Abstract
Gastroesophageal junction carcinoma is a rare but often lethal condition with increasing importance as a public health problem in recent decades. While diagnosis of this disease has been complicated historically by the lack of uniform classification standards, available data from the Surveillance, Epidemiology, and End Results (SEER) cancer registry program in the United States show an approximate 2.5-fold increase in the incidence of gastroesophageal junction adenocarcinoma (GEJAC) from 1973–1992, with rates stabilizing in the last two decades. Similar proportional trends are observed among subgroups defined by race and gender, but rates are significantly higher in males relative to females, and in white males relative to black males. Smoking, obesity, and gastroesophageal reflux disease are significant risk factors for GEJAC, and may account for a substantial fraction of total disease burden. Infection with Helicobacter pylori has been associated with reduced incidence, and high dietary fiber intake has also been linked to lower disease risk. Ongoing studies continue to explore a potential role for non-steroidal anti-inflammatory drugs in chemoprevention.
Introduction
The gastroesophageal (GE) junction forms the border between the distal esophagus and the proximal stomach, and normally is where squamous epithelium of the esophagus transitions into columnar epithelium of the gastric cardia.1 The vast majority of neoplasms arising at the GE junction are classified as adenocarcinomas, with the remainder predominantly categorized as squamous cell carcinomas or carcinomas not otherwise specified.
Classification of GE junction carcinoma has been complicated historically by variable definitions and interpretations of site of origin. In 1996, Siewert et al. proposed a classification scheme that defined adenocarcinomas of the GE junction as tumors that have their center within 5 cm proximal and distal of the anatomic gastric cardia. Three subtypes were further described: a) Type I, tumors of the distal esophagus that may infiltrate the junction from above; b) Type II, true carcinoma of the cardia, arising from cardiac epithelium or metaplastic junctional epithelium, and c) Type III, subcardial gastric carcinoma that in filtrates the GE junction and distal esophagus from below. However this schema is not universally agreed upon. The primary ICD-O-3 topography classification currently used by SEER for tumors in this area is “C16.0 Cardia”, which includes tumors of the gastric cardia, and cardioesophageal/gastroesophageal junction as defined by the diagnosing physician or facility.
