. S1B). Mice on a protein-free diet also tended to be more active than those on a control diet, particularly during the dark phase (fig. S1C). We then applied 35 min of bilateral renal ischemia, using microvascular clamps to occlude blood flow in or out of each kidney (ischemia) followed by clamp release and return of blood flow (reperfusion). Kidney function was monitored by measuring waste products in the blood (urea and creatinine) normally cleared by the kidneys. Serum urea was significantly reduced in the protein-free group compared to the complete diet or pair-fed groups on both days 1 and 2 after reperfusion (Fig. 1C). On day 2, serum creati-nine was significantly lower in the protein-free group than in the complete diet group (Fig. 1D). Postoperative weight loss was reduced in the protein-free group compared to the complete diet or pair-fed groups (fig. S1D). Similar results were obtained with a shorter preconditioning period of 6 days for total food intake, weight loss, and blood glucose before surgery (Fig. 1, E and F, and fig. S1, E and F), and preservation of organ function (Fig. 1G) and weight change after renal IR (fig. S1G). Protein deficiency also imparted a significant survival advantage over ad libitum or pair-feeding of the control diet (Fig. 1H). Therefore, short-term, ad libitum feeding of a protein-deficient diet protected against renal IR. Although mice on a protein-free diet ate less food per mouse than those on a complete diet (fig. S1, A and E, bottom), food intake was similar when corrected for their smaller body size (fig. S1, A and E, top), a phenomenon common to both DR and methionine restriction (27) in rodents.
Fig. 1.
Protection against renal IR by dietary protein deficiency in the absence of reduced calorie intake.(A to H) Wild-type male B6D2F1 mice were given ad libitum (AL) access to complete or protein (Prot)-free chow, or pair-fed (PF) to the protein-free chow group with complete chow, for 14 days (A to D) or 6 days (E to H) before induction of renal IR injury. (A and E) Body weight of mice expressed as percent initial body weight: (A) n = 9 to 14 per group; (E) n = 12 per group. (B and F) Daily food intake expressed as weight of food eaten per total weight of animals in the cage: (B) n = 2cages per group; (F) n = 3 to 5 cages per group. (C and G) Serum urea on the indicated day before (day 0) or 1 or 2 days after renal IR: (C) n = 7 to 9 per group; (G) n = 7 to 8 per group. (D) Serum creatinine on day 2 after renal IR (n = 3 to 4 per group). (H) Kaplan-Meier survival curves of the indicated groups over 7 days after renal IR (n = 7 to 8 per group). Survival in the protein-free group was significantly improved over both complete diet groups (log-rank test: P = 0.0022 versus AL; P = 0.0001 versus PF). Error bars indicate SEM. Asterisks indicate the significance of the difference between the indicated groups within a given day after IR according to a one-way ANOVA followed by Tukey's multiple comparison test comparing all pairs of values. *P < 0.05; **P < 0.01; ***P < 0.001.
Protection against renal IR by isolated EAA deprivation
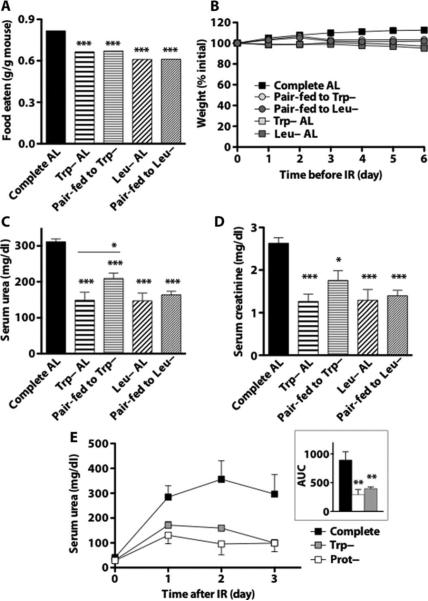
We next asked whether depletion of individual EAA could have effects similar to those of total protein depletion by giving mice ad libitum access to diets deficient in methionine (Met–), tryptophan (Trp–), or leucine (Leu–) for 6 days. Aversion toward each incomplete diet was observed, the greatest being toward Met– (fig. S2, A and B). Despite variable food intake, mice on all four incomplete diets had similar reductions in body weight and blood glucose (fig. S2, C and D) relative to those on the complete diet, as well as improved urea clearance, postoperative weight loss, and survival after 35 min of bilateral renal IR (fig. S2, E to G). To isolate the effects of EAA deprivation from reduced food intake, which may independently modulate resistance to ischemic injury (15), we tested the two least aversive amino acid–deficient diets, Trp– and Leu–, in comparison to pair-fed controls. Mice on Trp– and Leu– diets ate 18 and 25% less, respectively, than those on the complete diet (Fig. 2A) and lost slightly but significantly more weight than the corresponding pair-fed groups (Trp– 97.2% initial weight versus 103.3% for corresponding pair-fed control, P < 0.001; Leu– 95.1% initial versus 102.0% for pair-fed, P < 0.0001; Fig. 2B). Mice were then subjected to 25 min of bilateral renal IR to better determine functional differences (rather than measuring the differences in mortality associated with longer ischemia times). Although kidney function was significantly improved in all groups relative to the ad libitum complete diet group (Fig. 2, C and D), tryptophan deficiency protected kidney function relative to its pair-fed control (serum urea significantly lower than pair-fed) more than did leucine deficiency relative to its pair-fed control. Thus, a modest DR of 18 to 25% for 6 days alone protected against IR, and additional benefits of deficiency of at least one EAA, tryptophan, could be experimentally separated from those of reduced food intake alone.
Fig. 2.
Protection against renal IR by isolated EAA deprivation. (A) Total food intake over 6-day preoperative period expressed as weight of food eaten per total weight of animals in the cage (n = 2 cages per group). (B) Body weights of mice fed complete, Trp– or Leu– chow ad libitum (AL) or pair-fed (PF) to Trp– or Leu– animals with complete chow over the 6-day preoperative period (n = 8 per group) expressed as percent initial weight. (C and D) Kidney function as measured by serum urea (C) and creatinine (D) 1 day after 25 min of renal IR. (E) Effect of isocaloric protein- (Prot–) and tryptophan-deficient diets on kidney function, as measured by serum urea. Mice were preconditioned for 1 week on the indicated diet restricted daily to 0.28 kcal per gram of initial weight (~35% DR) before and up to 3 days after 30 min of renal ischemia (n = 5 per group). Inset: Area under the curve (AUC) analysis. Error bars indicate SEM. Asterisks indicate the significance of the difference between the indicated group and the complete diet group according to a one-way ANOVA followed by Dunnett's multiple comparison test, or between the Trp– or Leu– groups and their respective pair-fed controls as indicated with Bonferroni'smultiple comparison test. *P <0.05; **P < 0.01; ***P < 0.001.
