Abstract
Antitumor agents that bind to tubulin and disrupt microtubule dynamics have attracted considerable attention in the last few years. To extend our knowledge of the thiazole ring as a suitable mimic for the cis-olefin present in combretastatin A-4, we fixed the 3,4,5-trimethoxyphenyl at the C4-position of the thiazole core. We found that the substituents at the C2- and C5-positions had a profound effect on antiproliferative activity. Comparing compounds with the same substituents at the C5-position of the thiazole ring, the moiety at the C2-position influenced antiproliferative activities, with the order of potency being NHCH3> Me ≫ N(CH3)2. The N-methylamino substituent significantly improved antiproliferative activity on MCF-7 cells with respect to C2-amino counterparts. Increasing steric bulk at the C2-position from N-methylamino to N,N-dimethylamino caused a 1–2 log decrease in activity. The 2-N-methylamino thiazole derivatives 3b, 3d and 3e were the most active compounds as antiproliferative agents, with IC50 values from low micromolar to single digit nanomolar, and, in addition, they are also active on multidrug-resistant cell lines over-expressing P-glycoprotein. Antiproliferative activity was probably caused by the compounds binding to the colchicines site of tubulin polymerization and disrupting microtubule dynamics. Moreover, the most active compound 3e induced apoptosis through the activation of caspase-2, -3 and -8, but 3e did not cause mitochondrial depolarization.
Keywords: Tubulin, Thiazole, Combretastatin-A4, Colchicine site, Apoptosis
1. Introduction
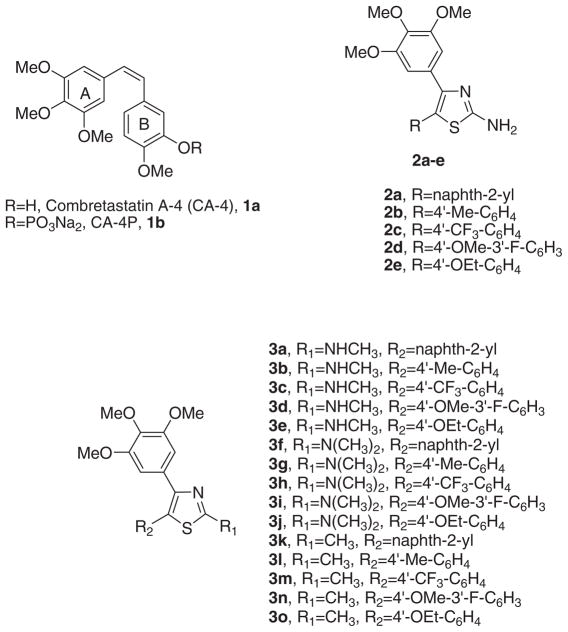
Microtubules are key components of the cytoskeleton and are involved in a wide range of critical cellular functions, such as cell division, where they are responsible for mitotic spindle formation and proper chromosomal separation.1 Antimitotic agents are one of the main class of cytotoxic drugs for cancer treatment, and tubulin represents a known target for numerous small natural and synthetic molecules that inhibit the formation of the mitotic spindle. 2,3 Combretastatin A-4 (CA-4, 1a; Chart 1) isolated from the bark of the South African tree Combretum caffrum,4 is one of the well-known natural molecules that inhibits the spindle formation through its interaction with tubulin at the colchicine site.5 CA-4 inhibits cell growth at nanomolar concentrations, exhibiting inhibitory effects even on multidrug resistant cancer cell lines.6 The disodium phosphate prodrug of CA-4, named CA-4P (1b),7 has been prepared as a water-soluble derivative, and there have been promising results with 1b as a tumor vascular disrupting agent in phase II clinical trials.8
Chart 1.
