Summary
Bradykinin can enhance skeletal muscle glucose uptake (GU), and exercise increases both bradykinin production and muscle insulin sensitivity, but bradykinin’s relationship with post-exercise insulin action is uncertain. Our primary aim was to determine if the B2 receptor of bradykinin (B2R) is essential for the post-exercise increase in GU by insulin-stimulated mouse soleus muscles. Wildtype (WT) and B2R knockout (B2RKO) mice were sedentary or performed 60 minutes of treadmill exercise. Isolated soleus muscles were incubated with [3H]-2-deoxyglucose ±insulin (60 or 100 μU/mL). GU tended to be greater for WT vs. B2RKO soleus with 60μU/mL insulin (P=0.166) and was significantly greater for muscles with 100μU/mL insulin (P<0.05). Both genotypes had significant exercise-induced reductions (P<0.05) in glycemia and insulinemia, and the decrements for glucose (~14%) and insulin (~55%) were similar between genotypes. GU tended to be greater for exercised vs. sedentary soleus with 60μU/mL insulin (P=0.063) and was significantly greater for muscles with 100μU/mL insulin (P<0.05). There were no significant interactions between genotype and exercise for blood glucose, plasma insulin or GU. These results indicate that the B2R is not essential for the exercise-induced decrements in blood glucose or plasma insulin or for the post-exercise increase in GU by insulin-stimulated mouse soleus muscle.
Keywords: glucose transport, insulin sensitivity, insulin resistance, kinin, physical activity
Introduction
A single exercise bout can lead to subsequently improved whole body insulin sensitivity, and skeletal muscle is the major tissue that accounts for this exercise-induced improvement in glucose disposal (Henriksen 2002, Richter et al. 1982). The increased glucose uptake by muscle is evident several hours after exercise cessation in vivo (Richter et al. 1989), and persists when rodent skeletal muscles are dissected out after exercise and studied in vitro (Cartee et al. 1989, Hamada et al. 2006).
The mechanisms whereby prior exercise initiates the improvement in skeletal muscle insulin sensitivity remain incompletely understood. Presumably, events that occur during exercise trigger the processes that subsequently lead to increased insulin-stimulated glucose transport. Exercise induces increased production of a circulating nonapeptide bradykinin (Blais et al. 1999, Boix et al. 2005, Langberg et al. 2002, Stebbins et al. 1990, Taguchi et al. 2000). Bradykinin can favor a subsequent increase in insulin-stimulated glucose uptake (Beard et al. 2006, Duka et al. 2001, Henriksen and Jacob 1995, Henriksen et al. 1996, Henriksen et al. 1998, Henriksen et al. 1999, Miyata et al. 1998). These observations raised the possibility that bradykinin may participate in the post-exercise increase in skeletal muscle insulin sensitivity.
Skeletal muscle cells express the B2 receptor of bradykinin, B2R (Duka et al. 2006, Figueroa et al. 1996, Rabito et al. 1996), which is important for bradykinin’s influence on glucose uptake (Beard et al. 2006, Duka et al. 2001, Figueroa et al. 1996). We studied mice that were null for B2R (B2 receptor of bradykinin knockout, B2RKO) and normal, wildtype (WT) mice under sedentary and post-exercise conditions to test the hypothesis that the B2R is essential for the post-exercise increase in glucose uptake by insulin-stimulated skeletal muscle.
Methods
Materials
Human recombinant insulin was from Eli Lilly (Indianapolis, IN). 2-Deoxy-[3H]glucose and [14C]mannitol were fromPerkin-Elmer (Boston, MA). Reagents and apparatus for SDS-PAGE, nonfat dry milk, and nitrocellulose membranes were from Bio-Rad Laboratories (Hercules, CA). A bicinchoninic acid assay kit for total protein determination and SuperSignal WestDura Extended Duration Substrate for immunodetection were from Pierce Biotechnology (Rockford, IL). Anti-phospho-Akt Thr308 and secondary antibody (horseradish peroxidase-conjugated anti-rabbit IgG) was from Cell Signaling Technology. Other reagents were from Sigma-Aldrich (St. Louis, MO).
Animals
Animal care was approved by the University of Michigan Committee on Use and Care for Animals. Male mice null for the B2 receptor of bradykinin (B2RKO; strain 002641) and wildtype (WT) control mice (strain 101045; B6129SF2/J) were from the Jackson Laboratory (Bar Harbor, ME). All mice were housed in plastic cages and provided a standard diet (Lab Diet, PMI Nutrition International, Richmond, IN) and water ad libitum.