To compare the protection afforded by total protein and single EAA (tryptophan) deficiency to that afforded by a complete diet, we restricted each dietary group to 0.28 kcal per gram of body weight per day on the basis of their initial weights (~35% DR). Under these conditions, all food was consumed each day, thus normalizing calorie intake among groups independent of changes in weight due to diet. Kidney function as measured by serum urea for 3 days after 30 min of bilateral renal IR was significantly improved in both protein- and tryptophan-free diet groups relative to the complete restricted diet, with no significant difference between them (Fig. 2E). Thus, at 35% DR, single EAA (tryptophan) deficiency was as effective as total protein deprivation in protecting kidney function from ischemic stress, and both were significantly better than a complete diet.
Requirement for Gcn2 in dietary preconditioning against renal and hepatic ischemia
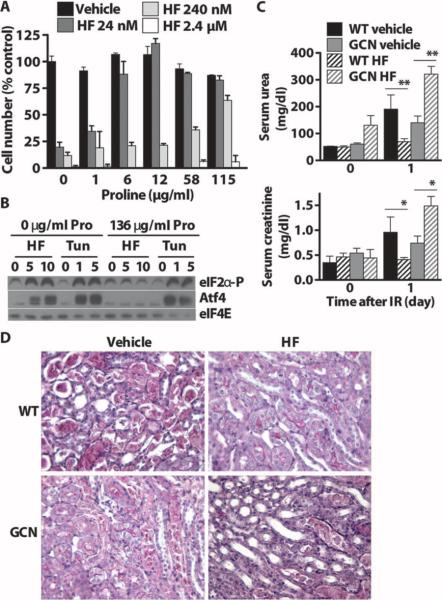
Because GCN2-based amino acid sensing can in theory respond to deficiency of any amino acid, whereas mTOR-based sensing detects primarily the presence of leucine, we tested the genetic requirement for Gcn2 in protection from ischemic injury by isolated tryptophan deficiency. Wild-type males showed significant weight loss on the Trp– diet, whereas Gcn2−/− males did not (Fig. 3A) despite eating less than Gcn2−/− animals on the complete diet (fig. S3A). Both groups had significantly reduced blood glucose compared to their own pair-fed controls (fig. S3B). One day after 25 min of bilateral renal IR, Trp– wild-type mice had significantly reduced serum creatinine (Fig. 3B) and urea (fig. S3C) relative to wild-type mice pair-fed a complete diet. On a complete diet, Gcn2−/− mice had reduced serum urea and creatinine, consistent with constitutive protection against renal IR relative to wild-type mice. However, the benefit of the Trp– diet observed in wild-type animals was absent in Gcn2−/− mice (Fig. 3B). Damage marker kidney injury molecule 1 (Kim1) mRNA (Fig. 3C) and acute tubular necrosis as assessed histologically for necrosis of tubular epithelial cells and loss of PAS-stained brush border at the corticomedullary junction (Fig. 3D and fig. S3D) 1 day after IR showed changes consistent with renal functional readouts (creatinine).
Fig. 3.
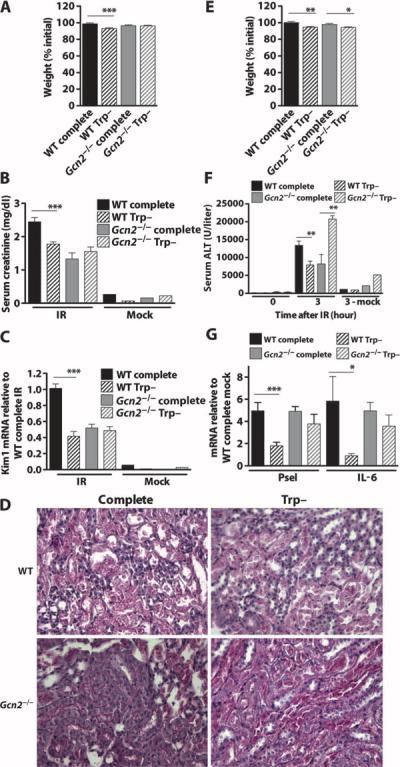
Requirement for Gcn2 in tryptophan deficiency-mediated protection against renal and hepatic IR. (A to D) Gcn2-mediated protection by tryptophan deficiency against renal IR. (A) Body weights of male mice (n = 12per group) fed the indicated diet for 6 days expressed as percent initial weight. (B) Renal function as indicated by serum creatinine 1 day after renal IR (n = 10 per group) or mock ischemia (n = 2 per group). (C) Kidney damage as indicated by mRNA abundance of kidney damage marker Kim1 mRNA as detected by quantitative PCR (qPCR) in kidney samples harvested 1 day after renal IR (n = 9 to 10 per group) or mock ischemia (n = 2 per group) and expressed relative to the wild-type (WT) complete IR group. (D) Representative images of PAS-stained kidney sections 1 day after reperfusion showing the corticomedullary junction, where most tubular damage occurs. (E to G) Gcn2-mediated protection by tryptophan deficiency against hepatic IR. (E) Body weights of female mice (n = 12 to 13 per group)fed the indicated diet for 6 days expressed as percent initial weight. (F) Serum ALT before (0 hours) or 3 hours after 45 min of hepatic ischemia (n = 5 to 10 per group) or mock IR (n = 2 to 3 per group). (G) mRNA abundance of proinflammatory markers P-selectin (Psel) and IL-6 as detected by qPCR in liver samples harvested 3 hours after hepatic ischemia (n = 4 to 5 per group) expressed relative to the WT mock-treated group. Error bars indicate SEM. Asterisks indicate the significance of the difference between the indicated groups by Student's t test for effect of diet within the same genotype. *P < 0.05; **P < 0.01; ***P < 0.001.