Lead structures of tubulin polymerization inhibitors
Structure–activity relationship (SAR) studies of CA-4 have underlined that the presence of the 3,4,5-trimethoxy substituted A-ring and the 4-methoxy substituted B-ring separated by a double- bond with cis-configuration are fundamental for optimal antiproliferative activity.9 It has also been reported that 3-hydroxy group on the B-ring is not necessary for potent activity.10 The activity of CA-4 is hampered by isomerization of the active cis-stibene configuration into the corresponding inactive trans analogue. Replacement of the olefinic bond with a five-membered heterocyclic ring permitted retention of the correct geometric orientation of the two phenyl rings of CA-4, placing them at an appropriate distance for efficient interaction with the colchicines site of tubulin.11
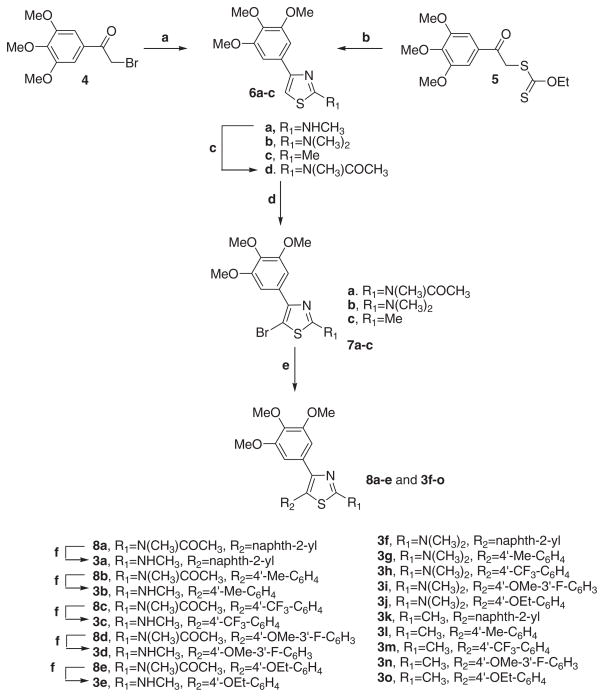
In our previous study, we reported the biological evaluation of a series of 2-amino-4,5-diarylthiazoles with general structure 2, with the 2-aminothiazole moiety retaining the cis-olefin configuration of CA-4, with the 3′,4′,5′-trimethoxyphenyl ring of CA-4 at the C4-position of the thiazole ring.12 On the basis of in vitro antiproliferative activity data, the best substituents for the C5-position of the thiazole ring, corresponding to the B-ring of CA-4, were a naphth-2-yl (2a) or a phenyl substituted at its para-position with a Me (2b), CF3 (2c) MeO (2d) and EtO (2e) moiety.
Taking into consideration these results, in the present study we have synthesized a new series of analogues with general structure 3, all of which retain at the C4- and C5-positions of the thiazole ring the same substituents as derivatives 2a–e, examining the effect on biological activity derived by replacement of the amino group at the C2-position of the thiazole nucleus with different moieties, such as N-methylamine (3a–e), N,N-dimethylamine (3f–j) and methyl (3k–o).
2. Chemistry
2-Substituted-4-(3′,4′,5′-trimethoxyphenyl)-5-aryl thiazole derivatives with general structure 3 were prepared following the reaction sequence shown in Scheme 1. 4-Substituted thiazoles 6a and 6c were formed by condensation of 2-bromo- 1-(3′,4′,5′-trimethoxyphenyl)ethanone 4 with N-methylthiourea or thioacetamide, respectively, in refluxing ethanol. The former compound 6a was transformed into the corresponding N-acetyl analogue 6d by treatment with a refluxing mixture of acetic anhydride and sodium acetate. The 2-N,N-dimethylamino thiazole 6b were prepared by ‘one-pot’ cyclization in refluxing ethanol of N,N-dimethylcyanamide with the anion of α-mercapto 3,4,5-trimethoxyacetophenone, generated in situ by treating O-ethyl-S-[2-oxo-2-(3,4,5-trimethoxyphenyl)-ethyl] dithiocarbonate 5 with piperidine.13 Position 5 of C2-substituted thiazoles 6b–d was then brominated using N-bromosuccinimide in CHCl3, to yield the 5-bromothiazole derivatives 7a–c. These molecules were subjected to Suzuki–Miyaura coupling conditions in the presence of various commercially available arylboronic acids, giving rise to the corresponding 5-aryl thiazole derivatives 3f–o and 8a–e. These latter compounds were transformed by ethanolysis into the final products 3a–e.
Scheme 1.
Reagents and conditions: (a) N-Methylthiourea or thioacetamide for 6a and 6c, respectively, EtOH, reflux; (b) (CH3)2NCN, piperidine, EtOH, reflux; (c) AcONa, Ac2O, reflux; (d) NBS, CHCl3, rt for 6b–d; (e) PdCl2(DPPF), ArB(OH)2, CsF, 1,4-dioxane and 65 °C for 7a and 7c or toluene and 75 °C for 7b; (f) 1 N NaOH, EtOH, reflux.
