Abstract
Background
Pancreatic islet transplantation has the potential to cure Type 1 Diabetes (T1D), a chronic lifelong disease, but its clinical applicability is limited by allograft rejection. Nuclear factor κB (NF-κB) is a transcription factor important for survival and differentiation of T cells. In this study, we tested whether NF-κB in T cells is required for the rejection of islet allografts.
Methods
Mice expressing a super-repressor form of NF-κB selectively in T cell (IκBαΔN-Tg mice) with or without the anti-apoptotic factor Bcl-xL, or mice with impaired TCR-and BCR-driven NF-κB activity (CARMA1-KO mice) were rendered diabetic and transplanted with islet allografts. Secondary skin transplantation in long-term acceptors of islet allografts was used to test for development of donor-specific tolerance. Immune infiltration of the transplanted islets was examined by immunofluorescence. TCR-transgenic CD4+ T cells were used to follow T cell priming and differentiation.
Results
Islet allograft survival was prolonged in IκBαΔN-Tg mice, although the animals did not develop donor-specific tolerance. Reduced NF-κB activity did not prevent T cell priming or differentiation but rather reduced survival of activated T cells, as transgenic expression of Bcl-xL restored islet allograft rejection in IκBαΔN-Tg mice. Abolishing TCR- and BCR-driven activation of NF-κB selectively via CARMA1 deficiency prevented T cell priming and islet allograft rejection.
Conclusions
Our data suggest that T cell-NF-κB plays an important role in the rejection of islet allografts. Targeting NF-κB selectively in lymphocytes appears a promising approach to facilitate acceptance of transplanted islets.
Keywords: islet transplantation, NF-κB, T cells
Introduction
T1D results from the autoimmune destruction of insulin-producing β cells contained in pancreatic islets. Islet transplantation is a minimally invasive procedure that can achieve insulin independence in most patients for at least one year (1, 2). However, β-cell function is progressively lost over time and more than 80% of patients revert to insulin-dependence within 5 years, likely because of alloimmunity in addition to recurrence of autoimmunity.
NF-κB is a transcription factor central for T cell activation that is a prime candidate to control alloimmune responses in vivo (3–7). It is targeted by immunosuppressive agents such as steroids or proteasome inhibitors (8), but current treatments inhibit NF-κB in all cell types causing unwanted side-effects. Whether inhibition of NF-κB selectively in T cells/lymphocytes can promote survival of islet allografts and should be a focus of future drug development for this transplanted tissue remains to be established. The NF-κB family of transcription factors consists of five members: RelA (p65), RelB, c-Rel, p50 and p52 that can homo- and hetero-dimerize (9). In naive T cells, NF-κB dimers are retained in the cytoplasm by the NF-κB inhibitor IκBα (10). TCR and BCR engagement results in the assembly of an adaptosome complex comprising CARMA1, Bcl-10 and Malt-1that is required for the downstream phosphorylation and degradation of IκBα, allowing NF-κB dimers to translocate into the nucleus and drive gene transcription (10). In addition to the TCR, other cell surface receptors in T cells can activate NF-κB. These include Toll-like receptor (TLR) and tumor necrosis factor receptor (TNFR) family members. These families of receptors rely on adaptors other than the CARMA1/Bcl-10/Malt-1 used by the TCR, such as TNFR-associated factor (TRAF) family members or myeloid differentiation primary response gene 88 (MyD88) (9).
NF-κB has been shown to play a role in the rejection of pancreatic islet allografts as mice globally deficient in c-Rel developed delayed rejection with 40% of mice accepting islet allografts long-term (11). However, whether these effects are due to inhibition of NF-κB in T cells and/or other cell types is not known. To study NF-κB specifically in T cells, we have taken advantage of the IκBαΔN-Tg mice previously developed (3), in which the Lck proximal promoter/CD2 locus drives expression in T cells of a non-degradable IκBα transgene that dominantly sequesters NF-κB dimers in the cytoplasm, reducing NF-κB-dependent gene transcription. In addition, we have used mice deficient in CARMA-1 such that NF-κB activity is selectively impaired downstream of the TCR and the BCR, but not of other receptors in T cells (12). Our results show that NF-κB in T cells plays a crucial role in the rejection of allogeneic pancreatic islets.