Treadmill exercise protocol
All mice (8–18 wk old) were familiarized with treadmill (Columbus Instruments, Columbus, OH) running for 10min on 2 consecutive days (1st day at 12-22m/min; 2nd day at 12-25 m/min). On the morning after the 2nd familiarization day, the WT and B2RKO mice were assigned to either a sedentary or exercised group. The exercise protocol consisted of 3 consecutive 20min-periods of progressive interval exercise (5min at 15m/min, 10min at 20m/min, and 5min at 25m/min with 0% slope) totaling 60min of running (Hamada et al. 2006). Access to food was removed for all mice at the time that the protocol began. All of the exercised mice completed the 60min protocol, after which exercised and sedentary mice were anesthetized (intraperitoneal injection of pentobarbital sodium, 50mg/kg body wt).
Blood glucose and plasma insulin
Blood was collected from the tail using heparinized capillary tubes, prior to anesthetization, in sedentary mice and in exercise mice immediately following the 60min treadmill protocol. Blood glucose was determined using an Accu-Check® Aviva (Roche Diagnostics, Indianapolis, IN) hand-held blood glucose meter. Blood was transferred to microcentrifuge tubes, centrifuged, and the plasma collected was used to assay insulin with the ALPCO Diagnostics™ Insulin (Mouse) Ultrasensitive EIA kit, catalog no. 80-INSMSU-E01 (Alpco Diagostics, Salem, NH).
Tissue dissection and in vitro soleus incubation
Paired soleus muscles from anesthetized mice were excised and incubated using a 2-step incubation protocol. During the 1st step, muscles were placed in vials containing 1.5mL of Krebs-Henseleit Buffer (KHB) supplemented with 0.1% bovine serum albumin (BSA), 2mM sodium pyruvate, and 6mM mannitol in the absence or presence of insulin (60μU/mL insulin was used for mice in Experiment 1; 100μU/mL insulin was used for mice in Experiment 2) for 60min. During all incubation steps, vials were placed in a heated (35°C), shaking water bath and continuously gassed from above (95% O2-5% CO2).
After the 1st incubation step, muscles were transferred to a 2nd vial containing KHB with 0.1% BSA, 1mM 2-deoxyglucose (2-deoxy-[3H]glucose, 6 mCi/mmol), 9mM mannitol ([14C]mannitol, 0.053 mCi/mmol), and the same insulin concentration as the previous step. Muscles were incubated at 35°C for 15min and then rapidly blotted on ice-cold filter paper, trimmed, freeze clamped, and stored at −80°C until processed.
Epididymal fat pads and gastrocnemius muscles were removed from some mice and weighed.
Muscle homogenization
Frozen muscles were weighed, transferred to prechilled glass tubes and homogenized in: 1) 0.5ml of perchloric acid (muscles from Experiment 1) or 2) ice-cold lysis buffer (0.5mL) containing 20mM Tris-HCl, 150mM NaCl, 1% NP-40, 1mM activated Na3VO4, 2mM EDTA, 2mM EGTA, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1μg/ml leupeptin (muscles from Experiment 2). Lysis buffer was used for the muscles analyzed for both glucose uptake and immunoblotting. Samples homogenized in perchloric acid were centrifuged (15,000g, 15min), and samples homogenized in the lysis buffer were rotated for ~1hr before being centrifuged (15,000g, 15min). Aliquots from supernatants were quantified for [3H] and [14C] using a liquid scintillation counter, and glucose uptake was calculated (Cartee and Bohn 1995).
Immunoblotting
Portions of samples processed in lysis buffer were analyzed by immunoblotting for phospho-Akt Thr308. Total protein concentration of the supernatants used for immunoblotting was determined by the bicinchoninic acid assay. Samples were resolved on a 10% SDS-PAGE gel and transferred to nitrocellulose in electrotransfer buffer overnight at 4°C. Blots were incubated in blocking solution [Tris-buffered saline (TBS) with 0.1% Tween 20 (TBST) and 5% nonfat dry milk] for 1hr at room temperature, washed with TBST and then incubated with anti-phospho-Akt Thr308 overnight at 4°C. Blots were washed with TBST and incubated with secondary antibody (horseradish peroxidase-conjugated anti-rabbit IgG). Blots were washed of excess antibody with TBST and then subjected to SuperSignal enhanced chemiluminesence (Thermo Scientific, Rockford, IL). Immunoreactive protein was quantified by densitometry (Alpha Innotech, San Leandro, CA). The mean value for WT sedentary samples on each immunoblot, expressed in densitometry units relative to total protein, was adjusted to equal 1.0. Each sample value was expressed relative to the adjusted mean value for the WT sedentary control.
Statistical analyses
Statistical analyses used Sigma Stat version 2.0 (San Rafael, CA). Data are expressed as means ± SE. Two-way ANOVA was used to determine significant differences, and a Tukey post hoc test was used to identify the source of significant variance. A P value ≤0.05 was considered statistically significant.
Results
There were no statistically significant interactions (exercise x genotype) for any of the measurements that were made.
Body and tissue masses
Body mass was similar between genotypes, but the epididymal fat pad/ body mass ratio was ~30-37% lower (P<0.001) for B2RKO compared to WT mice (Table 1). The soleus/body mass ratio was ~8-12% greater (P<0.05) for B2RKO vs. WT mice, but there was not a significant effect of genotype on gastrocnemius/body mass ratio.