3. Biological results and discussion
3.1. In vitro antiproliferative activities
The series of 2-substituted-4-(3′,4′,5′-trimethoxyphenyl)-5-arylthiazoles 3a–o were evaluated for their inhibition of the growth of a panel of six different human cancer cell lines in comparison with the 2-aminothiazole analogues 2a–e and the reference compound CA-4 (1a) as positive controls. From the antiproliferative data reported in Table 1, the potency of the new synthesized molecules 3a–o was found to be highly dependent on the substituent at the 2-position of thiazole ring, and, in addition, some interesting trends were noted. The substituent at the 2-position of the thiazole ring clearly affected inhibitory effects on cell growth, with decreasing activity generally observed as follows: NH2 > NHCH3 > CH3 ≫ N(CH3)2.
Table 1.
In vitro cell growth inhibitory effects of compounds 2a–e, 3a–o and CA-4 (1)
| Compd | IC50a(nM)
|
|||||
|---|---|---|---|---|---|---|
| HeLa | A549 | HL-60 | Jurkat | MCF-7 | HT-29 | |
| 2ab | 1.6 ± 0.6 | 3.8 ± 0.6 | 2.7 ± 0.3 | 3.3 ± 0.9 | 51.2 ± 9.2 | 23.9 ± 3.9 |
| 2bb | 0.73 ± 0.2 | 42.8 ± 5.7 | 3.9 ± 1.1 | 3.9 ± 0.6 | 279.5 ± 69.8 | 12.4 ± 5.1 |
| 2cb | 3.2 ± 0.4 | 357 ± 65 | 5.1 ± 1.1 | 8.5 ± 1.2 | 0.4 ± 0.1 | 221 ± 50.2 |
| 2db | 2.3 ± 0.2 | 20.2 ± 1.7 | 3.2 ± 1.2 | 2.1 ± 0.4 | 60.9 ± 8.4 | 11.7 ± 3.9 |
| 2eb | 0.03 ± 0.005 | 0.09 ± 0.01 | 0.9 ± 0.3 | 0.14 ± 0.05 | 44.0 ± 6.3 | 38.2 ± 11.4 |
| 3a | 128 ± 49 | 151 ± 36 | 143 ± 67 | 51.1 ± 13 | 21.1 ± 4.0 | 38.2 ± 6.1 |
| 3b | 69.1 ± 13 | 125 ± 22 | 18.1 ± 5.2 | 19.0 ± 8.2 | 10.1 ± 4.1 | 423 ± 107 |
| 3c | 3401 ± 292 | 4565 ± 536 | 3166 ± 188 | 3014 ± 769 | 7285 ± 851 | 1235 ± 149 |
| 3d | 70.1 ± 12.3 | 138 ± 26 | 27.3 ± 7.2 | 34.3 ± 7.2 | 37.6 ± 14.1 | 6.3 ± 1.0 |
| 3e | 13.2 ± 6.3 | 38.6 ± 7.3 | 1.8 ± 0.8 | 1.7 ± 0.8 | 24.5 ± 14.2 | 3.2 ± 0.4 |
| 3f | 1404 ± 222 | 2900 ± 320 | 2568 ± 834 | 836 ± 135 | 2385 ± 574 | 729 ± 108 |
| 3g | 4141 ± 165 | 3960 ± 465 | 3479 ± 359 | 6656 ± 1762 | >10,000 | 3916 ± 1470 |
| 3h | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 |
| 3i | 3371 ± 366 | >10,000 | 1893 ± 424 | 7599 ± 1262 | 8362 ± 1291 | 497 ± 72 |
| 3j | 3264 ± 937 | >10,000 | 303 ± 26 | 4150 ± 1242 | >10,000 | 6027 ± 190 |
| 3k | 82.1 ± 4.0 | 119 ± 22 | 205 ± 37 | 48.6 ± 11.1 | 33.4 ± 8.2 | 702 ± 142 |
| 3l | 1100 ± 58 | 1950 ± 156 | 1512 ± 45 | 517 ± 192 | 1743 ± 374 | 722 ± 235 |
| 3m | 3826 ± 311 | 4287 ± 258 | >10,000 | 7109 ± 1677 | >10,000 | 396 ± 164 |
| 3n | 500 ± 25.2 | 2563 ± 421 | 3427 ± 80 | 1755 ± 838 | 606 ± 12 | 1417 ± 250 |
| 3o | 1866 ± 145 | 3321 ± 352 | 253 ± 19 | 1744 ± 656 | 42.3 ± 8.2 | 487 ± 67 |
| CA-4 | 4 ± 1 | 180 ± 50 | 1 ± 0.2 | 5 ± 0.6 | 370 ± 100 | 3100 ± 100 |
IC50 = compound concentration required to inhibit tumor cell proliferation by 50%. Data are expressed as the mean ± SE from the dose–response curves of at least three independent experiments.