Results
Inhibiting NF-κB in T Cells Facilitates Islet Allograft Survival
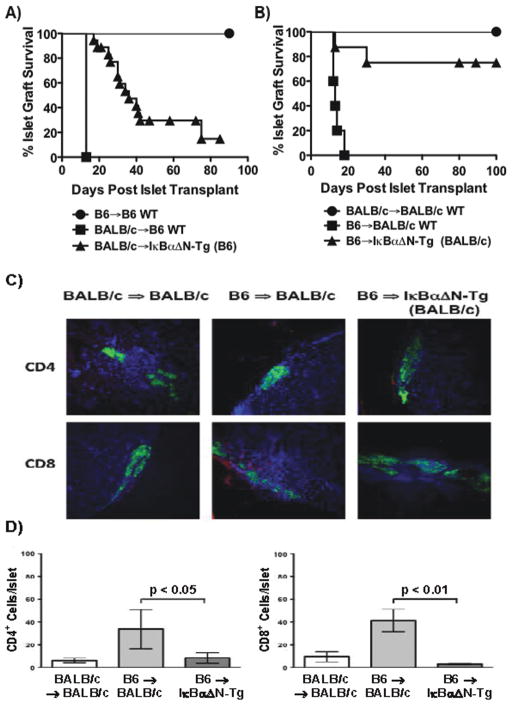
To investigate whether inhibition of NF-κB selectively in T cells can promote islet allograft survival, we first used IκBαΔN-Tg mice that express as a T cell-specific transgene a super-repressor form of the NF-κB inhibitor IκBα. Wildtype B6 or IκBαΔN-Tg (B6) mice (H-2b) were rendered diabetic with STZ, and transplanted with syngeneic or fully allogeneic BALB/c (H-2d) islets under the kidney capsule. In wildtype mice, syngeneic grafts survived long-term, while islet allografts were acutely rejected by 13 days. In contrast, islet allograft rejection was markedly delayed in IκBαΔN-Tg (B6) mice, with approximately 10% of the mice retaining allografts for more than 80 days (Figure 1A). Similar results were obtained using the reverse strain combination [B6 islets into IκBαΔN-Tg (BALB/c) mice], with a larger fraction of the mice (approximately 75%) accepting allografts long-term (Figure 1B). These data demonstrate that NF-κB in T cells plays an important role in the acute rejection of islet allografts.
Figure 1. NF-κB in T cells facilitates islet allograft rejection.
Wildtype or IκBαΔN-Tg mice on the BALB/c or B6 backgrounds were rendered diabetic with STZ and transplanted with syngeneic or allogeneic islets. A) Recipients on the B6 background [B6 => B6 (n=7, MST > 90); BALB/c => B6 (n=4, MST 13); BALB/c => IκBαΔN-Tg (B6) (n=17, MST 34)]. B) Recipients on the BALB/c background [BALB/c => BALB/c (n=5, MST > 100); B6 => BALB/c (n=5, MST > 13); B6 => IκBαΔN-Tg (BALB/c) (n=8, MST 80)]. Allograft survival was significantly prolonged in IκBαΔN-Tg mice from both backgrounds (p<0.01). C) Frozen sections of explanted kidneys containing islet grafts harvested from mice on the BALB/c background were immunostained using anti-insulin antibody (green) and anti-CD4 (red, upper panels) or anti-CD8 (red, lower panels) primary antibodies with DAPI (blue) as a counterstain to label nuclei. Immunostained sections were viewed at a 10x magnification using a fixed sample DSU confocal microscope. D) Quantification of the mean ± SEM number of CD4+ and CD8+ cell subsets/islet. Approximately 10 islets per mice were examined in 3–4 mice/group. *p<0.05; **p<0.01.
IκBαΔN-Tg (BALB/c) and wildtype recipients transplanted with B6 islets were sacrificed and frozen sections from allograft-containing kidneys were examined by immunofluorescence using anti-insulin and either anti-CD4 or anti-CD8 antibodies. Syngeneic islets were readily visualized on day 9 post-transplantation by strong insulin staining indicative of functioning β cells, whereas CD4+ or CD8+ cells were not detected (Figure 1C). As expected, wildtype CD4+ or CD8+ cells penetrated allogeneic grafts during acute rejection (d9 post-transplantation). In contrast, very few CD4+ or CD8+ cells were detected in the islet allografts of IκBαΔN-Tg (BALB/c) recipients even on day 45 post-transplantation. Quantification of the number of infiltrating T cell subsets/islet revealed a significantly lower number of both CD4+ and CD8+ cells in allogeneic islets retrieved from IκBαΔN-Tg mice that had not rejected by day 40 (Figure 1D), suggesting that the defect is prior to, or at the level of, migration. Of note, IκBαΔN-Tg mice that successfully rejected islet allografts showed intra-graft T cell infiltrates comparable to wildtype recipients (data not shown).