Incidence Trends
The incidence of GE junction carcinoma has risen significantly since the early 1970s in the United States, with a disease burden characterized by sharp disparities across race and gender.3 A number of studies have reported similar increases in incidence of this disease during this time period in many regions around the world, including Sweden, Canada, Great Britain, and much of Europe.4–7 Data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program from 1973–2008 were analyzed to evaluate incidence patterns over time. Gastroesophageal junction carcinoma was defined based on criteria of the World Health Organization’s International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3), with analysis restricted to adenocarcinomas (codes 8140–8576), as this histology comprises the vast majority (90%) of all GE junction tumors (14,870 of 16,527). Within the total pool of adenocarcinomas, the analysis was further limited to cases of white or black race, which comprised 94% of all cases (13,959 of 14,870).
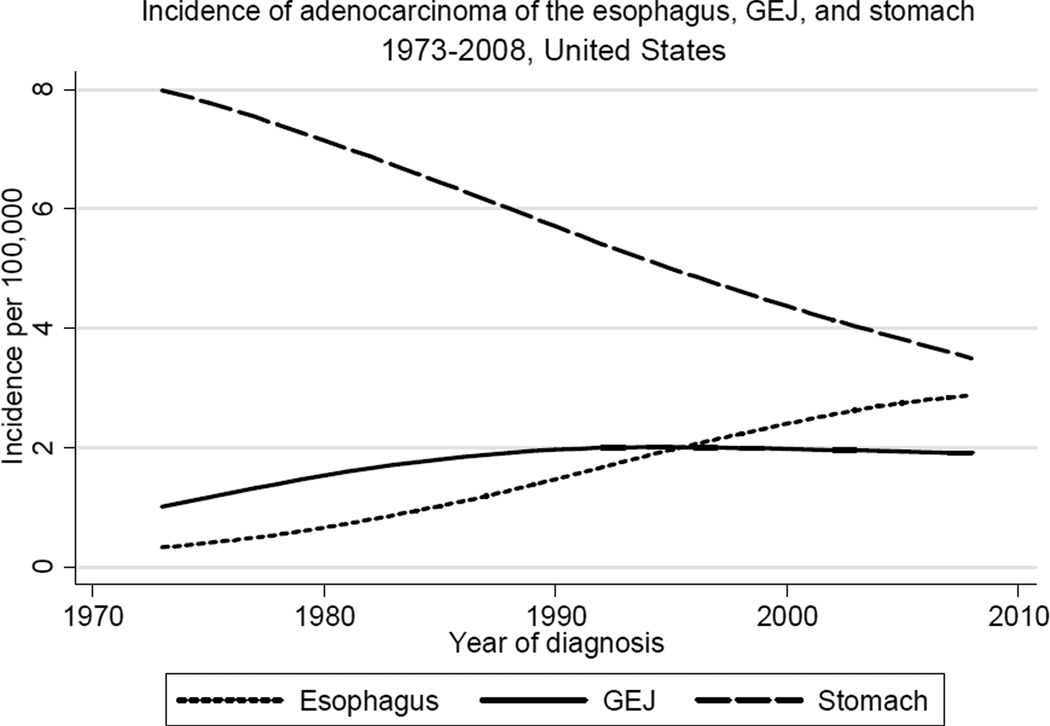
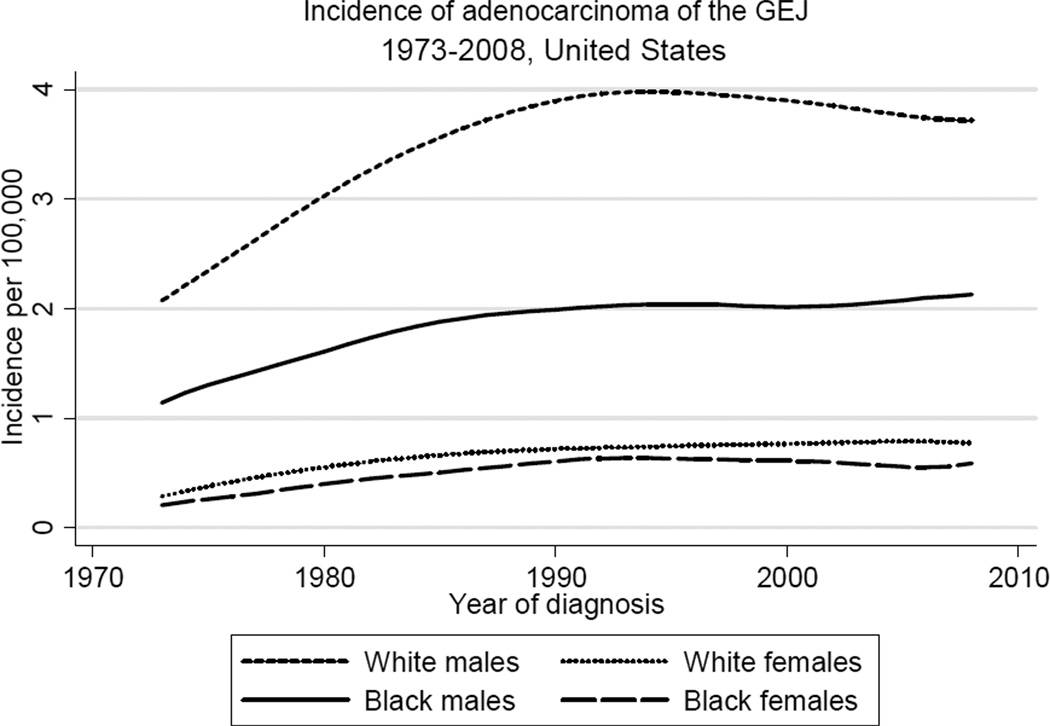
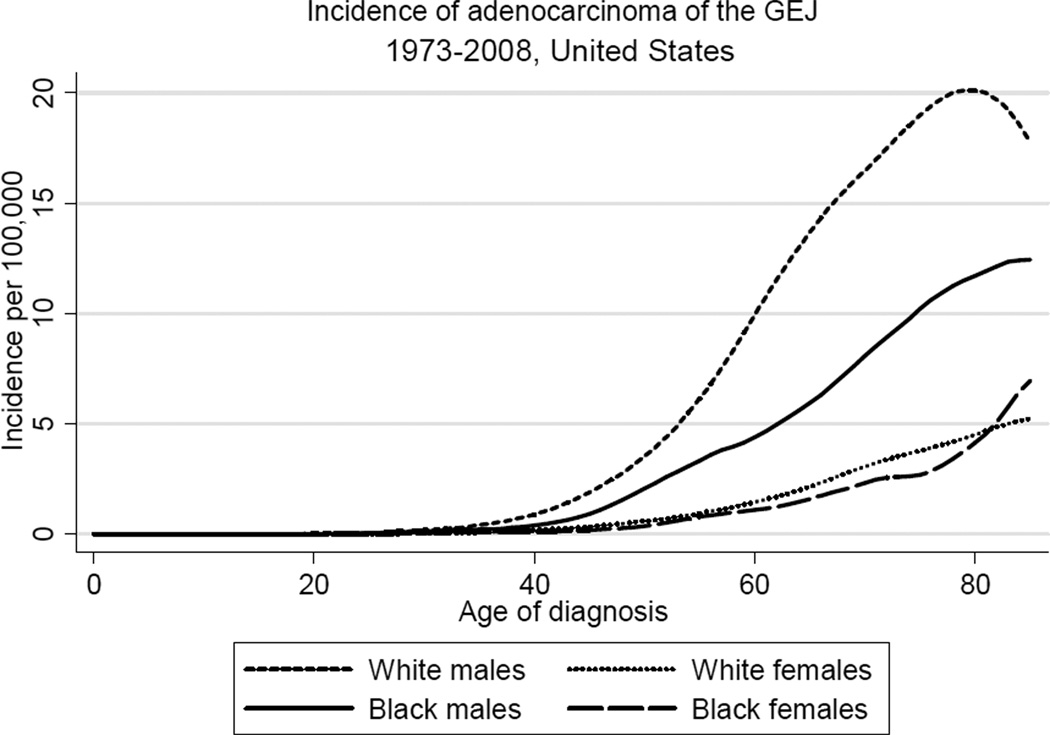
Figure 1 shows trends in incidence of adenocarcinoma of three major sites in the upper gastrointestinal tract, the esophagus, GE junction, and stomach. While incidence of (non-cardia) gastric adenocarcinoma has declined substantially in the last four decades, incidence of adenocarcinoma of both the esophagus and GE junction has risen considerably during this same period. The sharply increasing incidence of esophageal adenocarcinoma (EAC) outpaced the more gradual rise in the incidence of GEJAC, which stabilized in the early 1990s after increasing by nearly 2.5-fold since the early 1970s. As shown in Table 1 and Figure 2, rates of GEJAC are significantly higher in males relative to females (male:female ratio, 4.70), and in white males relative to black males (white male:black male ratio, 1.88). Incidence of GEJAC increased proportionally to a similar extent in all four subgroups defined by race and gender during the first twenty years of the study period (Figure 2). Since the early 1990s, rates have largely plateaued in all groups, with modest recent declines in white males. Incidence of GEJAC rises significantly with increasing age after the first three to four decades of life in all subgroups, before plateauing around the eighth decade (the absence of this plateau among black females may be due to the small number of cases) (Figure 3).