Table 1.
Body mass and tissue/body mass ratios and glucose and insulin concentrations.
| WT Sedentary |
WT Exercise |
B2RKO Sedentary |
B2RKO Exercise |
|
|---|---|---|---|---|
| Body mass, g | 26.4±0.6 | 26.3±0.7 | 26.8±0.5 | 26.4±0.6 |
| Fat pad/body mass ratio (mg/g) | 6.5±0.4 | 7.0±0.4 | 4.6±0.3* | 4.4±0.3* |
| Soleus/body mass ratio (mg/g) | 0.36±0.02 | 0.34±0.1 | 0.39±0.2* | 0.38±0.2* |
| Gastrocnemius/body mass ratio (mg/g) |
4.7±0.1 | 4.7±0.1 | 4.9±0.3 | 5.0±0.1 |
| Blood glucose, mmol·L-1 | 7.85±0.51 | 6.74±0.48† | 8.91±0.25* | 7.65±0.31†* |
| Plasma insulin, ng·mL-1 | 0.673±0.12 | 0.307±0.06† | 0.455±.06* | 0.205±.04†* |
Values are means±SE, n = 21-22 for body mass; n = 21-22 for soleus/body mass ratio; n = 8 for gastrocnemius/body mass ratio; and n= 14-16 for epididymal fat pad/body mass ratio and glucose and insulin concentrations.
P<0.05 (WT vs. B2RKO).
P<0.05 (Sedentary vs. Exercise).
Blood glucose and plasma insulin
Glycemia was 14% greater in B2RKO vs. WT mice under both sedentary and exercised conditions (P<0.01; Table 1). Within each genotype, exercise caused a similar ~14% decrease in blood glucose (P<0.05). Plasma insulin was ~32% lower (P<0.05) in the B2RKO vs. WT mice in sedentary and exercised conditions. In both genotypes, exercise caused a 55% decrease in plasma insulin (P<0.001).
Muscle glucose uptake
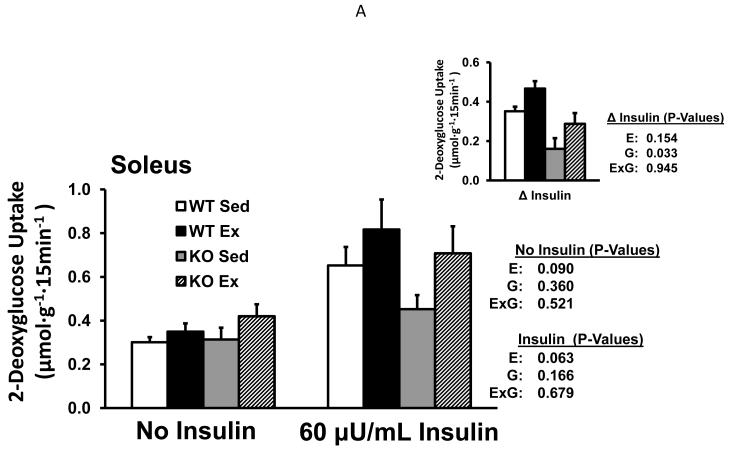
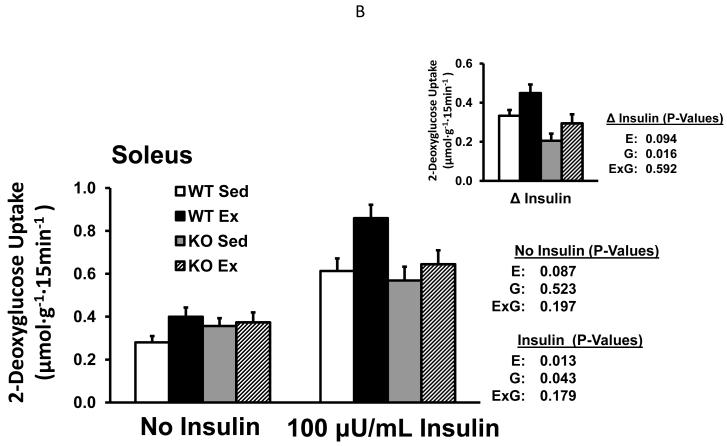
There was not an effect of genotype on glucose uptake without insulin in either experiment (Fig. 1A and 1B) or when the data from both experiments were pooled (data not shown). Glucose uptake with insulin was lower for B2RKO vs. WT mice with 100μU/mL insulin (P<0.05), and a non-significant trend (P=0.166) for lower values in the B2RKO mice was also evident with 60μU/mL insulin. For the pooled data from the insulin-treated soleus, glucose uptake was significantly lower (P<0.05) in the B2RKO compared to WT mice. Paired muscles were used for glucose uptake measurements, with one muscle from each mouse incubated without insulin and the contralateral muscle incubated with insulin. We calculated the insulin-stimulated increase in glucose uptake (delta insulin) by subtracting the basal value from the insulin-stimulated value. Delta insulin was significantly lower for B2RKO vs. WT mice with 60μU/mL (P<0.05), 100μU/mL (P<0.05) and when the data from both experiments were pooled (P<0.001).