Intact Priming of NF-κB-Impaired T Cells in Response to Alloantigen
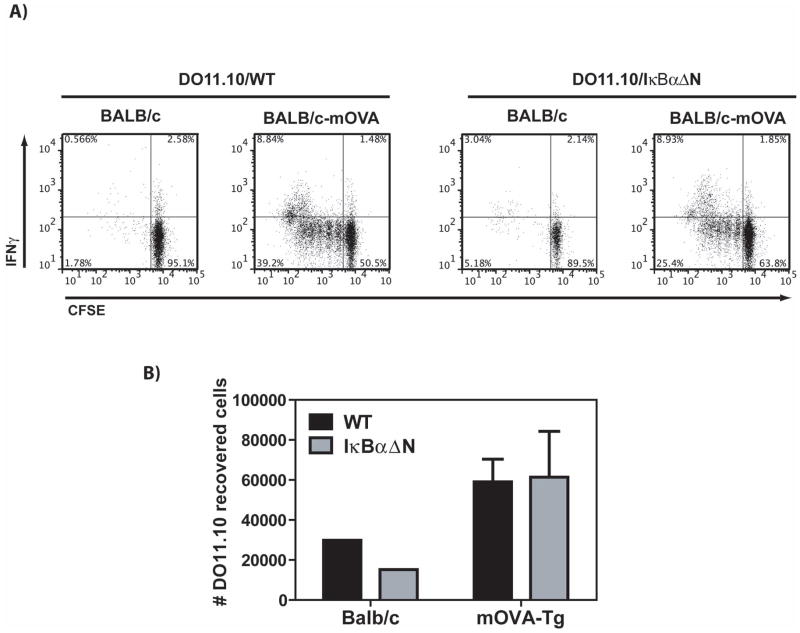
The combination of prolonged allograft survival in IκBαΔN-Tg mice with the decreased numbers of intra-graft T cells pointed towards reduced T cell priming/expansion, diminished T cell survival, or suboptimal migration of NF-κB-impaired T cells to the islet allografts. To investigate whether IκBαΔN-Tg T cells had a defect in T cell priming, CFSE-labeled DO11.10-Tg or DO11.10xIκBαΔN-Tg splenocytes containing the same number of OVA-specific T cells were adoptively transferred into syngeneic BALB/c recipients transplanted 1d later with BALB/c islets that did or did not express membrane-bound OVA. The kidney draining lymph nodes and grafts were harvested 5d after transplantation and cells were stimulated in vitro with PMA and ionomycin and analyzed by flow cytometry 4h later to assess their function. Surprisingly, T cell proliferation and IFN-γ production were readily detected in the draining lymph nodes of mice transplanted with mOVA-expressing islets, regardless of whether the transferred T cells were of DO11.10 or DO11.10xIκBαΔN-Tg origin (Figure 2A and 2B). Similarly, IFN-γ in response to allogeneic stimulators could be detected by ELISpots in the spleen of polyclonal IκBαΔN-Tg mice transplanted with BALB/c islets (data not shown). These results suggest that islet allograft acceptance in IκBαΔN-Tg mice is likely not due to lack of T cell priming.
Figure 2. Intact priming of NF-κB-impaired T cells in response to alloantigen.
CFSE-labeled DO11.10 wildtype (WT) or DO11.10xIκBαΔN-Tg splenocytes containing 2×106 CD4+ T cells were adoptively transferred into syngeneic BALB/c recipients transplanted 1 day later with BALB/c islets or with BALB/c islets expressing membrane-bound OVA (mOva). A) Proliferation (CFSE dilution) and IFN-γ production in DO11.10 cells (CD4+KJ126+-gated events) was assessed by flow cytometry on cells from the draining lymph nodes on day 5 post-transplantation following restimulation with PMA and ionomycin. B) Total numbers of OVA-specific T cells identified by flow cytometry as in A) were calculated by multiplying the percentage of CD4+KJ126+ cells by the number of live cells identified by the Trypan Blue exclusion method. Values represent the mean + SEM of 3 determinations. Results are representative of 2 independent experiments.
Islet Allograft Acceptance in IκBαΔN-Tg Mice Is at Least in Part Due to T Cell Deletion
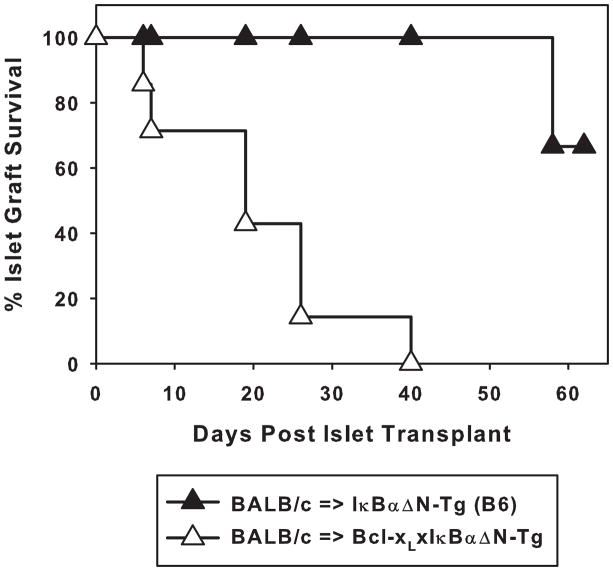
NF-κB activation plays a role in the survival of T cells following antigen recognition, in part via the upregulation of anti-apoptotic molecules such as Bcl-xL (13). Thus, it was possible that initial T cell priming was intact after graft-specific antigen recognition in vivo but that a proportion of dividing NF-κB-impaired T cells would later die because of impaired induction of anti-apoptotic factors, explaining prolonged islet allograft survival in IκBαΔN-Tg mice. To test this hypothesis, IκBαΔN-Tg mice (B6) were crossed with mice expressing Bcl-xL selectively in T cells and resulting mice were transplanted with BALB/c islets. As shown in Figure 3, expression of Bcl-xL in T cells restored the ability of IκBαΔN-Tg mice to reject islet allografts suggesting that T cell deletion is at least in part responsible for the prolonged survival of islet allografts.
Figure 3. Transgenic expression of Bcl-xL in T cells accelerates allograft rejection by IκBαΔN-Tg mice.
IκBαΔN-Tg (B6, n=6, MST > 50) and Bcl-xLxIκBαΔN-Tg (B6, n=7, MST 19) mice were rendered diabetic and transplanted with BALB/c islets. Graft survival time in these 2 strains was significantly different (p<0.001).