Figure 1.
Trends in incidence of adenocarcinoma of the esophagus, gastroesophageal junction (GEJ), and non-cardia stomach in the United States, 1973 to 2008 (per 100,000, adjusted for age, race, and sex to the 2000 US standard population, with lowess smoothing). Data from the National Cancer Institute’s SEER Program (SEER*Stat Database: Incidence: SEER 9 Regs Public Use, November 2010 submission).
Table 1.
Incidence of gastroesophageal junction adenocarcinoma in the United States by Race and Gender, 1973 to 2008.
| White |
Black |
Total* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||
| No. | Rate | No. | Rate | No. | Rate | No. | Rate | No. | Rate | |
| 1973–1978 | 975 | 2.48 | 198 | 0.40 | 37 | 1.35 | 13 | 0.34 | 1223 | 1.22 |
| 1979–1984 | 1,391 | 3.17 | 358 | 0.65 | 55 | 1.71 | 14 | 0.37 | 1818 | 1.63 |
| 1985–1990 | 1,851 | 3.96 | 435 | 0.72 | 84 | 2.07 | 32 | 0.61 | 2402 | 2.00 |
| 1991–1996 | 2,060 | 4.10 | 475 | 0.71 | 92 | 2.05 | 42 | 0.68 | 2669 | 2.05 |
| 1997–2002 | 2,101 | 3.87 | 546 | 0.77 | 98 | 2.05 | 46 | 0.65 | 2791 | 1.97 |
| 2003–2008 | 2,294 | 3.78 | 597 | 0.80 | 119 | 2.01 | 46 | 0.55 | 3056 | 1.94 |
Rates per 100,000, age-adjusted to the 2000 US standard population.
Total rates adjusted for age, race, and sex.
Figure 2.
Trends in incidence of adenocarcinoma of the gastroesophageal junction (GEJ) in the United States by race and gender, 1973 to 2008 (per 100,000, age-adjusted to the 2000 US standard population, with lowess smoothing). Data from the National Cancer Institute’s SEER Program (SEER*Stat Database: Incidence: SEER 9 Regs Public Use, November 2010 submission).
Figure 3.
Age-specific incidence of adenocarcinoma of the gastroesophageal junction (GEJ) in the United States by race and gender, 1973 to 2008 (per 100,000, with lowess smoothing). Data from the National Cancer Institute’s SEER Program (SEER*Stat Database: Incidence: SEER 9 Regs Public Use, November 2010 submission).
Misclassification has likely resulted in some fraction of esophageal and non-cardia gastric adenocarcinomas being labeled as junctional, and some junctional carcinomas being labeled as esophageal or non-cardia gastric. It is difficult to predict the extent to which misclassification has affected incidence rates for GE junction carcinoma. One study in Sweden estimated that due to the low accuracy in registering junctional cancers, the true incidence could be between 15% lower and 45% higher than reported.8 Another report raised the possibility that improved classification of gastric cancer sites over time, specifically a shift away from the “unspecified” category to the “cardia” category, might largely account for the apparent increase in GEJAC incidence in the United States during the 1970s and 1980s.9 Increasing acceptance and implementation of a common standard in diagnosis and classification of junctional cancers will allow for more definitive conclusions to be drawn regarding future incidence patterns.
Survival
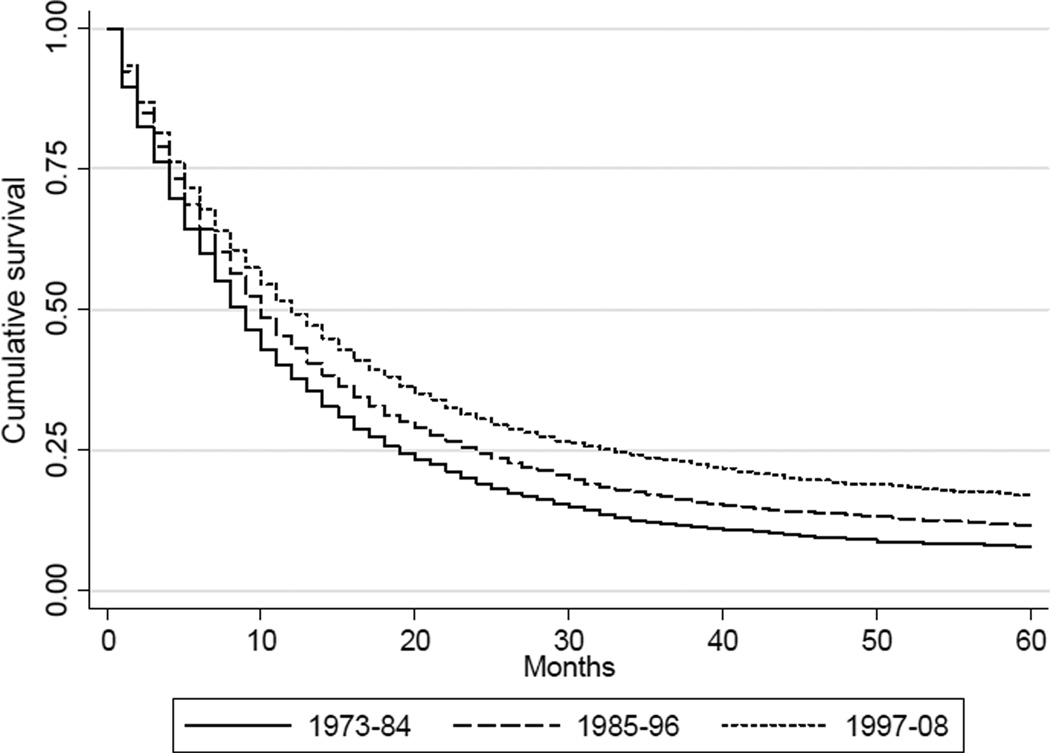
Data from the SEER Program from 1973–2008 were analyzed to evaluate survival patterns associated with GEJAC. The majority of GEJAC is diagnosed at the regional or distant stage, for which overall five-year survival rates during this period were 12% and 2%, respectively (Figure 4A). Modest improvements in overall five-year survival have been observed over time (Figure 4B), with cumulative survival increasing from 8% to 12% to 17% for GEJAC (of any stage) diagnosed from 1973–1984, 1985–1996, and 1997–2008, respectively. These increases in overall 5-year survival may be attributable to more effective detection and treatment of early stage disease.10 Quantitative confirmation of the trends described was obtained through proportional hazards Cox regression analysis, in which hazard ratios were calculated for overall mortality of GEJAC by stage or year of diagnosis (Table 2, A-B). This analysis also demonstrated a hazard ratio of 1.21 for blacks relative to whites for overall mortality of GEJAC (Table 2C). Kaplan-Meier curves showed reduced survival among blacks relative to whites in all three of the stage-specific strata (data not shown).