To test whether the benefits of a Trp– diet on ischemic injury were sexor organ-specific, we used a model of liver ischemia in female wild-type and Gcn2−/− animals. Six days of tryptophan deficiency resulted in significant weight loss in both wild-type and Gcn2−/− females (Fig. 3E), despite significant reduction in food intake only in wild type (fig. S3E). Serum markers of liver damage [alanine transaminase (ALT), aspartate transaminase (AST), and lactate dehydrogenase (LDH)] were significantly reduced in the wild-type Trp– group compared to wild type after 45 min of ischemia and 3 hours of reperfusion (Fig. 3F and fig. S3, F and G). However, ALT values in the Gcn2−/− Trp– group were significantly higher than controls. Inflammatory gene expression markers P-selectin and IL-6 were reduced in Trp– wild-type mice but not in Trp– Gcn2−/− mice(Fig.3G). Together, our data indicate that the benefits of isolated tryptophan deficiency on ischemic injury required Gcn2 but were not sex- or organ-specific.
Activation of the AASR in liver but not in kidney upon tryptophan deficiency
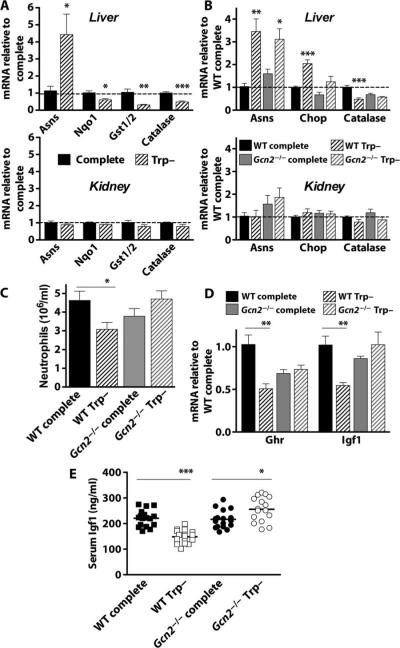
We hypothesized that activation of the ISR in both liver and kidney would lead to transcriptional up-regulation of metabolic and oxidative stress-related genes thought to be common to the ISR independent of which eIF2α kinase triggers the response (35–37). After 6 days of tryptophan deficiency, liver expression of the metabolic stress gene asparagine synthase (Asns) was significantly up-regulated. However, contrary to our hypothesis, oxidative stress resistance-related genes NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) dehydrogenase quinone 1 (Nqo1), glutathione synthetase (Gst1/2), and catalase were significantly down-regulated; none of these changes was observed in kidney (Fig. 4A). We next looked at the requirement for Gcn2. In Gcn2−/− livers, Asns was significantly differentially regulated upon tryptophan deficiency, whereas the ISR negative feedback control transcription factor Chop (C/EBP homologous protein) and catalase were not (Fig. 4B). No significant differences in expression of these genes were observed in the kidney of either genotype as a function of diet. A limitation of this study is that changes in protein levels could be different from those in mRNA levels. Nevertheless, at least as measured by mRNA concentrations, the response to amino acid starvation was tissue-specific and did not share up-regulation of oxidative stress resistance genes with other ISR triggers such as ER stress.
Fig. 4.
Organ-specific and systemic activation of the AASR by tryptophan deficiency. (A) Gene expression as determined by qPCR on material prepared from liver (top) and kidney (bottom) from wild-type (WT) male B6D2F1 mice preconditioned for 6 days with ad libitum access to tryptophan-deficient (Trp–) chow or pair-fed on complete chow (n = 4 to 5 per group). Gene expression is presented relative to complete diet treatment group (dashed line). (B) Gene expression in liver (top) and kidney (bottom) from WT or Gcn2−/−C57BL/6 male mice (n = 4 to 5 per group) on the indicated diet for 6 days. Gene expression is presented relative to the WT complete diet treatment group (dashed line). (C) Numbers of peripheral neutrophils in whole blood prepared from mice as in (B). (D) Expression of growth hormone receptor (Ghr) and insulin-like growth factor 1 (Igf1) mRNAs in liver as determined by qPCR (n = 4 to 5 per group) normalized to the WT complete diet treatment group. (E) Serum Igf1 protein levels after 6 days on the indicated diet (n = 16 per group). Error bars indicate SEM. Asterisks indicate the significance of the difference between the indicated groups by Student's t test for effect of diet within the same genotype. *P < 0.05; **P < 0.01; ***P < 0.001.
Systemic changes upon tryptophan deficiency mediated by Gcn2
We next looked for evidence of systemic Gcn2-dependent factors that may underlie protection from IR by tryptophan deficiency. Because protein/energy malnutrition can inhibit innate immune function, in part through reduced numbers of leukocytes, we measured the effects of isolated tryptophan deficiency on blood cell composition. In animals fed a complete diet, there were no differences between wild-type and Gcn2−/− mice in any circulating leukocyte compartment, although red blood cells (RBCs) and platelets were slightly but significantly decreased (fig. S4A). However, upon tryptophan deficiency, wild-type mice showed a significant reduction in circulating neutrophils that was dependent on Gcn2 (Fig.4C). Furthermore, the ratios of granulocytes in mice on a Trp– versus complete diet were significantly increased in Gcn2−/− animals relative to wild type for all three lineages (neutrophils, eosinophils, and basophils; fig. S4B). Igf1 is an important growth factor for myeloid lineages and can also prime immune and endothelial cells for activation upon damage. In liver, the major source of growth hormone–dependent Igf1 production, growth hormone receptor (Ghr) and Igf1 mRNA levels were significantly reduced upon tryptophan deficiency in wild-type but not Gcn2−/− mice (Fig. 4D). Serum Igf1 protein levels followed the same pattern (Fig. 4E). Together, our data show that tryptophan deficiency elicited a systemic repression of granulocyte numbers in a Gcn2-dependent manner, possibly through a reduction in serum Igf1.