TCR-NF-κB Is Essential for Islet Allograft Rejection but Not for Donor-Specific Tolerance
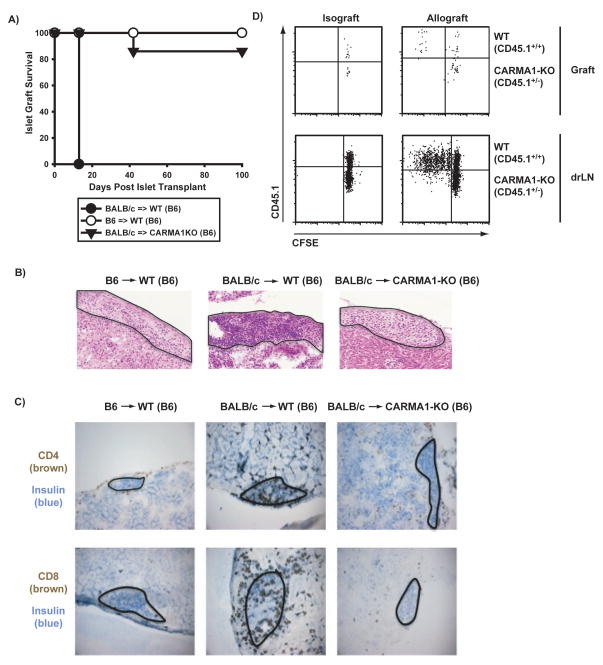
Our previous results indicate that T cell-specific NF-κB plays an important role in islet allograft rejection. In IκBαΔN-Tg T cells, NF-κB activation is theoretically impaired downstream of all receptors in T cells known to promote NF-κB activity, including TLR and TNFR family members, in addition to the TCR. To determine if more selective inhibition of NF-κB downstream of the TCR would be sufficient to promote long-term islet allograft acceptance, we used CARMA1-deficient mice that lack an adaptor essential for linking the TCR and BCR to NF-κB activity, but have normal NF-κB activation downstream of other receptors expressed in T cells (12). Similarly to IκBαΔN-Tg mice, CARMA1-deficient mice accepted fully allogeneic islet allografts long-term (Figure 4A). Histology and immunohistochemistry to detect CD4+ and CD8+ cells confirmed the presence of very few mononuclear cell infiltrates in grafts from CARMA1-deficient mice even at >40d post-transplantation, in contrast to very dense infiltrates in the allogeneic islets of wildtype mice on day 9 post-transplantation (Figure 4B, 4C). These data suggest that selective inhibition of TCR- and BCR-driven NF-κB activity is sufficient to allow islet allograft acceptance. Blockade of NF-κB upon TCR stimulation is more complete in CARMA1KO than IκBαΔN-Tg T cells, as assesses by electromobility shift assay (data not shown). To investigate the functional consequence of lack of CARMA1, CARMA1KO mice were crossed with TEa-Tg mice that express a TCR reactive to an I-Ed peptide presented by I-Ab. Congenically tagged TEaxCARMA1KO (CD45.1+/−) and wildtype (CD45.1+/+) purified T cells were CFSE labeled and coinjected into several CD45.2+/+ recipients of BALB/c islet allografts. The graft and renal draining lymph nodes were harvested on day 7 and analyzed by flow cytometry. As shown in Figure 4D, whereas wildtype T cells proliferated extensively, CARMA1KO T cells failed to divide at all, suggesting lack of priming and therefore a more proximal functional defect than in IκBαΔN-Tg T cells.
Figure 4. Permanent acceptance of islet allografts in CARMA1-deficient recipients.
A) Wildtype (B6) or CARMA1KO (B6) mice were rendered diabetic and transplanted with syngeneic (n=5, MST > 100) or fully allogeneic (BALB/c) islets (n=4, MST 16 for wildtype and n=7, MST > 90 for CARMA1KO recipients). B) Wildtype (CD45.1+/+) and CARMA1-KO (CD45.1+/−) TEa CD4+ cells (106) were coinjected into B6 (CD45.2+/+) recipients. One day later the mice were transplanted with B6 or BALB/c pancreatic islets. Mice were sacrificed seven days post-transplant and the spleen (not shown), draining lymph nodes and graft were processed for FACS analysis. Shown are the CD4+CD45.1+TCRVα2+TCRVβ6+-gated cells, displayed as CD45.1bright (wildtype) and CD45.1intermediate (CARMA1KO) versus CFSE. C) and D) H&E (C) and immunohistochemistry (D) staining of frozen sections obtained from graft-containing kidneys explanted on d9 (B6 => B6 and BALB/c => B6) and d40 [BALB/c => CARMA1KO (B6)] after transplantation were used to visualize mononuclear infiltrates in the grafts. A magnification of 20x is shown using an Olympus FSX100 microscope. Contour lines are drawn around the transplanted islets.