Data taken from Ref. 12.
With the exception of 4′-trifluoromethylphenyl derivative 3c, all N-methylamino derivatives 3a–e had significant antiproliferative activity against all tested human cancer cell lines, some of them even had IC50’s in the low to mid nanomolar range. Compound 3e, bearing 4′-ethoxyphenyl and N-methylamino moieties at the 2- and 5-positions, respectively, of thiazole ring displayed the greatest antiproliferative activity among the new synthesized compounds 3a–o, with IC50 values ranging from 1.7 to 38 nM against the six cell lines. Derivative 3e was 5-, 15- and 1000-fold more potent than CA-4 against A549, MCF-7 and HT-29 cells, but it was 2- to 4-fold less potent than CA-4 against the other three lines. However, compound 3e was one to two orders of magnitude less active than its 2-aminothiazole counterpart 2e in four of the six cancer cell lines, the exceptions being the MCF-7 and HT-29 cells, in which 3e was 2- and 10-fold more potent than 2e, respectively. In human colon adenocarcinoma HT-29 cells, compound 3e was 4- to 70-fold more potent than derivatives 2a–e.
Comparing compounds with the same substituent at the C5-position of the thiazole ring, replacement of the 2-amino group of thiazole derivatives 2a–e with a N-methylamino group (compounds 3a–e) decreased antiproliferative activity against four of the six cell lines, while with MCF-7 and HT-29 cells there were variable effects. With the exception of 4′-trifluoromethylphenyl derivative 3c, the MCF-7 breast cancer cell line was more sensitive toward N-methylamino analogues 3a–b and 3d–e than the corresponding amino counterparts 2a–b and 2d–e, with 3b being the most potent of all the newly synthesized derivatives 3a–o in this cell line. Moreover, 3b was almost 28-fold more potent than 2b against the MCF-7 cells. In HT-29 cells, the 4′-methoxyphenyl (3d) and 4′-ethoxyphenyl (3e) derivatives were 2- and 10-fold more potent than their 2-amino counterparts 2d and 2e.
Comparing compounds 3k–o, which shared a common methyl moiety at the thiazole C2-position, the napht-2-yl derivative 3k had the highest antiproliferative activities in five of the six human cancer cell lines, the exception being the HT-29 cells, in which 3k was less active than 3m and 3o. All the N,N-dimethylamino derivatives 3f–j showed lower antiproliferative activities than did their N-methylamino analogues 3a–e, and 3f–j had relatively little activity against all the cell lines examined.
3.2. Effect of compounds 3e and 3k on normal human cells
To obtain more insights into the cytotoxic potential of test compounds for normal human cells, the most active compounds were assayed in vitro against peripheral blood lymphocytes (PBL) from healthy donors as previously reported.14 Compounds 3e and 3k, proved moderately cytotoxic having a GI50 of 443 ± 29 and 1272 ± 307 nM in resting PBL, respectively, whereas in PHA-stimulated PBL we found a GI50 of 120 ± 24 (3e) and 285 ± 57 nM (3k) suggesting that this derivatives acts preferentially on proliferating cells. Nevertheless these results pointed out also that compounds 3e and 3k are less cytotoxic respect to the lymphoblastic cell line Jurkat (see Table 1).
3.3. Effect of compounds 3k, 3d and 3e on multidrug resistant cells
To investigate whether these derivatives are substrates of drug efflux pumps, some of the most active compounds (3d–e, 3k) were tested against a panel of drug resistant cell lines that either overexpress P-glycoprotein (LovoDoxo and CemVbl100)15,16 or are associated with tubulin gene mutations (A549-T12)17 that result in modified tubulin with impaired polymerization properties. As shown in Table 2, the tested compounds exhibited cytotoxic activity in all three of the drug resistant cell lines. The activity in the two P-glycoprotein overexpressing cell lines demonstrated that these derivatives are not substrates for this important drug pump.