Preconditioning against renal IR with HF
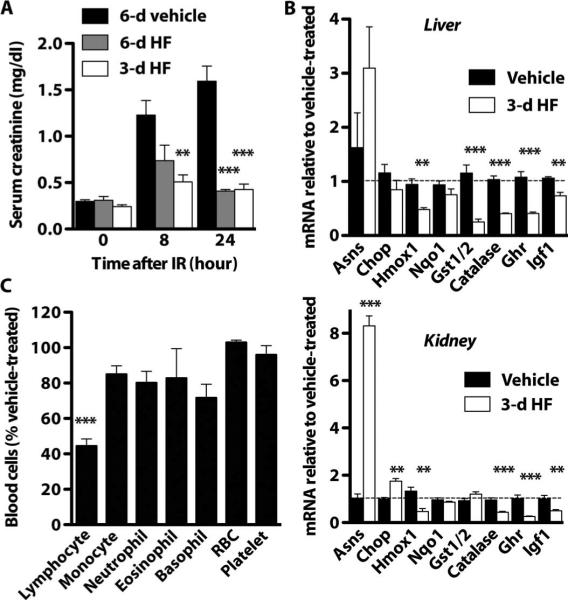
On the basis of our finding that dietary amino acid starvation protected against IR, we next tested a pharmacological compound, HF, that activates the AASR (46) as a potential dietary preconditioning mimetic against renal IR. Daily injection of HF (0.2 mg/kg) to wild-type mice for 3 to 6 days resulted in robust protection against renal IR as measured by preservation of renal function (serum creatinine, Fig. 5A; urea, fig. S5A) and reduced damage (serum LDH release, fig. S5B). The transcriptional response to HF resembled dietary tryptophan deficiency but was present in both liver and kidney (Fig. 5B). HF treatment for 3 days also resulted in a significant reduction of circulating lymphocytes (Fig. 5C). These changes occurred in the absence of significant differences in the percent initial body weight or blood glucose (fig. S5, C and D).
Fig. 5.
Protection against IR by pharmacological activation of the AASR. Preconditioning with HF for 3 to 6 days protected against renal IR. (A) Serum creatinine before (0 hours) or 8 to 24 hours after 25 min of bilateral renal IR (n = 5 per group). (B) Gene expression changes in liver (top) and kidney (bottom) upon 3 days of HF treatment as measured by qPCR and presented relative to the vehicle-treated group (n = 4 to 5 per group). (C)Complete blood cell counts upon 3 days of HF treatment expressed as a percentage of the vehicle-treated (n = 5 per group). Error bars indicate SEM. Asterisks indicate the significance of the difference between the indicated group and the vehicle-treated group by one-way ANOVA followed by Dunnett's multiple comparison test (A) or by Student's t test for effect of HF treatment (B and C). **P < 0.01; ***P < 0.001.
If HF mimics amino acid deprivation in vivo, which amino acids are involved and is Gcn2 required? In T cells, the effects of HF on TH17 cell differentiation in vitro are abrogated by excess amino acids (46). We used cytotoxicity of HF in spontaneously immortalized mouse embryonic fibroblasts (MEFs) as a biological endpoint. Individual amino acids were titrated against varying doses of HF, and cell survival was measured by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. Of 10 EAAs and 7 NEAAs tested, only l-proline rescued HF cytotoxicity; the biologically inactive D enantiomer of proline had no effect (Fig. 6A and fig. S6A). To confirm these findings with a different biological endpoint, we tested the effects of proline on the ability of HF to increase eIF2α phosphorylation and derepress Atf4 translation in MEFs. eIF2α phosphorylation and Atf4 stabilization by HF were dose-dependent, and both were abrogated by excess l-proline (Fig. 6B). As a control, eIF2α phosphorylation and Atf4 stabilization induced by tunicamycin, an ER stress–inducing agent that promotes eIF2α phosphorylation by a different kinase, PERK, were not affected by proline supplementation. We next asked whether the benefits of HF on renal IR were dependent on Gcn2. Three days of HF preconditioning did not affect weight loss in wild-type or Gcn2−/− mice (fig. S6B). One day after 25 min of bilateral renal IR, renal function was significantly better in HF-pretreated wild-type mice but worse in HF-pretreated Gcn2−/− mice (Fig. 6C). Histological evidence was consistent with functional protection by HF in the wild-type but not Gcn2−/− mice (Fig.6D). Thus, HF can function in vivo as a dietary preconditioning mimetic.
Fig. 6.
Block of HF activity by excess proline in vitro and requirementfor Gcn2 in vivo. (A) Cytostatic/cytotoxic effects of increasing concentrations of HF added to proliferating MEF cultures and blockade by addition of excess proline to the medium as measured by the MTT assay and expressed as a percentage of cell number in the vehicle-treated group with no additional proline. (B) Phospho-eIF2α and Atf4 immunoblots of extracts prepared from MEFs treated with HF (0, 5, or 10 nM) or tunicamycin (Tun) (0, 1, or 5 μg/ml) for 3 hours in complete medium with or without proline (Pro) supplementation. eIF4E was used as a loading control. (C) Protection by HF against renal IR required Gcn2. Serum urea (top) and creatinine (bottom) before (day 0) and 1 day after 25 min of bilateral renal IR. Error bars indicate SEM. Asterisks indicate the significance of the difference between the indicated groups by Student's t test for effect of diet within the same genotype. *P < 0.05; **P < 0.01. (D) Representative images of PAS-stained kidney sections 1 day after reperfusion showing the corticomedullary junction, where most tubular damage occurs.
DISCUSSION
Together, our data are consistent with the notion that ad libitum feeding of a nutritionally complete diet before surgical ischemia is a risk factor for inflammatory injury in preclinical models of IR injury. On the other hand, reduced intake of a complete diet, or ad libitum feeding of a diet deficient in protein or EAAs for as little as 6 days, provided significant protection. Pharmacological activation of the AASR with HF also provided protection. The protective effects of tryptophan deficiency and HF pretreatment were both dependent on the amino acid deprivation sensor Gcn2, indicating a role for translational control in AASR-induced stress resistance.