Fig. 1.
Glucose uptake by isolated soleus muscles (Experiment 1: without or with 60μU/mL insulin (A); Experiment 2: without or with 100μU/mL insulin (B) from WT and B2RKO mice that were sedentary (SED) or exercised (EX). Insets of (A) and (B) represent the insulin-stimulated increase in glucose uptake (delta insulin) calculated by subtracting the basal value from the insulin-stimulated value of paired muscles. Values are mean ± SE for 9 or 15-16 muscles per group (A and B, respectively). *P<0.05. E, main effect of exercise treatment; G, main effect of genotype; E x G, interaction between main effects.
Glucose uptake without insulin tended to be slightly higher for exercised vs. sedentary mice in both Experiment 1 (P=0.090; Fig. 1A) and Experiment 2 (P=0.087; Fig. 1B). The pooled values for glucose uptake without insulin from both experiments were significantly greater for the exercised vs. sedentary (P<0.05). In the insulin-stimulated soleus, glucose uptake tended to be greater for the exercised vs. sedentary mice in Experiment 1 (P=0.063 with 60μU/ml; Fig. 1A) and was significantly greater in Experiment 2 (P<0.05 with 100μU/ml; Fig. 1B). When data from both experiments were pooled, glucose uptake of insulin-stimulated muscles was significantly greater (P<0.005) for exercised vs. sedentary mice. Delta insulin tended to be greater for exercised vs. sedentary mice with 60μU/mL (P=0.150) or 100μU/mL (P=0.094), and the delta insulin values were significantly greater after exercise when the data from both experiments were pooled (P<0.05).
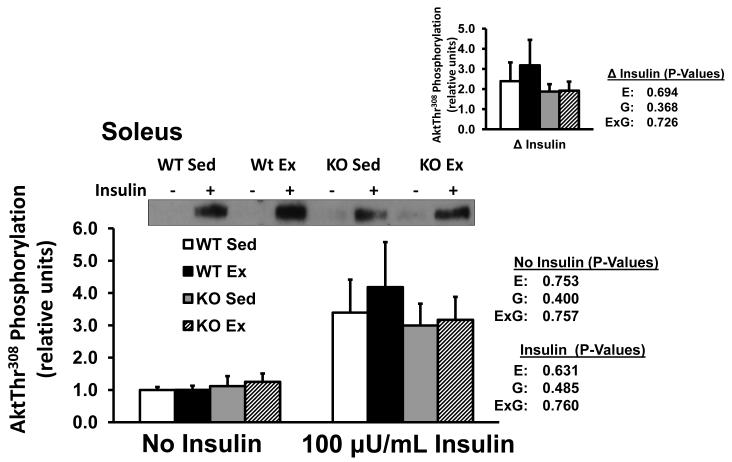
Akt Thr308 phosphorylation
Soleus Akt Thr308 phosphorylation was not significantly altered by exercise or genotype, with or without insulin, or for delta insulin (Fig. 2).
Fig. 2.
Akt threonine308 phosphorylation in isolated soleus from WT and B2RKO mice that were SED or EX. The insets represents the insulin-stimulated increase Akt threonine308 (delta insulin) calculated by subtracting the basal value from the insulin-stimulated value of paired muscles. Values are mean ±SE for 8 muscles per group.
Discussion
Improved whole body insulin sensitivity is a hallmark-effect of prior exercise. A post-exercise elevation in insulin-stimulated glucose uptake by skeletal muscle has been found in humans (Richter et al. 1989, Wojtaszewski et al. 2000), rats (Cartee and Holloszy 1990, Cartee et al. 1989, Richter et al. 1982), and mice (Bonen and Tan 1989, Bonen et al. 1984, Hamada et al. 2006). The primary aim of this study was to determine if the B2R is essential for the post-exercise increase in glucose uptake in insulin-stimulated skeletal muscle. Prior exercise resulted in an increased glucose uptake by muscles incubated with 100μU/mL insulin and a trend (P=0.063) for increased glucose uptake by muscles incubated with 60μU/mL insulin. The lack of a significant interaction between genotype and exercise on glucose uptake by muscles incubated with either insulin concentration indicates that the B2R is not essential for the post-exercise increase in glucose uptake. This study focused on glucose uptake, which is a rate-controlling process for skeletal muscle glucose metabolism. However, the current results do not eliminate the possibility that the B2R may influence other aspects of muscle metabolism, including glycogen synthesis, glucose oxidation, lipid synthesis and fatty acid esterification that we did not assess.