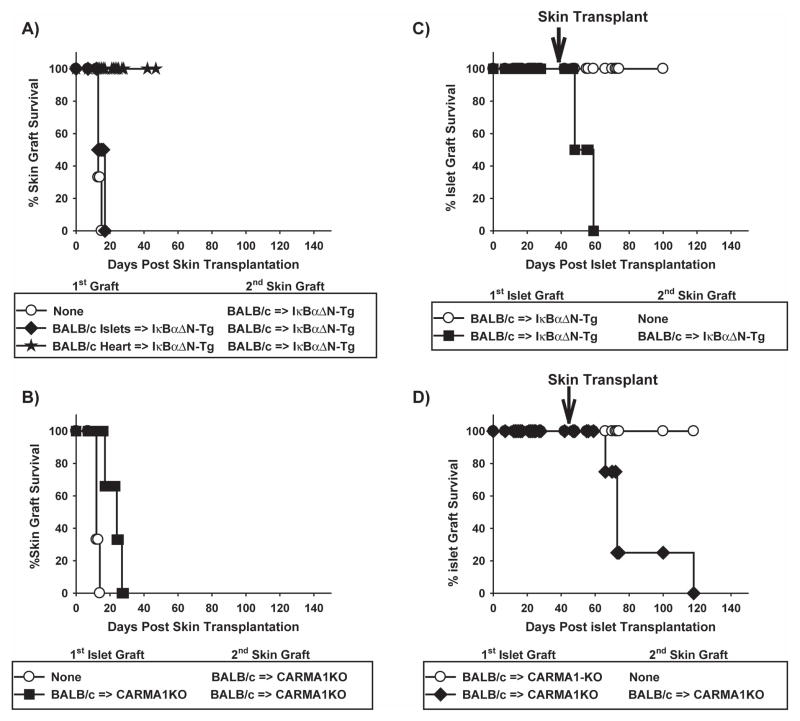
To determine whether islet transplantation in mice with reduced NF-κB activity in T cells resulted in the development of donor-specific tolerance, donor skin grafts were transplanted into IκBαΔN-Tg or CARMA1-KO mice that had accepted islet allografts long-term (>40d). Donor skin grafts were rapidly rejected by IκBαΔN-Tg (B6) (Figure 5A) and CARMA1-KO recipients of islet allografts (Figure 5B, difference in survival times not statistically significant), indicating that neither strain had developed the robust donor-specific tolerance achieved following heart transplantation [Figure 5A and (14)]. In addition, transplantation of donor skin precipitated the rejection of the primary islet allografts in both strains of mice (Figure 5C, D), indicating that the immune response elicited by the skin transplant could overcome the consequences of NF-κB impairment in T cells. Overall, our results demonstrate that reduced NF-κB activity in T cells can promote long-term acceptance, but not tolerance, of islet allografts.
Figure 5. Secondary donor skin transplantation precipitates rejection of primary islet allografts.
IκBαΔN-Tg (B6) and CARMA1KO (B6) mice with stable BALB/c islet allografts for 45–50d were left unmanipulated or were transplanted with secondary donor BALB/c skin allografts. A) Survival of skin allografts in naïve (MST 13) or allogeneic (MST 15) islet- or heart-bearing (MST >40) IκBαΔN-Tg mice. B) Survival of skin allografts in naïve (MST 12) or allogeneic (MST 24) islet-bearing CARMA1-deficient mice. C) Survival of the primary islet allografts in unmanipulated (MST >100) or skin-grafted (MST 53) IκBαΔN-Tg mice. D) Survival of the primary islet allografts in unmanipulated (MST>100) or skin-grafted (MST 73) CARMA1-deficient mice. Results represent 3 mice per group.
Discussion
Wider implementation of islet allograft transplantation in the clinic is limited by the progressive loss of islet mass in part due to alloimmunity and recurrent autoimmunity despite current immunosuppressive therapies. We have previously shown that inhibition of NF-κB in T cells results in long-term acceptance of cardiac allografts with development of donor-specific tolerance (14). To determine the promise of such an approach in islet transplantation, we examined islet allograft survival in 2 mouse models with impaired NF-κB activity in T cells. Our results show that T cell-NF-κB activity plays an important role in islet allograft rejection. However, reduced NF-κB activity in T cells did not promote donor-specific tolerance to islet allografts, in contrast to that observed following cardiac transplantation.
Previous results by our group and others indicate that IκBαΔN-Tg mice accept heart allografts long-term and develop donor-specific tolerance following cardiac transplantation, but efficiently reject skin allografts (14, 15). Our current study positions pancreatic islets in between heart and skin tissues with respect to their dependence on T cell-NF-κB for allograft rejection. Along with the Chong laboratory, we have previously proposed that organs such as skin, lung and intestine that are colonized with commensal microbes may be more susceptible to rejection because of microbial signals enhancing alloresponses (16). Although pancreatic islets are not colonized by commensal bacteria, the process of islet isolation likely releases endogenous damage-associated molecular patterns, which may also increase alloresponses (17). We (16) and others (18, 19) have shown that elimination of MyD88 to reduce signaling by microbial patterns facilitates costimulation blockade-mediated acceptance of skin allografts. Whether such an approach could synergize with inhibition of T cell-NF-κB for acceptance of islet allografts, remains to be established.
The percentage of IκBαΔN-Tg mice that accept islet allografts long-term was greater on the BALB/c than the B6 background. Although the exact factors responsible for these differences are not clear, it is well known that the genetic background has a major impact on the strength and type of immune responses. For instance, BALB/c mice are thought to be more susceptible to Leishmania major infection than B6 mice because of their greater predisposition towards Th2 responses (20). A lower ratio of Th1:Th2 differentiation in IκBαΔN-Tg (BALB/c) than IκBαΔN-Tg (B6) mice may conceivably be protective for islet allograft survival.