DR can be beneficial in humans and experimental organisms as measured by a number of endpoints, including acute stress resistance and longevity. In humans, these benefits include clinically relevant metabolic parameters such as blood pressure, glucose homeostasis, and blood lipid profiles (16–18). Clinical applications of DR against acute stress are practically nonexistent, perhaps because of the difficulty of self-imposed food restriction and an assumption that DR benefits take a long time to accrue. Contrary to this notion, we and others have shown rapid onset of benefits of dietary preconditioning, including DR and fasting, in models of renal and hepatic ischemia (15, 57), and therapy with chemotherapeutic agents (58). Here, we have shown that the benefits of reduced total food/calorie intake and reduced protein/EAAs are separable. Thus, reduced food intake per se is not required for the benefits of dietary preconditioning, obviating the difficulty of adherence to reduced food intake. Nevertheless, our data also suggest that reduced nutrient intake combined with reduced calorie intake may together be the most efficacious preconditioning regimen.
Our results are also consistent with a body of literature from lower, nonmammalian organisms, indicating that nutrient restriction rather than calorie restriction per se is a key variable in the benefits of DR (19, 21). Investigations of this issue in mammals have yielded equivocal results (59–61), likely a result of the reliance on life span as an experimental endpoint, which constraints the experimental diets to those compatible with long-term survival. Here, we separated the effects of nutrient deprivation from calorie intake using clinically relevant, acute stress endpoints (instead of longevity) and short-term interventions compatible with incomplete diets. We conclude that nutrient restriction can contribute to DR benefits in mammals as in flies, consistent with evolutionary conservation of the nutritional basis of DR. Our data also support the idea that coordinately regulated reductions in food intake and body size are common both to DR (imposed through reduced food availability) and amino acid restriction (likely through initial aversion to food intake).
We also identified a role for Gcn2 in mediating the beneficial effects of amino acid starvation on stress resistance. Our data indicate that the response to dietary amino acid starvation is tissue-specific and does not involve transcriptional up-regulation of ARE-containing genes such as Nqo1. Instead, we revealed a requirement for Gcn2 in the systemic response to dietary tryptophan deficiency, which included reduced serum Igf1 and reduced numbers of peripheral granulocytes. Reduced insulin/IGF-1 signaling is synonymous with extended longevity and increased stress resistance (62, 63). In lower organisms, these effects depend on activation of Foxo transcription factors and presumably increased expression of Foxo target genes, such as those involved in oxidative stress resistance. Our data showed no significant increase in expression of candidate antioxidant genes in the liver after tryptophan deficiency. However, in mammals, Igf1 also plays a prominent role in regulation of the immune system as a growth factor for myeloid lineages (64). Igf1 can also contribute to inflammation by enhancing expression of endothelial cell adhesion molecules (65), priming granulocytes for activation (66), and suppressing granulocyte apoptosis (67). We observed fewer circulating neutrophils after tryptophan deficiency and reduced expression of proinflammatory and cell adhesion markers after ischemic insult. Thus, reduced Igf1 caused by tryptophan deficiency may have a greater effect on inflammatory capacity than on oxidative stress resistance. Although there is precedent for Gcn2-dependent immunosuppression of the adaptive immune system [for example, in Ido-mediated T cell anergy(68) and asparaginase-based immunosuppression (69)], direct effects on granulocytes have not been previously reported. T cells are also implicated in ischemic injury, because severe combined immunodeficient (SCID) or Rag−/− mice lacking functional B or T lymphocytes are partially protected from damage (70). HF inhibits T cell–mediated proinflammatory cytokine secretion by suppression of nuclear factor κB(NF-κB) activity (71) and prevents in vitro differentiation of proinflammatory TH17 cells (46). IL-17 has been implicated in renal IR injury, although neutrophils rather than T cells appear to be the major source (72). Future studies will be required to clarify in which tissues and cells GCN2 activation is required for adaptive stress resistance.
The observation that the lack of Gcn2 on its own, in the absence of any dietary intervention, led to protection against IR is paradoxical because activation of Gcn2 by either tryptophan deficiency or HF also led to protection. This could be a result of different roles of Gcn2 before and after acute stress. Although Gcn2 activation before stress resulted in potentially beneficial systemic changes (reduced Igf1, reduced circulating leukocytes), the lack of Gcn2 may be beneficial after stress for different reasons. In the case of renal damage by the ER stress activator tunicamycin, the lack of the proapoptotic transcription factor Chop, a downstream target of Atf4 activation, is protective (73). Thus, activation of Gcn2 after stress may actually be detrimental if it increases apoptotic signaling and results in more cell death. Another nonmutually exclusive possibility is that the lack of Gcn2 results in compensatory activation of aspects of Gcn2 signal transduction through a different eIF2α kinase, for example, PERK. Recent studies indicate that Gcn2 may function as a repressor of mTOR (74). De-repression of mTOR in the absence of Gcn2 could potentially increase protein synthesis, increase ER stress, and activate the unfolded protein response, resulting in the activation of PERK and stabilization of Atf4.
A useful strategy for harnessing the health benefits of DR could be pharmacological activation of DR target pathways by DR mimetics, abrogating the need for dietary changes. Known DR mimetics such as resveratrol and metformin are thought to work by activating pathways that regulate the response to reduced energy, including the NAD-dependent sirtuins and adenosine monophosphate (AMP)–activated protein kinase. Here, we identified a pathway involved in dietary preconditioning, the AASR, through manipulation of diet and then tested compounds already known to activate this response in the absence of dietary intervention. Our data suggest that HF may act as a dietary preconditioning mimetic. In addition to its ability to precondition against renal IR, we demonstrated the dependence of its activity on Gcn2 and the ability of l-proline, but not d-proline or any of 16 other amino acids, to competitively inhibit its biological activity. Consistent with our finding, dietary l-proline can mitigate the skin-weakening effects of HF in chickens (75). Further studies will be required to clarify the role of proline in the activation of AASR by HF.
Alternate day calorie restriction for 2 months is well tolerated and is highly effective in reducing levels of oxidative stress and inflammation in human subjects (76, 77). Short-term, preoperative dietary interventions are feasible and safe in patients participating in living organ donation for transplantation (78). However, dietary recommendations are not a routine component of medical management around the time of surgery (79), with the exception of preoperative overnight fasting, which serves a different purpose (80). What is still required is a demonstration that short-term dietary preconditioning can reduce surgical stress in humans. For this test, vascular procedures with high risk of ischemic injury such as carotid endarterectomy may be appropriate. In addition, we need to know what diet works best and for how long to apply it before surgery. Our data indicate that isolated protein or amino acid deficiency can modulate stress resistance independent of DR; however, the benefits of energy and nutrient restriction may be additive or even synergistic. Finally, our studies were performed in young, healthy rodents; it will be necessary to determine whether this approach will work when needed most, such as in elderly or obese individuals. For example, in some experimental models of ischemic preconditioning, cardioprotection is lost as a function of age (81).