Earlier research demonstrated that circulating bradykinin can be increased by exercise or muscle contraction (Blais et al. 1999, Boix et al. 2005, Langberg et al. 2002, Stebbins et al. 1990, Taguchi et al. 2000). Furthermore, there is substantial evidence that bradykinin can elevate glucose uptake in both intact animals (Damas et al. 1999, Duka et al. 2001, Kohlman et al. 1995, Uehara et al. 1994) and isolated cells or tissues (Beard et al. 2006, Damas et al. 2004, Henriksen et al. 1999, Isami et al. 1996, Kudoh et al. 2000, Miyata et al. 1998). It seemed possible that bradykinin could be involved in the post-exercise increase in insulin-stimulated glucose transport. However, previous studies indicated that electrically stimulated contractions by isolated rat skeletal muscle in the presence of exogenous bradykinin did not cause a subsequent increase in glucose transport of insulin-stimulated muscles (Dumke et al. 2002). Furthermore, HOE-140, a B2R inhibitor, did not reduce the ability of prior contraction in serum to increase insulin-dependent glucose transport (Dumke et al. 2002). These results did not support a role for bradykinin in the post-contraction increase in insulin sensitivity, but because bradykinin is very rapidly degraded by kininases, experiments using exogenous bradykinin should be interpreted cautiously. Accordingly, in the current study, we used in vivo exercise by mice lacking the B2R as a novel approach to assess bradykinin’s potential importance for the persistent increase in insulin-stimulated glucose uptake after in vivo exercise. The current results for in vivo exercise extend the earlier findings using exogenous bradykinin or B2 receptor inhibitors with ex vivo muscle contractions. These results using several different approaches do not support the idea that exercise or contraction lead to subsequent elevation in insulin sensitivity as the result of a B2R-dependent mechanism.
The ~14% decline in blood glucose in both genotypes compares to an ~12-29% decrease in glycemia previously reported for normal mice after 60min of treadmill exercise (Fritsche et al. 2010, Hoene et al. 2009, Howlett et al. 2002, Wojtaszewski et al. 1999). Treadmill exercise caused an ~32% decline in plasma insulin concentration in both genotypes, which compares to published results indicating an ~33-52% reduction in circulating insulin after 60min of treadmill exercise by normal mice (Fritsche et al. 2010, Hamada et al. 2006, Higaki et al. 1999, Hoene et al. 2009). The absence of significant exercise x genotype interactions in the current study suggests that the expression of the B2R was not essential for exercise effects on either glycemia or insulinemia.
Glucose uptake by isolated muscles in the absence of insulin was not different for B2RKO compared to WT mice. However, for muscles with 100μU/mL insulin there was a genotype-associated reduction in glucose uptake for B2RKO vs. WT mice. These results are reminiscent of the previously reported results of a study using the euglycemic-hyperinsulinemic-clamp in which Duka et al. (2001) reported a lower glucose infusion rate for B2RKO compared to WT mice. Duka et al. (2001) reported a non-significant trend for a 13% increase in fasting glycemia for B2RKO vs. WT mice which corresponds to the 14% increase in blood glucose that we found in B2RKO mice. We found a lower value for plasma insulin concentration in the B2RKO vs. WT mice, whereas Duka et al. (2001) did not find a significant difference between the genotypes for fasting insulin. Rather they found a non-significant trend for higher fasting insulin for B2RKO mice. The mice in the earlier study had been treated for 3 days prior to the clamp with captopril, an ACE inhibitor which also inhibits kininase II (the enzyme which degrades bradykinin). Furthermore, the mice in the current study were only fasted ~1hr prior to sampling plasma for the insulin assay. Duka et al. (2001) did not explicitly describe the duration of the fast, but they cited the method used in an earlier euglycemic-hyperinsulinemic clamp study in which the animals underwent an overnight fast. The age and sex of the mice in the earlier study were not described. It is uncertain if these or other differences in experimental design account for the different effect of B2RKO on circulating insulin concentration. The lower plasma insulin concentration for B2RKO vs. WT mice suggests that there may have been decreased insulin secretion in the B2RKO animals.
The current study provides the first insulin signaling data for B2RKO mice. Muscle Akt Thr308 phosphorylation was similar between B2RKO and WT mice implicating an Akt-independent mechanism for the insulin resistance found in the B2RKO muscles. Muscle Akt Thr308 phosphorylation was also not different for exercised compared to sedentary groups, consistent with earlier data after exercise by normal mice (Hamada et al. 2006) and providing evidence that the increased insulin sensitivity after exercise, regardless of B2R expression, does not require enhanced Akt Thr308 phosphorylation.