Figure 4.
Kaplan-Meier curves comparing percent survival for 5 years among cases of gastroesophageal junction adenocarcinoma by (A) stage, and (B) year of diagnosis (1973–1984, 1985–1996, 1997–2008). Unstaged cases were excluded from analysis. Data from the National Cancer Institute’s SEER Program (SEER*Stat Database: Incidence: SEER 9 Regs Public Use, November 2010 submission).
Table 2.
Adjusted hazard ratios for overall mortality of gastroesophageal junction adenocarcinoma by (A) stage, (B) year of diagnosis (1973–1984, 1985–1996, 1997–2008), and (C) race. Unstaged cases were excluded from analysis. Data from the National Cancer Institute’s SEER Program (SEER*Stat Database: Incidence: SEER 9 Regs Public Use, November 2010 submission).
| A. | |||
|---|---|---|---|
| Stage | Hazard Ratio (#) | 95% CI | |
| Localized | 1.00 | ||
| Regional | 1.71 | 1.62 | 1.81 |
| Distant | 3.99 | 3.75 | 4.24 |
| B. | |||
|---|---|---|---|
| Year of diagnosis | Hazard Ratio (*) | 95% CI | |
| 1973–1984 | 1.00 | ||
| 1985–1996 | 0.86 | 0.82 | 0.91 |
| 1997–2008 | 0.73 | 0.70 | 0.77 |
| C. | |||
|---|---|---|---|
| Race | Hazard Ratio (**) | 95% CI | |
| White | 1.00 | ||
| Black | 1.21 | 1.09 | 1.34 |
Adjusted for age at diagnosis, gender, registry, race, and year of diagnosis.
Adjusted for age at diagnosis, gender, registry, race, and stage.
Adjusted for age at diagnosis, gender, registry, stage, and year of diagnosis.
Risk Factors
Smoking
Multiple studies have found that smoking is associated with increased risk of GEJAC, with odds ratios ranging from 1.5 to 3.5.11–13 A recent analysis from the International BEACON (Barrett’s and Esophageal Adenocarcinoma) consortium pooled 10 population-based case-control studies and two cohort studies to obtain a total of 1450 GEJACs and 9453 controls for analysis.14 Using meta-analytic models, the authors arrived at a summary OR of 2.18 (95% CI 1.84–2.58). A strong dose response effect was observed for pack-years of smoking and associated risk of GEJAC, while smoking cessation was associated with reduced risk. No evidence of effect modification by gender was identified. Similar findings were reported by Tramacere and colleagues, who performed a meta-analysis of 33 studies published up to January 2010.15 Relative to never-smokers, the pooled relative risk for current-smokers was reported as 2.32 (95% CI 1.96–2.75), and that for ex-smokers as 1.62 (95% CI 1.40–1.87).
Smoking rates in the US began to decline in 1965, yet rates of GEJAC increased through the subsequent two and a half decades, before stabilizing in the early 1990s. By contrast, incidence of esophageal squamous cell carcinoma and non-cardia gastric carcinoma, diseases strongly linked to smoking, have declined significantly.16,17 Further, smoking rates among men and women have been converging over time, while rates of GEJAC have remained much higher among males than females. These observations would not seem to support a strong role for smoking prevalence shifts in influencing GEJAC incidence. Nevertheless, it should be emphasized that a long latency period for this disease, as has been speculated for EAC14, could delay any impact of smoking patterns on observed incidence trends.
Alcohol
Studies investigating an association between alcohol consumption and risk for GEJAC have yielded inconsistent results and have been hampered by small sample sizes. A recent analysis carried out by the BEACON consortium pooled nine case-control studies and two cohort studies to obtain a total of 1837 GEJAC cases and 10,854 controls.18 Using similar approaches employed by Cook et al. in evaluating the effects of smoking on GEJAC risk, the authors report that no increase in risk for GEJAC was observed for any of the alcohol intake measures analyzed. The OR for >=7 drinks/day was 0.77 (95% CI 0.54–1.10). Moderate intake (0.5 to <1 drink/day) was also associated with reduced risk of GEJAC (OR 0.78, 95% CI 0.62–0.99), although the wide confidence interval and potential for false positives due to multiple comparisons point to the need for additional studies. Similar findings were recently reported by Tramacere and colleagues in a meta-analysis of twenty case-control and four cohort studies.19 The relative risk for drinkers versus non-drinkers was 0.89 (95% CI 0.76–1.03) for GEJAC.