In conclusion, we report that dietary activation of an evolutionarily conserved response to amino acid starvation protected mice against IR injury. This protection was mediated by the Gcn2 kinase, implicating translational control in dietary preconditioning. We also showed that HF, a drug already in clinical trials as a chemotherapeutic agent, can act as a dietary preconditioning mimetic against surgical stress in preclinical models. Modulation of the AASR by brief dietary or pharmacological interventions may thus be a promising approach for mitigation of surgical stress, including IR injury.
MATERIALS AND METHODS
Mice
Eight-week-old C57BL/6 and B6D2F1 mice were purchased from the Jackson Laboratory. Gcn2 knockout and control mice on a C57BL/6 background were bred at our facility. Animals were kept under standard laboratory conditions and allowed free access to water and food except as noted. All experiments were performed with the approval of the appropriate institutional animal care and use committee.
Preconditioning regimens
Mice were given ad libitum access to isocaloric protein or single EAA-deficient diets (Research Diets) or pair-fed a complete diet for 6 to 14 days before induction of ischemia or tissue harvest. No morbidity or mortality was observed as a function of the diets alone. HF hydrochloride (Santa Cruz Biotechnology) was solubilized in dimethyl sulfoxide and diluted in phosphate-buffered saline (PBS).
IR models
Mice were anesthetized by isoflurane inhalation, and body temperature was maintained on a water-circulating heat pad until recovery from anesthesia. Ischemia was induced after a midline abdominal incision. For renal IR, the left renal pedicle was localized and the renal artery and vein were occluded for 25 to 35 min with an atraumatic microvascular clamp (Roboz). The procedure was repeated immediately on the right kidney. Liver IR was performed by visualizing the liver hilus and clamping the portal vein, hepatic artery, and bile duct to the median and left hepatic lobes for 45 min. In this model, 70% of the liver tissue becomes ischemic, and blood outflow from the small intestine is preserved through the right anterior and caudate liver lobes. After inspection for signs of ischemia (purple color in kidney, pale color in liver), the incision was covered with PBS-soaked cotton and the animal was placed under a heating pad and an aluminum foil blanket to maintain body temperature. After release of the clamp, restoration of blood flow was inspected by return of the ischemic organ to normal color. Animals were returned to clean cages and allowed ad libitum access to a complete diet after surgery.
Blood measurements
Blood glucose determinations were performed on fresh blood with an Easy Check Diabetes Meter Kit (Home Aide Diagnostics) according to the manufacturer's instructions. Complete blood cell counts were analyzed with a Hemavet blood analyzer according to the manufacturer's instructions. Serum urea and creatinine levels were measured with QuantiChrom assay kits (BioAssay Systems) based on the improved Jung and Jaffe methods, respectively. Serum ALT, AST, and LDH levels were determined by kinetic analysis in a 96-well format with a BioTek microplate reader. Serum Igf1 levels were determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems).
Histology
Organs were harvested, fixed in formalin, and embedded in paraffin. Sections (3 μm) were stained with periodic acid–Schiff (PAS) or hematoxylin and eosin (H&E).
Quantitative real-time polymerase chain reaction
Total RNA was extracted from frozen tissue with RNA Bee (Qiagen), and hexamer-primed complementary DNA (cDNA) was synthesized with Verso cDNA Kit (Thermo Scientific) according to the manufacturer's instructions. Quantitative real-time polymerase chain reaction (PCR) was performed with a MyIQ (Bio-Rad) with SYBR Green. Relative expression was calculated with the ΔΔCt method. Each sample was tested in duplicate at least twice.
Immunoblotting
Cells were lysed in a nonionic detergent buffer, cleared by centrifugation, resolved by SDS–polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes for blotting with antibodies against Atf4 (Santa Cruz Biotechnology), phopho-eIF2α, and eIF4E (Cell Signaling).
Statistics
Data are expressed as the means ± SEM. Statistical analyses were performed in GraphPad Prism with an unpaired, two-tailed Student's t test, one-way analysis of variance (ANOVA), or Kaplan-Meier survival analysis as indicated.
Supplementary Material
Acknowledgments
We thank R. de Bruin and M. Verweij for insightful discussions; C.-H. Lee for critical reading of the manuscript; K. Brand and Harvard Catalyst for help with statistical analyses; K. Inouye and A. White for technical assistance; and D. Ron and G. Hotamisligil for sharing mouse strains. Funding: Supported by NIH (National Institute on Aging, AG036712; National Institute of Diabetes and Digestive and Kidney Diseases, DK090629), Ellison Medical Foundation, American Federation for Aging Research, and the William F. Milton Fund.
Footnotes
SUPPLEMENTARY MATERIAL www.sciencetranslationalmedicine.org/cgi/content/full/4/118/118ra11/DC1
Author contributions: W.P., L.R., S.V., P.M., J.G., A.C., T.C., and J.R.M. designed and performed the experiments. W.P. and J.R.M. wrote the paper. J.R.M. provided funding.
Competing interests: J.R.M. has been a consultant for L-Nutra, a company that develops medical food to fight diseases, including cancer.