The current results are consistent with previous studies (Cervenka et al. 1999, Schanstra et al. 2003) in which body mass was not different between B2RKO and WT mice. Schanstra et al. (2003) also found no difference between B2RKO and WT mice for food intake. A more recent study reported that B2RKO vs. WT mice had greater energy intake and energy expenditure concomitant with a ~25% reduction in total body fat content (per g body mass) determined by carcass analysis (de Picoli Souza et al. 2010). The results for lower body fat are similar to the reduction in epididymal fat pad/body mass ratio for the B2RKO compared to WT mice in the current study. They also found the gastrocnemius mass (per g body mass) of the B2RKO mice to be ~19% greater than control mice. Although gastrocnemius mass was not significantly different between genotypes in the current study, the soleus/body mass ratio was greater for B2RKO versus WT mice. The metabolic phenotype of B2RKO mice occurs despite moderate decrements in body fat and increments in skeletal muscle mass which are typically expected to favor improved insulin sensitivity.
In conclusion, mice lacking the B2R compared to WT controls had a small reduction in glucose uptake by insulin-stimulated soleus muscles concomitant with an undiminished insulin-stimulated increase in Akt phosphorylation. Earlier in vivo results from B2RKO mice undergoing a hyperinsulinemic clamp demonstrated insulin resistance, but they had not directly evaluated skeletal muscle or assessed insulin signaling. The current results demonstrate that insulin resistance of skeletal muscles lacking the B2R persists ex vivo and suggest this defect is secondary to an Akt-independent mechanism. The absence of the B2R also did not alter the effects of exercise on circulating glucose or insulin concentrations in vivo indicating that the B2R is not a major modulator of the important effects of acute exercise on either glycemia or insulinemia. Finally, the lack of a significant interaction between exercise and genotype for glucose uptake by insulin-stimulated skeletal muscle after exercise demonstrates that the B2R is not essential for the increased glucose uptake in insulin-stimulated soleus muscle after exercise by mice.
Acknowledgements
This research was supported by National Institutes of Health grant DK-071771 (GDC).
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- BEARD KM, LU H, HO K, FANTUS IG. Bradykinin augments insulin-stimulated glucose transport in rat adipocytes via endothelial nitric oxide synthase-mediated inhibition of Jun NH2-terminal kinase. Diabetes. 2006;55:2678–2687. doi: 10.2337/db05-1538. [DOI] [PubMed] [Google Scholar]
- BLAIS C, JR., ADAM A, MASSICOTTE D, PERONNET F. Increase in blood bradykinin concentration after eccentric weight-training exercise in men. J Appl Physiol. 1999;87:1197–1201. doi: 10.1152/jappl.1999.87.3.1197. [DOI] [PubMed] [Google Scholar]
- BOIX F, ROE C, ROSENBORG L, KNARDAHL S. Kinin peptides in human trapezius muscle during sustained isometric contraction and their relation to pain. J Appl Physiol. 2005;98:534–540. doi: 10.1152/japplphysiol.01340.2003. [DOI] [PubMed] [Google Scholar]
- BONEN A, TAN MH. Dissociation between insulin binding and glucose utilization after intense exercise in mouse skeletal muscles. Horm Metab Res. 1989;21:172–178. doi: 10.1055/s-2007-1009184. [DOI] [PubMed] [Google Scholar]
- BONEN A, TAN MH, WATSON-WRIGHT WM. Effects of exercise on insulin binding and glucose metabolism in muscle. Can J Physiol Pharmacol. 1984;62:1500–1504. doi: 10.1139/y84-248. [DOI] [PubMed] [Google Scholar]
- CARTEE GD, BOHN EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol. 1995;268:E902–909. doi: 10.1152/ajpendo.1995.268.5.E902. [DOI] [PubMed] [Google Scholar]
- CARTEE GD, HOLLOSZY JO. Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am J Physiol. 1990;258:E390–393. doi: 10.1152/ajpendo.1990.258.2.E390. [DOI] [PubMed] [Google Scholar]
- CARTEE GD, YOUNG DA, SLEEPER MD, ZIERATH J, WALLBERG-HENRIKSSON H, HOLLOSZY JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol. 1989;256:E494–499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- CERVENKA L, HARRISON-BERNARD LM, DIPP S, PRIMROSE G, IMIG JD, EL-DAHR SS. Early onset salt-sensitive hypertension in bradykinin B(2) receptor null mice. Hypertension. 1999;34:176–180. doi: 10.1161/01.hyp.34.2.176. [DOI] [PubMed] [Google Scholar]