Gastroesophageal Reflux Disease
Multiple studies have shown an association between gastroesophageal reflux disease (GERD) and elevated risk for GEJAC.20–23 Estimated odds ratios range from 2 to 13. Population-based case-control studies in Sweden and Australia have both reported a dose-response relationship, with level of risk tracking with frequency and/or severity and duration of reflux.22,23 In the Australian study, Pandeya et al. reported that the risk associated with frequent GERD symptoms and heavy smoking in combination was more than two times higher than that predicted from a simply additive model of the risks from GERD and smoking alone.24
Chronic reflux is associated with Barrett’s esophagus, a squamous-to-columnar metaplastic precursor lesion predisposing to esophageal adenocarcinoma. GERD has also been linked to intestinal metaplasia in the GE junction, which may arise from a multilayered epithelium transition state and is believed to represent the biological precursor of dysplasia and cancer.25 Acid and bile have been shown to exert genotoxic effects through direct oxidative damage and possible reaction with ingested nitrites to form nitric oxide. Smoking may further promote the action of inflammatory pathways while also potentially reducing esophageal sphincter tone and hence exacerbating reflux.24
Conflicting evidence exists regarding time trends in reflux symptoms and/or GERD, with some reports but not others indicating rising prevalence of this condition.26 The potential influence of reflux prevalence on GEJAC incidence remains unclear.
Obesity
Obesity has been consistently identified as a risk factor for GEJAC. In a population-based case-control study, Chow et al. reported that risk was significantly elevated for males (but not females) in the heaviest quartile relative to the lightest quartile of body mass index (BMI) (OR 1.8, 95% CI 1.1–2.9).27 A population-based study in Sweden reported an odds ratio of 2.3 (95% CI 1.5–3.6) for the heaviest versus lightest quartile.28 A more recent prospective cohort study of 218,854 subjects in the US found that compared to people with a normal-range BMI, a BMI >=35 was associated with a hazard ratio of 3.67 (95% CI 2.00 to 6.71).29 In a systematic review and meta-analysis, Kubo and colleagues reported that a high BMI was associated with a summary OR for GEJAC of 1.5 (95% 1.3–1.8).30 A recent analysis by the BEACON consortium pooled 12 studies to obtain 1900 GEJAC cases and 11,159 controls. The authors reported a summary odds ratio of 3.07 (95% CI 1.89–4.99) associated with a BMI>=40 relative to a BMI<25, with similar results for men and women.31
Speculation regarding the specific role of abdominal obesity was addressed in a nested case-control study by Corley et al., who reported an association between abdominal diameter and risk for EAC but not GEJAC.32 The prospective cohort by Abnet and colleagues further reported an association between waist-to-hip ratio and risk of EAC but not GEJAC.29 Whiteman and colleagues found that obesity and frequent reflux in combination were associated with considerably higher risk for GEJAC than either single factor alone, although additive effects appeared most likely, in contrast to synergistic effects in EAC.23
Increased body weight may predispose to reflux through mechanical means, but may also act through independent pathways involving inflammatory or hormonal mediators.33 The prevalence of overweight and obesity have increased significantly in recent decades. While this trend could partially account for the rising incidence of GEJAC in the late 1970s and 1980s, obesity levels have continued to increase while rates of GEJAC have plateaued. Furthermore, an analysis of data from the Connecticut SEER registry suggests that the incidence of GEJAC actually began rising in the 1950s, well before the onset of the obesity epidemic.17
Diet
Several studies have suggested that high dietary fiber is associated with reduced risk of GEJAC, while high fat intake is associated with increased risk.13,34 A multi-center case control study in the US reported an odds ratio of 0.43 (95% CI 0.30–0.61) associated with total fiber intake.35 It has been proposed that dietary fiber may act to limit hiatal hernias, promote cleansing of toxins from the epithelium, or enhance clearance of damaged epithelial cells. A population-based case-control study in Sweden explored the influence of three major dietary patterns on disease risk.36 The authors reported that a diet high in processed meat, red meat, sweets, and high-fat dairy, relative to a diet low in these foods, was associated with an odds ratio for GEJAC of 1.8 (95% CI 1.1–2.9). High intake of fruits, vegetables, fish, and poultry was associated with reduced risk, without reaching statistical significance.
Shifts in dietary practices in recent decades towards increased fat intake and consumption of meats in Western countries may have contributed in part to the rising incidence of GEJAC, although it is not clear that healthier dietary trends have taken hold in the last 20 years, while rates of GEJAC have stabilized. It remains unknown whether the effects of diet act principally through influencing levels of obesity, or whether dietary practices impact cancer risk independent of body weight.