REFERENCES AND NOTES
- 1.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 2.Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, Schulz R, Erbel R. Coronary microembolization: From bedside to bench and back to bedside. Circulation. 2009;120:1822–1836. doi: 10.1161/CIRCULATIONAHA.109.888784. [DOI] [PubMed] [Google Scholar]
- 3.Selim M. Perioperative stroke. N. Engl. J. Med. 2007;356:706–713. doi: 10.1056/NEJMra062668. [DOI] [PubMed] [Google Scholar]
- 4.Kikura M, Bateman BT, Tanaka KA. Perioperative ischemic stroke in non-cardiovascular surgery patients. J. Anesth. 2010;24:733–738. doi: 10.1007/s00540-010-0969-3. [DOI] [PubMed] [Google Scholar]
- 5.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl. Health Stat. Report. 2010;29:1–20. 24. [PubMed] [Google Scholar]
- 6.Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- 7.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 8.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson A, Liu F, Adamo ML, Van Remmen H, Nelson JF. The role of insulin and insulin-like growth factor-I in mammalian ageing. Best Pract. Res. Clin. Endocrinol. Metab. 2004;18:393–406. doi: 10.1016/j.beem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: Possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 12.Chandrasekar B, Nelson JF, Colston JT, Freeman GL. Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2094–H2102. doi: 10.1152/ajpheart.2001.280.5.H2094. [DOI] [PubMed] [Google Scholar]
- 13.Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: Evidence for a preconditioning mechanism. J. Neurosci. Res. 1999;57:830–839. [PubMed] [Google Scholar]
- 14.Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Müller C, de Jong M, van IJcken W, IJzermans JN, Hoeijmakers JH, de Bruin RW. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010;9:40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E, Pennington CALERIE Team Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- 18.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Washington University School of Medicine CALERIE Group, Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: A randomized controlled trial. Am. J. Clin. Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech. Ageing Dev. 2006;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of life-span by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troen AM, French EE, Roberts JF, Selhub J, Ordovas JM, Parnell LD, Lai CQ. Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet. Age. 2007;29:29–39. doi: 10.1007/s11357-006-9018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: From life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J. Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 25.Segall PE, Timiras PS. Patho-physiologic findings after chronic tryptophan deficiency in rats: A model for delayed growth and aging. Mech. Ageing Dev. 1976;5:109–124. doi: 10.1016/0047-6374(76)90012-9. [DOI] [PubMed] [Google Scholar]
- 26.Ooka H, Segall PE, Timiras PS. Histology and survival in age-delayed low-tryptophan-fed rats. Mech. Ageing Dev. 1988;43:79–98. doi: 10.1016/0047-6374(88)90099-1. [DOI] [PubMed] [Google Scholar]
- 27.Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajo T, Yamatsuji T, Ban H, Shigemitsu K, Haisa M, Motoki T, Noma K, Nobuhisa T, Matsuoka J, Gunduz M, Yonezawa K, Tanaka N, Naomoto Y. Glutamine is a key regulator for amino acid-controlled cell growth through the mTOR signaling pathway in rat intestinal epithelial cells. Biochem. Biophys. Res. Commun. 2005;326:174–180. doi: 10.1016/j.bbrc.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Xu G, Kwon G, Marshall CA, Lin TA, Lawrence JC, Jr., McDaniel ML. Branched-chain amino acids are essential in the regulation of PHAS-I and p70 S6 kinase by pancreatic β-cells. A possible role in protein translation and mitogenic signaling. J. Biol. Chem. 1998;273:28178–28184. doi: 10.1074/jbc.273.43.28178. [DOI] [PubMed] [Google Scholar]
- 30.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 31.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 32.Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2α protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell. Biol. 1995;15:4497–4506. doi: 10.1128/mcb.15.8.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong J, Qiu H, Garcia-Barrio M, Anderson J, Hinnebusch AG. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell. 2000;6:269–279. doi: 10.1016/s1097-2765(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 34.Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol. Metab. 2009;20:436–443. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JI, Dominy JE, Jr., Sikalidis AK, Hirschberger LL, Wang W, Stipanuk MH. HepG2/C3A cells respond to cysteine deprivation by induction of the amino acid deprivation/integrated stress response pathway. Physiol. Genomics. 2008;33:218–229. doi: 10.1152/physiolgenomics.00263.2007. [DOI] [PubMed] [Google Scholar]
- 36.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 37.Tan S, Somia N, Maher P, Schubert D. Regulation of antioxidant metabolism by translation initiation factor 2α. J. Cell Biol. 2001;152:997–1006. doi: 10.1083/jcb.152.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AM, Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 39.Hayes JD, McMahon M. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 2001;174:103–113. doi: 10.1016/s0304-3835(01)00695-4. [DOI] [PubMed] [Google Scholar]
- 40.Nath KA. Heme oxygenase-1: A provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70:432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 41.Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J. Biol. Chem. 2004;279:36553–36561. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- 42.Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent l-asparaginase. J. Biol. Chem. 2009;284:32742–32749. doi: 10.1074/jbc.M109.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang S, Zeng Q, Gettayacamin M, Tungtaeng A, Wannaying S, Lim A, Hansukjariya P, Okunji CO, Zhu S, Fang D. Antimalarial activities and therapeutic properties of febrifugine analogs. Antimicrob. Agents Chemother. 2005;49:1169–1176. doi: 10.1128/AAC.49.3.1169-1176.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, Lefebvre RE, Unutmaz D, Mazitschek R, Waldner H, Whitman M, Keller T, Rao A. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Granot I, Halevy O, Hurwitz S, Pines M. Halofuginone: An inhibitor of collagen type I synthesis. Biochim. Biophys. Acta. 1993;1156:107–112. doi: 10.1016/0304-4165(93)90123-p. [DOI] [PubMed] [Google Scholar]