Table 2.
In vitro cell growth inhibitory effects of compound 3d–e, 3k on drug resistant cell lines
| Compd | IC50
a (nM)
|
Resistance ratiob | |
|---|---|---|---|
| LoVo | LoVoDoxo | ||
| 3d | 190 ± 70 | 152 ± 14 | 0.8 |
| 3e | 31 ± 4.5 | 14.5 ± 1.2 | 0.5 |
| 3k | 72 ± 16 | 150 ± 25 | 2.1 |
| Doxorubicinc | 120 ± 30 | 13150 ± 210 | 109.6 |
| CEM | CEMVbl100 | Resistance ratiob | |
| 3d | 10 ± 3.5 | 15 ± 4.6 | 1.5 |
| 3e | 0.8 ± 0.1 | 1.2 ± 0.2 | 2.9 |
| 3k | 61 ± 18 | 88 ± 12 | 1.4 |
| Vinblastine | 0.8 ± 0.1 | 205 ± 46 | 256.2 |
| A549 | A549-T12 | Resistance ratiob | |
| 3d | 138 ± 26 | 125 ± 23 | 0.9 |
| 3e | 38.6 ± 7.3 | 21.5 ± 9.0 | 0.5 |
| 3k | 119 ± 22 | 121 ± 36 | 1.0 |
| Taxolc | 7.2 ± 0.1 | 75.2 ± 12.5 | 10.4 |
IC50 = compound concentration required to inhibit tumor cell proliferation by 50%. Data are expressed as the mean ± SE from the dose–response curves of at least three independent experiments.
The values express the ratio between the IC50’s determined in resistant and non-resistant cell lines.
Data from Ref. 8.
3.4. Inhibition of tubulin polymerization and colchicine binding
To investigate whether the antiproliferative activities of compounds 3a–b, 3d–k and 3o derived from an interaction with tubulin, these agents were evaluated for their inhibition of tubulin polymerization and for effects on the binding of [3H]colchicine to tubulin.18,19 For comparison, 2-amino thiazoles 2a–b and 2d–e, as well as CA-4, were examined in contemporaneous experiments. Data for inactive compounds in the assembly assay (IC50 >20 μM) are not shown in Table 3. All the N,N-dimethylamino derivatives 3f–j did not inhibit tubulin assembly at a concentration as high as 20 μM. Compound 3e was found to be the most active derivative in the in vitro tubulin polymerization assay (IC50, 0.89 μM). This is in agreement with 3e being the compound with the greatest antiproliferative activity. Compounds 3a–b, 3d, 3k and 3o had IC50 values of 0.96–1.3 μM, essentially equivalent to that of CA-4 (IC50, 1.2 μM), although 3o was less effective as an inhibitor of cell growth than CA-4.
Table 3.
Inhibition of tubulin polymerization and colchicine binding by compounds 2a–b, 2d–e, 3a–b, 3d–e, 3k, 3o and CA-4
| Compd | Tubulin assemblya IC50 (μM) | Colchicine bindingb (%) |
|---|---|---|
| 2a | 0.74 ± 0.01 | 94 ± 0.49 |
| 2b | 0.44 ± 0.01 | 88 ± 0.71 |
| 2d | 1.1 ± 0.07 | 79 ± 0.21 |
| 2e | 0.61 ± 0.05 | 95 ± 0.71 |
| 3a | 0.96 ± 0.01 | 78 ± 2.1 |
| 3b | 1.2 ± 0.07 | 68 ± 1.4 |
| 3d | 1.3 ± 0.07 | 61 ± 0.28 |
| 3e | 0.89 ± 0.06 | 83 ± 0.28 |
| 3k | 1.1 ± 0.07 | 66 ± 0.21 |
| 3o | 1.2 ± 0.07 | 51 ± 2.8 |
| CA-4 (1) | 1.2 ± 0.07 | 98 ± 0.42 |
Inhibition of tubulin polymerization. Tubulin was at 10 μM.