Helicobacter Pylori
Infection with the bacterium H. pylori is associated with increased risk for peptic ulcer disease and gastric cancer, via mechanisms of chronic inflammation, atrophy, and dysplasia.37 By contrast, an inverse association has been reported between H. pylori infection and risk for EAC and GEJAC.38 A nested case-control study using subjects from the ATBC trial in Finland reported that H. pylori seropositivity was associated with an odds ratio for GEJAC of 0.31 (95% CI 0.11–0.89).39 In their Australian case-control study, Whiteman and colleagues reported an odds ratio of 0.41 (95% CI 0.27–0.60) associated with infection.40
It is not well understood how H. pylori infection may confer a protective effect, if the observed associations are causal. Proposed mechanisms include reduced gastric acid production, cytokine or hormonal deregulation, and micro biome alteration.41 Declining prevalence of H. pylori infection in Western populations over previous decades may have played a role in the rising incidence of EAC and GEJAC.17
Medications
Use of non-steroidal anti-inflammatory drugs (NSAIDs) has been linked to reduced risk for EAC and GEJAC.42 A recent analysis carried out by the BEACON international consortium pooled five case-control studies and one cohort study to obtain a total of 1140 GEJAC cases and 5314 controls.43 Using a meta-analytic model, the authors arrived at a summary odds ratio for NSAID users relative to non-users of 0.83 (95% CI 0.66–1.03), while the OR associated with current use was 0.57 (95% CI 0.39–0.83). Similar results were reported for aspirin compared to overall NSAID use. Evidence of a dose-response relationship between frequency and duration of use and disease risk was obtained for EAC, but not for GEJAC. Results from one of the individual studies included in this analysis found that aspirin use is associated with more significant reductions in risk for EAC in subjects with increasing frequency of reflux symptoms, but evidence for this potential interaction was not obtained for GEJAC.44 A recent prospective cohort study in the US (NIH-AARP Diet and Health Study) did not report a statistically significant association between aspirin or NSAID use and risk of GEJAC.45 In an accompanying meta-analysis, the authors reported an odds ratio associated with non-aspirin NSAID use of 0.80 (95% CI 0.67–0.95), and an OR associated with aspirin use of 0.82 (0.65–1.04).
Collectively, the evidence suggests a modest association between NSAID use and reduced risk of GEJAC, although the magnitude and significance of this association is weaker than for EAC. Reverse causality cannot be ruled out. NSAIDs inhibit the activity of cyclooxygenase (COX) enzymes and thereby block production of prostaglandins and other inflammatory mediators. Chronic inflammation represents a common mechanism through which several risk factors for GEJAC may act (reflux, obesity, smoking). Agents such as NSAIDs are hypothesized to reduce disease risk by decreasing inflammation, and potentially modulating levels of apoptosis and proliferation. An ongoing randomized trial in the United Kingdom is currently evaluating the effectiveness of NSAIDs as chemo preventive agents in esophageal adenocarcinoma.46
Bisphosphonates are often prescribed for the prevention and treatment of osteoporosis, and are known to cause esophageal irritation and erosion.47 Studies investigating a potential association between use of bisphosphonates and risk of esophageal cancer have yielded inconsistent results, and junctional cancers were not specifically identified.48,49 Additional studies with larger sample sizes and longer durations of follow-up are required to definitively evaluate this potential risk factor.
Other
The significant gender disparity observed in the incidence of EAC/GEJAC has prompted speculation about a potential role for reproductive and sex hormonal factors in disease etiology. A recent analysis by the BEACON consortium pooled all female subjects from four population-based case-control studies to obtain a total of 218 cases of EAC or GEJAC and 862 controls.50 Among parous women, the authors reported a pooled odds ratio of 0.58 (95% CI 0.37–0.92) associated with breastfeeding relative to never breastfeeding, with longer durations of breastfeeding associated with further reduction in risk. Parity, menstruation, pregnancy history, and use of oral contraceptives or hormone replacement therapy (HRT) were not found to be associated with risk of EAC/GEJAC. A recent analysis of women enrolled in the Women’s Health Initiative clinical trials and observational studies also did not find associations between most reproductive/hormonal factors or HRT and EAC risk, although a non-significant inverse association with breast feeding (hazard ratio 0.44, 95% CI 0.18–1.07) again was observed.51
Given that EAC and GEJAC cases were combined in the BEACON analysis, it is not clear that the association between breastfeeding and disease risk would also be observed in GEJAC-only cases relative to controls. The mechanisms by which breastfeeding may modulate risk for EAC/GEJAC are not well understood, although such an effect has been reported in cancers of the breast, endometrium, and ovary. Expression of estrogen receptors has been reported in EAC, and oxytocin receptors are also expressed in the GI tract. It is speculated that the high levels of prolactin and oxytocin and the drop in estrogen associated with breastfeeding might lead to long-term modification of hormonal signaling.
Concluding remarks
In the last two decades, the incidence of gastroesophageal junction adenocarcinoma has remained stable in the US, but only after an apparent 2.5-fold increase in the twenty years prior. The study of this disease has been complicated historically by the lack of a clear and uniform classification system, resulting in a high likelihood of many esophageal and non-cardia gastric cancers being misclassified as junctional, and many junctional cancers being misclassified as esophageal or non-cardia gastric. While the epidemiology of GEJAC shares important features with that of EAC, clear differences are also evident. Several key risk factors have been linked to both conditions, notably obesity, reflux, and smoking, but the strength of these associations is consistently weaker for junctional carcinoma than for EAC, with the exception of smoking. Furthermore, while the incidence of both cancers increased significantly through the early 1990s, rates of GEJAC have since plateaued, but rates of EAC have continued to rise. These disparities suggest that the biological mechanisms underlying these diseases, while similar, are not identical. Improvements in classification consistency over time will facilitate further epidemiologic delineation of the differences between these two cancers.
By one estimate, more than half of the GEJAC disease burden is attributable to smoking and elevated BMI.52 Combinations of smoking, elevated BMI, and reflux may account for almost 70% of total cases.53 The incidence of this cancer has remained stable in recent years, but significant reductions in incidence likely remain within reach, through primary prevention efforts targeted at these important risk factors. Smoking rates have declined in the past several decades, but the prevalence of smoking among Americans continues to hover at 20–25%, and little progress has been made in slowing or reversing the sharp rise in obesity. Reflux remains undiagnosed in a large portion of the population, while the vast majority of those with the diagnosis will not develop cancer.