- DAMAS J, BOURDON V, LEFEBVRE PJ. Insulin sensitivity, clearance and release in kininogen-deficient rats. Exp Physiol. 1999;84:549–557. doi: 10.1111/j.1469-445x.1999.01812.x. [DOI] [PubMed] [Google Scholar]
- DAMAS J, GARBACKI N, LEFEBVRE PJ. The kallikrein-kinin system, angiotensin converting enzyme inhibitors and insulin sensitivity. Diabetes Metab Res Rev. 2004;20:288–297. doi: 10.1002/dmrr.489. [DOI] [PubMed] [Google Scholar]
- DE PICOLI SOUZA K, BATISTA EC, SILVA ED, REIS FC, SILVA SMA, ARAUJO RC, LUZ J, SANTOS EL, PESQUERO JB. Effect of kinin B2 receptor ablation on skeletal muscle development and myostatin gene expression. Neuropeptides. 2010;44:209–214. doi: 10.1016/j.npep.2009.12.001. [DOI] [PubMed] [Google Scholar]
- DUKA A, DUKA I, GAO G, SHENOUDA S, GAVRAS I, GAVRAS H. Role of bradykinin B1 and B2 receptors in normal blood pressure regulation. Am J Physiol Endocrinol Metab. 2006;291:E268–274. doi: 10.1152/ajpendo.00382.2005. [DOI] [PubMed] [Google Scholar]
- DUKA I, SHENOUDA S, JOHNS C, KINTSURASHVILI E, GAVRAS I, GAVRAS H. Role of the B(2) receptor of bradykinin in insulin sensitivity. Hypertension. 2001;38:1355–1360. doi: 10.1161/hy1201.096574. [DOI] [PubMed] [Google Scholar]
- DUMKE CL, KIM J, ARIAS EB, CARTEE GD. Role of kallikrein-kininogen system in insulin-stimulated glucose transport after muscle contractions. J Appl Physiol. 2002;92:657–664. doi: 10.1152/japplphysiol.00854.2001. [DOI] [PubMed] [Google Scholar]
- FIGUEROA CD, DIETZE G, MULLER-ESTERL W. Immunolocalization of bradykinin B2 receptors on skeletal muscle cells. Diabetes. 1996;45(Suppl 1):S24–28. doi: 10.2337/diab.45.1.s24. [DOI] [PubMed] [Google Scholar]
- FRITSCHE L, HOENE M, LEHMANN R, ELLINGSGAARD H, HENNIGE AM, POHL AK, HARING HU, SCHLEICHER ED, WEIGERT C. IL-6 deficiency in mice neither impairs induction of metabolic genes in the liver nor affects blood glucose levels during fasting and moderately intense exercise. Diabetologia. 2010;53:1732–1742. doi: 10.1007/s00125-010-1754-4. [DOI] [PubMed] [Google Scholar]
- HAMADA T, ARIAS EB, CARTEE GD. Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. J Appl Physiol. 2006;101:1368–1376. doi: 10.1152/japplphysiol.00416.2006. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN EJ. Invited review: Effects of acute exercise and exercise training on insulin resistance. J Appl Physiol. 2002;93:788–796. doi: 10.1152/japplphysiol.01219.2001. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN EJ, JACOB S. Effects of captopril on glucose transport activity in skeletal muscle of obese Zucker rats. Metabolism. 1995;44:267–272. doi: 10.1016/0026-0495(95)90276-7. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN EJ, JACOB S, AUGUSTIN HJ, DIETZE GJ. Glucose transport activity in insulin-resistant rat muscle. Effects of angiotensin-converting enzyme inhibitors and bradykinin antagonism. Diabetes. 1996;45(Suppl 1):S125–128. doi: 10.2337/diab.45.1.s125. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN EJ, JACOB S, FOGT DL, DIETZE GJ. Effect of chronic bradykinin administration on insulin action in an animal model of insulin resistance. Am J Physiol. 1998;275:R40–45. doi: 10.1152/ajpregu.1998.275.1.R40. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN EJ, JACOB S, KINNICK TR, YOUNGBLOOD EB, SCHMIT MB, DIETZE GJ. ACE inhibition and glucose transport in insulinresistant muscle: roles of bradykinin and nitric oxide. Am J Physiol. 1999;277:R332–336. doi: 10.1152/ajpregu.1999.277.1.R332. [DOI] [PubMed] [Google Scholar]
- HIGAKI Y, WOJTASZEWSKI JF, HIRSHMAN MF, WITHERS DJ, TOWERY H, WHITE MF, GOODYEAR LJ. Insulin receptor substrate-2 is not necessary for insulin- and exercise-stimulated glucose transport in skeletal muscle. J Biol Chem. 1999;274:20791–20795. doi: 10.1074/jbc.274.30.20791. [DOI] [PubMed] [Google Scholar]