Our results demonstrate that transgenic expression of the anti-apoptotic factor Bcl-xL in T cells was sufficient to accelerate islet allograft rejection in IκBαΔN-Tg mice, suggesting that impaired survival of activated T cells is a major mechanism by which IκBαΔN-Tg mice have delayed rejection of islet allografts. This is similar to our previous results using cardiac allograft IκBαΔN-Tg recipients (21) in which we had shown that death of alloreactive NF-κB-impaired T cells was mediated by Fas (22) and suggest that a dominant function of T cell-NF-κB in vivo is to enable survival of activated T cells.
Lack of CARMA1 resulted in more universal acceptance of islet allografts than overexpression of the IκBαΔN super-repressor in T cells. We speculate that this is due to the more complete inhibition of TCR-driven NF-κB activity in CARMA1-deficient than IκBαΔN-Tg T cells (our unpublished observations). This is consistent with the lack of proliferation observed when TEa-TgxCARMA1KO T cells were transferred into islet allograft-bearing mice, prompting the hypothesis that partial reduction in NF-κB results in abortive proliferation and subsequent apoptosis of T cells, whereas complete ablation of TCR-NF-κB can prevent T cell activation altogether. In addition, CARMA1 also links the BCR to NF-κB activity and B cells in CARMA1-deficient mice display an immature phenotype (12). B cells have been implicated in the rejection of islet allografts in mice with autoimmune diabetes (23), such that it is possible that the reduced antigen-presenting and/or antibody-secreting function of CARMA1-deficient B cells may play a role in the lack of T cell priming and universal acceptance of pancreatic islets by CARMA1-deficient mice.
Transplantation of primary skin allografts was able to promote rejection of secondary donor islets in IκBαΔN-Tg and CARMA1KO mice. We have previously shown that skin Langerhans cells can prime and sustain survival of NF-κB-impaired T cells (24), such that skin grafts may allow sufficient T cell priming and survival to enable islet allograft rejection by IκBαΔN-Tg and CARMA1KO mice.
Overall, our results indicate that NF-κB in T cells may be a promising therapeutic target for facilitating acceptance of pancreatic islets. Given the unique signaling pathway that links the TCR/BCR to NF-κB, it may be possible to identify small molecule inhibitors that disrupt TCR/BCR-driven NF-κB selectively, using compounds that inhibit phosphorylation of CARMA1, which is required for its downstream effects (25, 26), or that disrupt assembly of the CARMA1/Bcl-10/Malt-1 adaptosome complex. Such drugs would have major advantages over current immunosuppressive agents that inhibit NF-κB activity in all cell types as they would be expected to have fewer side-effects. However, whether they would prevent the recurrence of autoimmunity which is likely also a major barrier to long-term acceptance of allografts in T1D patients remains to be studied.
Materials and Methods
Mice
Six to 8-weeks old C57BL/6 (B6), BALB/c and C3H/HEJ (C3H) mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). IκBαΔN-Tg mice that express a non-degradable IκBα transgene driven by the Lck/CD2 locus control region (3) and backcrossed to either the B6 or the BALB/c background for more than ten generations were a gift from Mark Boothby (Vanderbilt University). Bcl-xL-Tg mice (H-2b) (27) were a gift from Craig Thomson (when at the University of Chicago). IκBαΔN-Tg mice on a BALB/c background were crossed to DO11.10-Tg mice (28) to obtain DO11.10xIκBαΔN-double transgenic mice. Mice on a BALB/c background expressing membrane-bound OVA (29) under the control of the actin promoter were a gift from Elizabeth Ingulli (when at the University of Minnesota). CARMA1-deficient mice (12) were a gift from Dan Littman (New York University) and were backcrossed to the B6 background for more than six generations. TEa-Tg mice (30) in which Vα2Vβ6 T cells recognized a peptide of I-Ed presented on I-Ab were obtained from Alexander Rudensky (University of Washington, WA) and crossed to CARMA1KO animals. All animals were used according to the procedures outlined by the University of Chicago’s Institutional Animal Care and Use Committee in compliance with the National Institutes of Health guidelines for animal use.
Organ Transplantation
Islets were isolated following collagenase P injection into the common bile duct. The concentration of collagenase P (0.375 mg/ml–0.55 mg/ml; Sigma-Aldrich) was titrated for each lot. Islets at the 1.096/1.069 and 1.069/1.037 interfaces of a discontinuous Ficoll gradient were collected and hand-picked for transplantation. Recipient mice were rendered diabetic by an ip injection of streptozotocin (STZ, Sigma-Aldrich, 180 – 225 mg/kg body weight, titrated for each lot and mouse strain) in 0.05M citrate buffer at ph 4.5. Diabetic mice (non-fasting glucose levels > 300 mg/dl for two consecutive days) were transplanted with approximately 400 islets injected underneath the kidney capsule. Mice with corrected glucose levels (< 200 mg/dl) within the first 4 days post transplantation were included in the study. The latter of 2 consecutive days of high blood glucose (> 250 mg/dl) was defined as the day of graft rejection. Skin and heart transplantation were performed as previously described (14, 21).