Given that the case burden of GEJAC in the last 35 years actually exceeded that of EAC (13,959 vs. 12,848), a renewed research focus on the pathophysiology and epidemiology of this condition is warranted. Additional progress is required to identify those individuals at highest risk of developing GEJAC, through improved molecular markers and advances in imaging technologies. Directing endoscopic surveillance programs and chemoprevention agents to this select pool of individuals will be an important element of a comprehensive prevention strategy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rusch VW. Are cancers of the esophagus, gastroesophageal junction, and cardia one disease, two, or several? Semin Oncol. 2004;31(4):444–449. doi: 10.1053/j.seminoncol.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85(11):1457–1459. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 3.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265(10):1287–1289. [PubMed] [Google Scholar]
- 4.Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29(4):645–654. doi: 10.1093/ije/29.4.645. [DOI] [PubMed] [Google Scholar]
- 5.Hansson LE, Sparen P, Nyren O. Increasing incidence of carcinoma of the gastric cardia in Sweden from 1970 to 1985. Br J Surg. 1993;80(3):374–377. doi: 10.1002/bjs.1800800338. [DOI] [PubMed] [Google Scholar]
- 6.Parfitt JR, Miladinovic Z, Driman DK. Increasing incidence of adenocarcinoma of the gastroesophageal junction and distal stomach in Canada -- an epidemiological study from 1964–2002. Can J Gastroenterol. 2006;20(4):271–276. doi: 10.1155/2006/175751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wayman J, Forman D, Griffin SM. Monitoring the changing pattern of esophago-gastric cancer: data from a UK regional cancer registry. Cancer Causes Control. 2001;12(10):943–949. doi: 10.1023/a:1013756531219. [DOI] [PubMed] [Google Scholar]
- 8.Ekstrom AM, Signorello LB, Hansson LE, Bergstrom R, Lindgren A, Nyren O. Evaluating gastric cancer misclassification: a potential explanation for the rise in cardia cancer incidence. J Natl Cancer Inst. 1999;91(9):786–790. doi: 10.1093/jnci/91.9.786. [DOI] [PubMed] [Google Scholar]
- 9.Corley DA, Kubo A. Influence of site classification on cancer incidence rates: an analysis of gastric cardia carcinomas. J Natl Cancer Inst. 2004;96(18):1383–1387. doi: 10.1093/jnci/djh265. [DOI] [PubMed] [Google Scholar]
- 10.Pera M, Manterola C, Vidal O, Grande L. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92(3):151–159. doi: 10.1002/jso.20357. [DOI] [PubMed] [Google Scholar]
- 11.Lagergren J, Bergstrom R, Lindgren A, Nyren O. The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. Int J Cancer. 2000;85(3):340–346. [PubMed] [Google Scholar]
- 12.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States) Cancer Causes Control. 2001;12(8):721–732. doi: 10.1023/a:1011290704728. [DOI] [PubMed] [Google Scholar]
- 13.Kabat GC, Ng SK, Wynder EL. Tobacco, alcohol intake, and diet in relation to adenocarcinoma of the esophagus and gastric cardia. Cancer Causes Control. 1993;4(2):123–132. doi: 10.1007/BF00053153. [DOI] [PubMed] [Google Scholar]
- 14.Cook MB, Kamangar F, Whiteman DC, Freedman ND, Gammon MD, Bernstein L, Brown LM, Risch HA, Ye W, Sharp L, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst. 2010;102(17):1344–1353. doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tramacere I, La Vecchia C, Negri E. Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology. 2011;22(3):344–349. doi: 10.1097/EDE.0b013e31821092cd. [DOI] [PubMed] [Google Scholar]
- 16.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17(1):2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Abrams JA, Sharaiha RZ, Gonsalves L, Lightdale CJ, Neugut AI. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut Tumor Registry data, 1940–2007. Cancer Epidemiol Biomarkers Prev. 2011;20(1):183–186. doi: 10.1158/1055-9965.EPI-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman ND, Murray LJ, Kamangar F, Abnet CC, Cook MB, Nyren O, Ye W, Wu AH, Bernstein L, Brown LM, et al. Alcohol intake and risk of oesophageal adenocarcinoma: a pooled analysis from the BEACON Consortium. Gut. 2011;60(8):1029–1037. doi: 10.1136/gut.2010.233866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tramacere I, Pelucchi C, Bagnardi V, Rota M, Scotti L, Islami F, Corrao G, Boffetta P, La Vecchia C, Negri E. A meta-analysis on alcohol drinking and esophageal and gastric cardia adenocarcinoma risk. Ann Oncol. 2012;23(2):287–297. doi: 10.1093/annonc/mdr136. [DOI] [PubMed] [Google Scholar]
- 20.Chow WH, Finkle WD, McLaughlin JK, Frankl H, Ziel HK, Fraumeni JF., Jr The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA. 1995;274(6):474–477. [PubMed] [Google Scholar]
- 21.Crane SJ, Locke GR, 3rd, Harmsen WS, Diehl NN, Zinsmeister AR, Melton LJ, 3rd, Romero Y, Talley NJ. Subsite-specific risk factors for esophageal and gastric adenocarcinoma. Am J Gastroenterol. 2007;102(8):1596–1602. doi: 10.1111/j.1572-0241.2007.01234.x. [DOI] [PubMed] [Google Scholar]
- 22.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340(11):825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 23.Whiteman DC, Sadeghi S, Pandeya N, Smithers BM, Gotley DC, Bain CJ, Webb PM, Green AC. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57(2):173–180. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 24.Pandeya N, Webb PM, Sadeghi S, Green AC, Whiteman DC. Gastro-oesophageal reflux symptoms and the risks of oesophageal cancer: are the effects modified by smoking, NSAIDs or acid suppressants? Gut. 2010;59(1):31–38. doi: 10.1136/gut.2009.190827. [DOI] [PubMed] [Google Scholar]