- 48.Elkin M, Reich R, Nagler A, Aingorn E, Pines M, de-Groot N, Hochberg A, Vlodavsky I. Inhibition of matrix metalloproteinase-2 expression and bladder carcinoma metastasis by halofuginone. Clin. Cancer Res. 1999;5:1982–1988. [PubMed] [Google Scholar]
- 49.Haran N, Leschinski L, Pines M, Rapoport J. Inhibition of rat renal fibroblast proliferation by halofuginone. Nephron Exp. Nephrol. 2006;104:e35–e40. doi: 10.1159/000093674. [DOI] [PubMed] [Google Scholar]
- 50.Karakoyun B, Yüksel M, Ercan F, Salva E, Işik I, Yeğen BC. Halofuginone, a specific inhibitor of collagen type 1 synthesis, ameliorates oxidant colonic damage in rats with experimental colitis. Dig. Dis. Sci. 2010;55:607–616. doi: 10.1007/s10620-009-0798-0. [DOI] [PubMed] [Google Scholar]
- 51.Nagler A, Firman N, Feferman R, Cotev S, Pines M, Shoshan S. Reduction in pulmonary fibrosis in vivo by halofuginone. Am. J. Respir. Crit. Care Med. 1996;154:1082–1086. doi: 10.1164/ajrccm.154.4.8887611. [DOI] [PubMed] [Google Scholar]
- 52.Pines M, Knopov V, Genina O, Lavelin I, Nagler A. Halofuginone, a specific inhibitor of collagen type I synthesis, prevents dimethylnitrosamine-induced liver cirrhosis. J. Hepatol. 1997;27:391–398. doi: 10.1016/s0168-8278(97)80186-9. [DOI] [PubMed] [Google Scholar]
- 53.Elkin M, Miao HQ, Nagler A, Aingorn E, Reich R, Hemo I, Dou HL, Pines M, Vlodavsky I. Halofuginone: A potent inhibitor of critical steps in angiogenesis progression. FASEB J. 2000;14:2477–2485. doi: 10.1096/fj.00-0292com. [DOI] [PubMed] [Google Scholar]
- 54.Abramovitch R, Dafni H, Neeman M, Nagler A, Pines M. Inhibition of neovascularization and tumor growth, and facilitation of wound repair, by halofuginone, an inhibitor of collagen type I synthesis. Neoplasia. 1999;1:321–329. doi: 10.1038/sj.neo.7900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elkin M, Ariel I, Miao HQ, Nagler A, Pines M, de-Groot N, Hochberg A, Vlodavsky I. Inhibition of bladder carcinoma angiogenesis, stromal support, and tumor growth by halofuginone. Cancer Res. 1999;59:4111–4118. [PubMed] [Google Scholar]
- 56.de Jonge MJ, Dumez H, Verweij J, Yarkoni S, Snyder D, Lacombe D, Marréaud S, Yamaguchi T, Punt CJ. A. van Oosterom; EORTC New Drug Development Group (NDDG), Phase I and pharmacokinetic study of halofuginone, an oral quinazolinone derivative in patients with advanced solid tumours. Eur. J. Cancer. 2006;42:1768–1774. doi: 10.1016/j.ejca.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 57.Sumimoto R, Southard JH, Belzer FO. Livers from fasted rats acquire resistance to warm and cold ischemia injury. Transplantation. 1993;55:728–732. doi: 10.1097/00007890-199304000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. U.S.A. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masoro EJ. Caloric restriction and aging: Controversial issues. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:14–19. doi: 10.1093/gerona/61.1.14. [DOI] [PubMed] [Google Scholar]
- 60.Ross MH. Length of life and nutrition in the rat. J. Nutr. 1961;75:197–210. doi: 10.1093/jn/75.2.197. [DOI] [PubMed] [Google Scholar]
- 61.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J. Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 62.Longo VD, Finch CE. Evolutionary medicine: From dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 63.Kenyon C. The plasticity of aging: Insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Heemskerk VH, Daemen MA, Buurman WA. Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation. Cytokine Growth Factor Rev. 1999;10:5–14. doi: 10.1016/s1359-6101(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 65.Che W, Lerner-Marmarosh N, Huang Q, Osawa M, Ohta S, Yoshizumi M, Glassman M, Lee JD, Yan C, Berk BC, Abe J. Insulin-like growth factor-1 enhances inflammatory responses in endothelial cells: Role of Gab1 and MEKK3 in TNF-α–induced c-Jun and NF-κB activation and adhesion molecule expression. Circ. Res. 2002;90:1222–1230. doi: 10.1161/01.res.0000021127.83364.7d. [DOI] [PubMed] [Google Scholar]
- 66.Bjerknes R, Aarskog D. Priming of human polymorphonuclear neutrophilic leukocytes by insulin-like growth factor I: Increased phagocytic capacity, complement receptor expression, degranulation, and oxidative burst. J. Clin. Endocrinol. Metab. 1995;80:1948–1955. doi: 10.1210/jcem.80.6.7775645. [DOI] [PubMed] [Google Scholar]
- 67.Kooijman R, Coppens A, Hooghe-Peters E. IGF-I inhibits spontaneous apoptosis in human granulocytes. Endocrinology. 2002;143:1206–1212. doi: 10.1210/endo.143.4.8725. [DOI] [PubMed] [Google Scholar]
- 68.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 69.Bunpo P, Cundiff JK, Reinert RB, Wek RC, Aldrich CJ, Anthony TG. The eIF2 kinase GCN2 is essential for the murine immune system to adapt to amino acid deprivation by asparaginase. J. Nutr. 2010;140:2020–2027. doi: 10.3945/jn.110.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant. Rev. 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leiba M, Cahalon L, Shimoni A, Lider O, Zanin-Zhorov A, Hecht I, Sela U, Vlodavsky I, Nagler A. Halofuginone inhibits NF-κB and p38 MAPK in activated T cells. J. Leukoc. Biol. 2006;80:399–406. doi: 10.1189/jlb.0705409. [DOI] [PubMed] [Google Scholar]
- 72.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-γ-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, Meng Q, Cheng Y, Gao X, Li J, Liu Y, Guo F. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60:746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christensen KD, Zimmermann NG, Wyatt CL, Goodman TN, Buhr RJ, Twining PF. Mitigating the effects of halofuginone on skin strength by feeding L-proline to broiler chickens. Poult. Sci. 1995;74:1610–1621. doi: 10.3382/ps.0741610. [DOI] [PubMed] [Google Scholar]
- 76.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Ginhoven TM, de Bruin RW, Timmermans M, Mitchell JR, Hoeijmakers JH, Ijzermans JN. Pre-operative dietary restriction is feasible in live-kidney donors. Clin. Transplant. 2011;25:486–494. doi: 10.1111/j.1399-0012.2010.01313.x. [DOI] [PubMed] [Google Scholar]
- 79.Voûte MT, Winkel TA, Poldermans D. Optimal medical management around the time of surgery. Heart. 2010;96:1842–1848. doi: 10.1136/hrt.2008.151480. [DOI] [PubMed] [Google Scholar]
- 80.Crenshaw JT, Winslow EH. Preoperative fasting: Old habits die hard. Am. J. Nurs. 2002;102:36–44. doi: 10.1097/00000446-200205000-00033. [DOI] [PubMed] [Google Scholar]
- 81.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc. Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.