Inhibition of [3H]colchicine binding. Tubulin, colchicine and tested compound were at 1, 5 and 5 μM, respectively. Data are expressed as the mean ± SE of two independent experiments.
When comparing inhibition of tubulin polymerization with antiproliferative effect, we found a good correlation for most of the active compounds, but not for all of them. Thus, compounds 3b, 3d and 3o were similar as inhibitors of tubulin assembly, but 3b and 3d were much more active than 3o as antiproliferative agents.
The free amino group on compounds 2a and 2e is not critical for inhibition of tubulin assembly. Substituting the amino group with a methyl, as in compounds 3k and 3o, results in retention of activity.
In the colchicine binding studies, derivative 3e was almost (83% inhibition) as potent as CA-4, which in these experiments inhibited colchicine binding by 98%. Inhibition of colchicine binding by compounds 3b, 3d, 3k and 3o was lower, varying within the 51–68% range, and they were also less potent than 3a, which inhibited colchicine binding by 78%. Although, many agents in the present series have activities comparable (3b, 3d, 3k and 3o) or superior (3a and 3e) to that of CA-4 as inhibitors of tubulin assembly, none was as active as CA-4 as an inhibitor of colchicine binding to tubulin.
The results are consistent with the conclusion that inhibition of cell growth of these new compounds derives from an interaction with the colchicine site of tubulin and interference with microtubule assembly.
Comparing the antiproliferative activity of 2-N-methylaminothiazole derivatives 3d–e with those of 2-amino counterparts 2d–e, the data indicated that a free amino group at the thiazole C2-position was an essential requisite for potent activity. In the inhibition of tubulin assembly, compounds 2d and 3d, as well as 2e and 3e, exhibited similar antitubulin activity, although both 2-amino derivatives had somewhat greater activity than their methylated analogues. The discrepancy between antiproliferative activity and antitubulin potency has been reported previously. The lower antiproliferative activities of 3d–e as compared with 2d–e may result from poor permeability into cells, poor solubility in the tissue culture medium or any other mechanism limiting the accessibility of molecules 3d–e to cellular tubulin. Nevertheless, we cannot exclude the possibility that 2d–e may affect other molecular targets in addition to microtubules and thus cause their enhanced antiproliferative activity.
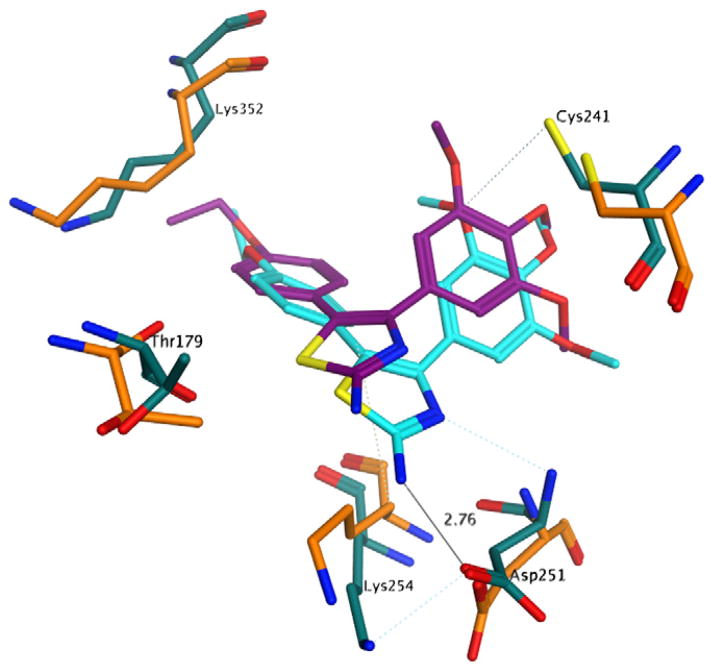
3.5. Molecular modeling