- 25.Odze RD. Pathology of the gastroesophageal junction. Semin Diagn Pathol. 2005;22(4):256–265. doi: 10.1053/j.semdp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Lagergren J. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005;54(1):i1–i5. doi: 10.1136/gut.2004.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow WH, Blot WJ, Vaughan TL, Risch HA, Gammon MD, Stanford JL, Dubrow R, Schoenberg JB, Mayne ST, Farrow DC, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90(2):150–155. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 28.Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130(11):883–890. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 29.O'Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut. doi: 10.1136/gutjnl-2011-300551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(5):872–878. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 31.Hoyo C, et al. Body mass index and esophageal and esophagogastric junction adenocarcinomas in men and women: a pooled analysis from the international BEACON consortium. doi: 10.1093/ije/dys176. (unpublished). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17(2):352–358. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett's oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10(2):87–101. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang ZF, Kurtz RC, Yu GP, Sun M, Gargon N, Karpeh M, Jr, Fein JS, Harlap S. Adenocarcinomas of the esophagus and gastric cardia: the role of diet. Nutr Cancer. 1997;27(3):298–309. doi: 10.1080/01635589709514541. [DOI] [PubMed] [Google Scholar]
- 35.Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Farrow DC, Schoenberg JB, Stanford JL, Ahsan H, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10(10):1055–1062. [PubMed] [Google Scholar]
- 36.Bahmanyar S, Ye W. Dietary patterns and risk of squamous-cell carcinoma and adenocarcinoma of the esophagus and adenocarcinoma of the gastric cardia: a population-based case-control study in Sweden. Nutr Cancer. 2006;54(2):171–178. doi: 10.1207/s15327914nc5402_3. [DOI] [PubMed] [Google Scholar]
- 37.Peek RM, Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208(2):233–248. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- 38.Chow WH, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, Perez-Perez GI, Schoenberg JB, Stanford JL, Rotterdam H, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58(4):588–590. [PubMed] [Google Scholar]
- 39.Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, Abnet CC, Albanes D, Virtamo J, Taylor PR. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98(20):1445–1452. doi: 10.1093/jnci/djj393. [DOI] [PubMed] [Google Scholar]
- 40.Whiteman DC, Parmar P, Fahey P, Moore SP, Stark M, Zhao ZZ, Montgomery GW, Green AC, Hayward NK, Webb PM. Association of Helicobacter pylori infection with reduced risk for esophageal cancer is independent of environmental and genetic modifiers. Gastroenterology. 2010;139(1):73–83. doi: 10.1053/j.gastro.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137(2):588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughan TL, Dong LM, Blount PL, Ayub K, Odze RD, Sanchez CA, Rabinovitch PS, Reid BJ. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett's oesophagus: a prospective study. Lancet Oncol. 2005;6(12):945–952. doi: 10.1016/S1470-2045(05)70431-9. [DOI] [PubMed] [Google Scholar]
- 43.Liao LM, Vaughan TL, Corley DA, Cook MB, Casson AG, Kamangar F, Abnet CC, Risch HA, Giffen C, Freedman ND, et al. Nonsteroidal Anti-Inflammatory Drug Use Reduces Risk of Adenocarcinomas of the Esophagus and Esophagogastric Junction in a Pooled Analysis. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadeghi S, Bain CJ, Pandeya N, Webb PM, Green AC, Whiteman DC. Aspirin, nonsteroidal anti-inflammatory drugs, and the risks of cancers of the esophagus. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1169–1178. doi: 10.1158/1055-9965.EPI-07-2852. [DOI] [PubMed] [Google Scholar]
- 45.Abnet CC, Freedman ND, Kamangar F, Leitzmann MF, Hollenbeck AR, Schatzkin A. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer. 2009;100(3):551–557. doi: 10.1038/sj.bjc.6604880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das D, Ishaq S, Harrison R, Kosuri K, Harper E, Decaestecker J, Sampliner R, Attwood S, Barr H, Watson P, et al. Management of Barrett's esophagus in the UK: over treated and under biopsied but improved by the introduction of a national randomized trial. Am J Gastroenterol. 2008;103(5):1079–1089. doi: 10.1111/j.1572-0241.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- 47.Haber SL, McNatty D. An evaluation of the use of oral bisphosphonates and risk of esophageal cancer. Ann Pharmacother. 2012;46(3):419–423. doi: 10.1345/aph.1Q482. [DOI] [PubMed] [Google Scholar]
- 48.Cardwell CR, Abnet CC, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA. 2010;304(6):657–663. doi: 10.1001/jama.2010.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;1(341) doi: 10.1136/bmj.c4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cronin-Fenton DP, Murray LJ, Whiteman DC, Cardwell C, Webb PM, Jordan SJ, Corley DA, Sharp L, Lagergren J. Reproductive and sex hormonal factors and oesophageal and gastric junction adenocarcinoma: a pooled analysis. Eur J Cancer. 2010;46(11):2067–2076. doi: 10.1016/j.ejca.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bodelon C, Anderson GL, Rossing MA, Chlebowski RT, Ochs-Balcom HM, Vaughan TL. Hormonal factors and risks of esophageal squamous cell carcinoma and adenocarcinoma in postmenopausal women. Cancer Prev Res. 2011;4(6):840–850. doi: 10.1158/1940-6207.CAPR-10-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engel LS, Chow WH, Vaughan TL, Gammon MD, Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Dubrow R, Rotterdam H, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95(18):1404–1413. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 53.Olsen CM, Pandeya N, Green AC, Webb PM, Whiteman DC. Population attributable fractions of adenocarcinoma of the esophagus and gastroesophageal junction. Am J Epidemiol. 2011;174(5):582–590. doi: 10.1093/aje/kwr117. [DOI] [PubMed] [Google Scholar]