In Vivo T Cell Proliferation
Splenocytes from DO11.10-Tg or DO11.10xIκBαΔN-Tg mice, mice were labeled with CFSE (4μM, Sigma-Aldrich) and anti-CD4 and analyzed by flow cytometry to adjust for the number of T cells. Splenocytes containing 2×106 CD4+ T cells were adoptively transferred into syngeneic BALB/c recipients transplanted 1d later with syngeneic islets that expressed membrane-bound OVA. Draining lymph nodes and grafts were harvested 5d after transplantation and live cells were counted using the Trypan blue exclusion method. Cells were incubated for 4h in tissue culture medium with brefeldin A (5μg/ml, Biolengend) in the presence or absence of phorbol myristoacetate (PMA, 150ng/ml, Sigma-Aldrich) and ionomycin (500ng/ml, Sigma-Aldrich) and then stained with anti-CD4, the clonotypic antibody KJ126 and anti-IFN-γ or control isotype for acquisition in an LSR II flow cytometer (Beckton-Dickinson). Results were analyzed with FlowJo software (Tree Star). TEa-Tg (CD45.1+/+) or TEaxCARMA1KO (CD45.1+/−) CD4+ cells were enriched by negative selection over magnetic columns and labeled with CFSE as above. TEa-Tg and TEaxCARMA1KO T cells were coinjected (106 each) into B6 recipients (CD45.2+/+) transplanted with BALB/c islets. Animals were sacrificed on day 7 and CFSE dilution in the graft and kidney draining lymph nodes was assessed by flow cytometry, upon gating on Vα2+Vβ6+CD45.1+ cells.
Microscopy
Kidneys containing the islet grafts were embedded in OCT (Tissue-Tek), snap frozen using dry ice and 2-methylbutane (Sigma-Aldrich) and later cut onto microslides using a microtome. Slides were dried, fixed in 4% PFA/PBS and stored at −80°C. Frozen sections (8 μm) were stained with hematoxylin/eosin (H&E) or immunostained using guinea-pig anti-insulin antibody (Dako) followed by donkey anti-guinea pig Dylight 488 antibody (Jackson ImmunoResearch), or with rat anti-CD4 or anti-CD8 antibodies (eBioscience) followed with donkey anti-rat Dylight 594 antibody (Jackson ImmunoResearch). Slides were mounted using Vectashield Hard Set Mounting Media containing Dapi (Vector Labs) and viewed using a fixed sample Disc Scanning Unit confocal microscope. Images analyzed using ImageJ software (NIH) to calculate the number of T cells present in the islet allografts.
Statistical Analyses
Comparisons between graft survival times were calculated using Kaplan-Meier survival curves coupled to the Logrank test. Multiple comparisons of the means were performed by ANOVA with correction of p values using the Tukey-Kramer method. For all tests, p values <0.05 were considered significant.
Acknowledgments
This work was supported by grants 1-2003-188 JDFRI and AI052352-01 to MLA, an AHA fellowship to LLM, and AI052352-01-03S1 and T32 HL 07605 to DLP.
We thank Mark Boothby, Craig Thompson, Dan Littman and Liz Ingulli for providing IκBαΔN-Tg, Bcl-xL-Tg, CARMA1-deficient and mOVA-Tg mice, respectively. We also thank the Integrated Microscopy and Flow Cytometry Core Facilities at the University of Chicago for their expert technical help, especially Vytas Bindokas for help with the ImageJ software analysis. We are indebted to the members of the Alegre laboratory for critical reading of the manuscript and helpful discussions.
Abbreviations
- APC
antigen-presenting cell
- C57Bl/6
B6
- CFSE
Carboxyfluorescein succinimidyl ester
- H&E
hematoxylin/eosin
- NF-κB
nuclear factor κ B
- OVA
ovalbumin
- PMA
Phorbol Myristate Acetate
- STZ
streptozotocin
- T1D
type 1 diabetes
- TLR
Toll-like receptor
- TNFR
tumor necrosis factor receptor
Footnotes
DLP designed and performed all experiments and participated in manuscript writing; YW and PZ transplanted the pancreatic islets; LLM participated in study design; MLA participated in study design and manuscript writing.
The authors declare no conflict of interest.