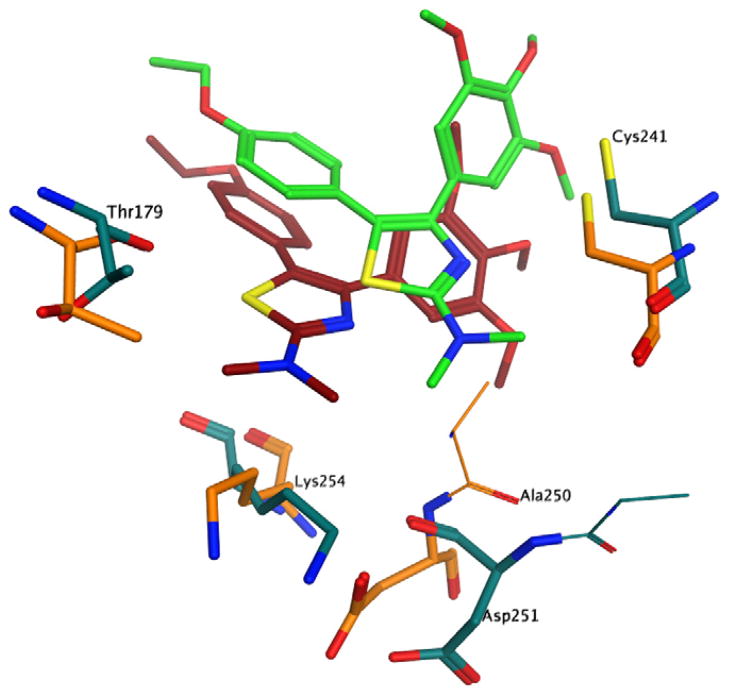
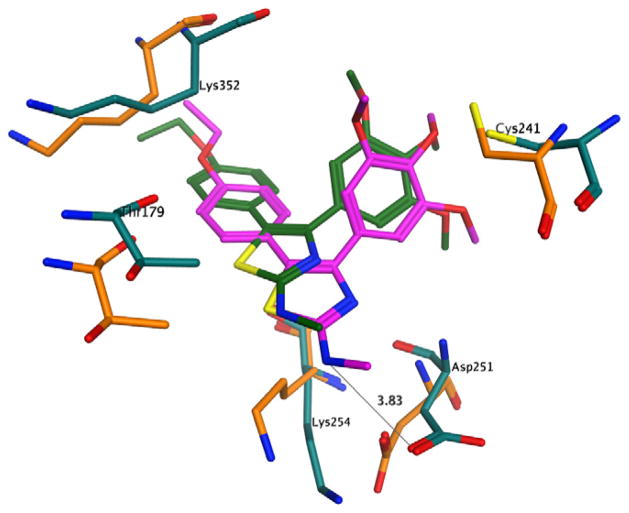
In an attempt to rationalize the biological results observed, a series of molecular docking simulations were carried out following the procedure used previously.12 Indeed, the binding mode observed for the compounds reported here is very similar to that observed for 2a–e, with the trimethoxyphenyl ring in close contact with Cys241 and the second aromatic moiety deep in the binding pocket (Fig. 1). Interestingly, the N,N-dimethylamino analogues 3f–j also presented the same binding mode, providing little structural rationale for the loss of biological activity observed with these compounds. More closely examining the docking results (Fig. 2), however, we observed a minor steric clash between the dimethylamino group and tubulin residue Thr179. To assess the significance of this observation, we performed a series of short molecular dynamics (MD) simulations on compounds 2e, 3e and 3j in complex with tubulin. The results obtained clearly show that the presence of the two methyl groups induce a significant conformational change of the binding site residues in close contact with the N,N-dimethylamino moiety (Fig. 2). Furthermore, there is a significant shift of the compound itself within the pocket, suggesting an overall binding instability for this compound.
Figure 1.
Proposed binding pose of 2e to tubulin. Before the molecular dynamics: carbon atoms of 2e in purple, carbon atoms of tubulin residues in orange; after the molecular dynamics: carbon atoms of 2e in cyan, carbon atoms of tubulin residues in turquoise. Other atoms indicated as follows: red, oxygen; blue, nitrogen; yellow, sulphur; Hydrogen atoms are not shown.
Figure 2.
Proposed binding pose of 3j to tubulin. Before the molecular dynamics: carbon atoms of 3j in brown, carbon atoms of tubulin residues in orange; after the molecular dynamics: carbon atoms of 3j in green, carbon atoms of tubulin residues in turquoise. Other atoms indicated as follows: red, oxygen; blue, nitrogen; yellow, sulphur; Hydrogen atoms are not shown.
Moreover, in the case of compound 2e, the binding complex remained stable during the simulation time, and 2e also established two hydrogen bonds between the aminothiazole ring and Asp251 (Fig. 1). The simulation on compound 3e presented a very similar profile to the one observed for 2e (Fig. 3), confirming the overall binding stability of this compound and providing a possible justification for the different inhibition of tubulin polymerization profiles of 2e, 3e and 3j.