- HOENE M, LEHMANN R, HENNIGE AM, POHL AK, HARING HU, SCHLEICHER ED, WEIGERT C. Acute regulation of metabolic genes and insulin receptor substrates in the liver of mice by one single bout of treadmill exercise. J Physiol. 2009;587:241–252. doi: 10.1113/jphysiol.2008.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWLETT KF, SAKAMOTO K, HIRSHMAN MF, ASCHENBACH WG, DOW M, WHITE MF, GOODYEAR LJ. Insulin signaling after exercise in insulin receptor substrate-2-deficient mice. Diabetes. 2002;51:479–483. doi: 10.2337/diabetes.51.2.479. [DOI] [PubMed] [Google Scholar]
- ISAMI S, KISHIKAWA H, ARAKI E, UEHARA M, KANEKO K, SHIROTANI T, TODAKA M, URA S, MOTOYOSHI S, MATSUMOTO K, MIYAMURA N, SHICHIRI M. Bradykinin enhances GLUT4 translocation through the increase of insulin receptor tyrosine kinase in primary adipocytes: evidence that bradykinin stimulates the insulin signalling pathway. Diabetologia. 1996;39:412–420. doi: 10.1007/BF00400672. [DOI] [PubMed] [Google Scholar]
- KOHLMAN O, JR., NEVES FDE A, GINOZA M, TAVARES A, CEZARETTI ML, ZANELLA MT, RIBEIRO AB, GAVRAS I, GAVRAS H. Role of bradykinin in insulin sensitivity and blood pressure regulation during hyperinsulinemia. Hypertension. 1995;25:1003–1007. doi: 10.1161/01.hyp.25.5.1003. [DOI] [PubMed] [Google Scholar]
- KUDOH A, DIETZE GJ, RABITO SF. Insulin enhances the bradykinin response in L8 rat skeletal myoblasts. Diabetes. 2000;49:190–194. doi: 10.2337/diabetes.49.2.190. [DOI] [PubMed] [Google Scholar]
- LANGBERG H, BJORN C, BOUSHEL R, HELLSTEN Y, KJAER M. Exercise-induced increase in interstitial bradykinin and adenosine concentrations in skeletal muscle and peritendinous tissue in humans. J Physiol. 2002;542:977–983. doi: 10.1113/jphysiol.2002.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYATA T, TAGUCHI T, UEHARA M, ISAMI S, KISHIKAWA H, KANEKO K, ARAKI E, SHICHIRI M. Bradykinin potentiates insulin-stimulated glucose uptake and enhances insulin signal through the bradykinin B2 receptor in dog skeletal muscle and rat L6 myoblasts. Eur J Endocrinol. 1998;138:344–352. doi: 10.1530/eje.0.1380344. [DOI] [PubMed] [Google Scholar]
- RABITO SF, MINSHALL RD, NAKAMURA F, WANG LX. Bradykinin B2 receptors on skeletal muscle are coupled to inositol 1,4,5-trisphosphate formation. Diabetes. 1996;45(Suppl 1):S29–33. doi: 10.2337/diab.45.1.s29. [DOI] [PubMed] [Google Scholar]
- RICHTER EA, GARETTO LP, GOODMAN MN, RUDERMAN NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982;69:785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER EA, MIKINES KJ, GALBO H, KIENS B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol. 1989;66:876–885. doi: 10.1152/jappl.1989.66.2.876. [DOI] [PubMed] [Google Scholar]
- SCHANSTRA JP, DUCHENE J, PRADDAUDE F, BRUNEVAL P, TACK I, CHEVALIER J, GIROLAMI J-P, BASCANDS J-L. Regulation of Cardiovascular Signaling by Kinins and Products of Similar Converting Enzyme Systems: Decreased renal NO excretion and reduced glomerular tuft area in mice lacking the bradykinin B2 receptor. Am J Physiol Heart Circ Physiol. 2003;284:H1904–1908. doi: 10.1152/ajpheart.01150.2002. %R 10.1152/ajpheart.01150.2002. [DOI] [PubMed] [Google Scholar]
- STEBBINS CL, CARRETERO OA, MINDROIU T, LONGHURST JC. Bradykinin release from contracting skeletal muscle of the cat. J Appl Physiol. 1990;69:1225–1230. doi: 10.1152/jappl.1990.69.4.1225. [DOI] [PubMed] [Google Scholar]
- TAGUCHI T, KISHIKAWA H, MOTOSHIMA H, SAKAI K, NISHIYAMA T, YOSHIZATO K, SHIRAKAMI A, TOYONAGA T, SHIRONTANI T, ARAKI E, SHICHIRI M. Involvement of bradykinin in acute exercise-induced increase of glucose uptake and GLUT-4 translocation in skeletal muscle: studies in normal and diabetic humans and rats. Metabolism. 2000;49:920–930. doi: 10.1053/meta.2000.6755. [DOI] [PubMed] [Google Scholar]
- UEHARA M, KISHIKAWA H, ISAMI S, KISANUKI K, OHKUBO Y, MIYAMURA N, MIYATA T, YANO T, SHICHIRI M. Effect on insulin sensitivity of angiotensin converting enzyme inhibitors with or without a sulphydryl group: bradykinin may improve insulin resistance in dogs and humans. Diabetologia. 1994;37:300–307. doi: 10.1007/BF00398058. [DOI] [PubMed] [Google Scholar]
- WOJTASZEWSKI JF, HANSEN BF, GADE, KIENS B, MARKUNS JF, GOODYEAR LJ, RICHTER EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- WOJTASZEWSKI JF, HIGAKI Y, HIRSHMAN MF, MICHAEL MD, DUFRESNE SD, KAHN CR, GOODYEAR LJ. Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice. J Clin Invest. 1999;104:1257–1264. doi: 10.1172/JCI7961. [DOI] [PMC free article] [PubMed] [Google Scholar]