References
- 1.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 3.Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J Exp Med. 1997;185:1897. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronica MA, Mora AL, Mitchell DB, et al. Preferential role for NF-kappa B/Rel signaling in the type 1 but not type 2 T cell-dependent immune response in vivo. J Immunol. 1999;163:5116. [PubMed] [Google Scholar]
- 5.Mora A, Youn J, Keegan A, Boothby M. NF-kappa B/Rel participation in the lymphokine-dependent proliferation of T lymphoid cells. J Immunol. 2001;166:2218. doi: 10.4049/jimmunol.166.4.2218. [DOI] [PubMed] [Google Scholar]
- 6.Corn RA, Aronica MA, Zhang F, et al. T cell-intrinsic requirement for NF-kappa B induction in postdifferentiation IFN-gamma production and clonal expansion in a Th1 response. J Immunol. 2003;171:1816. doi: 10.4049/jimmunol.171.4.1816. [DOI] [PubMed] [Google Scholar]
- 7.Mora AL, Corn RA, Stanic AK, et al. Antiapoptotic function of NF-kappaB in T lymphocytes is influenced by their differentiation status: roles of Fas, c-FLIP, and Bcl-xL. Cell Death Differ. 2003;10:1032. doi: 10.1038/sj.cdd.4401257. [DOI] [PubMed] [Google Scholar]
- 8.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 9.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 10.Thome M, Charton JE, Pelzer C, Hailfinger S. Antigen receptor signaling to NF-kappaB via CARMA1, BCL10, and MALT1. Cold Spring Harb Perspect Biol. 2010;2:a003004. doi: 10.1101/cshperspect.a003004. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, Thomas D, Boffa DJ, et al. Enforced c-REL deficiency prolongs survival of islet allografts1. Transplantation. 2002;74:291. doi: 10.1097/00007890-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Egawa T, Albrecht B, Favier B, et al. Requirement for CARMA1 in antigen receptor-induced NF-kappa B activation and lymphocyte proliferation. Curr Biol. 2003;13:1252. doi: 10.1016/s0960-9822(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 13.Stanic AK, Bezbradica JS, Park JJ, et al. NF-kappa B controls cell fate specification, survival, and molecular differentiation of immunoregulatory natural T lymphocytes. J Immunol. 2004;172:2265. doi: 10.4049/jimmunol.172.4.2265. (in eng) [DOI] [PubMed] [Google Scholar]
- 14.Zhou P, Hwang KW, Palucki DA, et al. Impaired NF-kB activation in T cells permits tolerance to primary heart allografts and to secondary donor skin grafts. Amer J Transplantation. 2003;3:139. doi: 10.1034/j.1600-6143.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 15.Finn PW, Stone JR, Boothby MR, Perkins DL. Inhibition of NF-kappaB-dependent T cell activation abrogates acute allograft rejection. J Immunol. 2001;167:5994. doi: 10.4049/jimmunol.167.10.5994. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Wang T, Zhou P, et al. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006;6:2282. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 17.Alegre ML, Goldstein DR, Chong AS. TLR signaling in Transplantation. Current Opinions in Organ Transplantation. 2009;13:358. doi: 10.1097/MOT.0b013e3283061149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker WE, Nasr IW, Camirand G, et al. Absence of innate MyD88 signaling promotes inducible allograft acceptance. J Immunol. 2006;177:5307. doi: 10.4049/jimmunol.177.8.5307. [DOI] [PubMed] [Google Scholar]
- 19.McKay D, Shigeoka A, Rubinstein M, Surh C, Sprent J. Simultaneous deletion of MyD88 and Trif delays major histocompatibility and minor antigen mismatch allograft rejection. Eur J Immunol. 2006;36:1994. doi: 10.1002/eji.200636249. [DOI] [PubMed] [Google Scholar]
- 20.Locksley RM, Pingel S, Lacy D, et al. Susceptibility to infectious diseases: Leishmania as a paradigm. J Infect Dis. 1999;179:S305. doi: 10.1086/513843. [DOI] [PubMed] [Google Scholar]
- 21.Zhou P, Balin SJ, Mashayekhi M, et al. Transplantation tolerance in NF-kappaB-impaired mice is not due to regulation but is prevented by transgenic expression of Bcl-xL. J Immunol. 2005;174:3447. doi: 10.4049/jimmunol.174.6.3447. [DOI] [PubMed] [Google Scholar]
- 22.Molinero LL, Wang Y, Zhou P, Yagita H, Alegre ML. Fas mediates cardiac allograft acceptance in mice with impaired T cell-intrinsinc NF-kB signaling. Transpl Int. 2009 doi: 10.1111/j.1432-2277.2009.00875.x. Accepted with minor revisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kupfer T, Beilke JN, Pham K, Buhrman J, Gill RG. “Indirect” acute islet allograft destruction in nonobese diabetic mice is independent of donor major histocompatibility complex and requires host B lymphocytes. Transplantation proceedings. 2008;40:462. doi: 10.1016/j.transproceed.2008.01.054. (in eng) [DOI] [PubMed] [Google Scholar]
- 24.Molinero L, Zhou P, Wang Y, et al. Epidermal Langerhans cells play a major role in skin allograft rejection in mice with NF-kB-impaired T cells. Am J Transplant. 2008;8:21. doi: 10.1111/j.1600-6143.2007.02038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner D, Brechmann M, Rohling S, et al. Phosphorylation of CARMA1 by HPK1 is critical for NF-kappaB activation in T cells. Proc Natl Acad Sci U S A. 2009;106:14508. doi: 10.1073/pnas.0900457106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thome M, Weil R. Post-translational modifications regulate distinct functions of CARMA1 and BCL10. Trends Immunol. 2007;28:281. doi: 10.1016/j.it.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Chao DT, Linette GP, Boise LH, et al. Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh CS, Macatonia SE, O’Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 30.Firpo EJ, Kong RK, Zhou Q, et al. Antigen-specific dose-dependent system for the study of an inheritable and reversible phenotype in mouse CD4+ T cells. Immunology. 2002;107:480. doi: 10.1046/j.1365-2567.2002.01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]