Figure 3.
Proposed binding pose of 3e to tubulin. Before the molecular dynamics: carbon atoms of 3e in dark green, carbon atoms of tubulin residues in orange; after the molecular dynamics: carbon atoms of 3e in purple, carbon atoms of tubulin residues in turquoise. Other atoms indicated as follows: red, oxygen; blue, nitrogen; yellow, sulphur; Hydrogen atoms are not shown.
3.6. Analysis of cell cycle effects
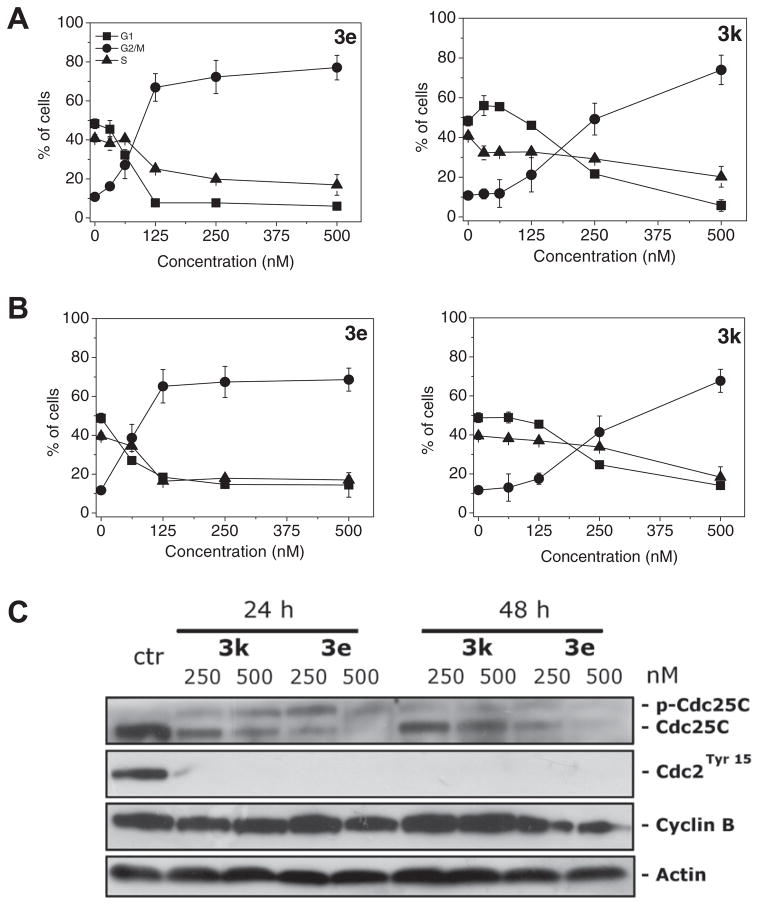
The effect of 3e and 3k on cell cycle progression was examined by flow cytometry in Hela and Jurkat cells. 3e treatment resulted in the rapid accumulation of cells in the G2/M phase, with a concomitant reduction in cells in both the S and G1 phases, in both cell lines. These changes occurred in a concentration-dependent manner (Fig. 4, panels A and B), but changes were observed even at the lowest concentrations (31 nM in HeLa cells, 62 nM in Jurkat cells) used. In contrast, compound 3k induced an increase in G2/M arrested cells only at higher concentrations (a small rise was first observed with 125 nM compound in both cell lines).
Figure 4.
Effect of compounds 3e and 3k cell on cycle distribution of HeLa (panel A) and Jurkat cells (panel B). Cells were treated with different compound concentrations ranging from 31 to 500 nM for 24 h. Then the cells were fixed and stained with PI to analyze DNA content by flow cytometry. Data are presented as mean ± SEM of three independent experiments. Panel C: Effect of 3e and 3k on G2/M regulatory proteins. HeLa cells were treated for 24 or 48 h with the indicated concentration of the compound. The cells were harvested and lysed for the detection of cyclin B, p-cdc2Y15 and cdc25c expression by western blot analysis. To confirm equal protein loading, each membrane was stripped and reprobed with anti-β-actin antibody.
Next, we investigated the association between 3e and 3k-induced G2/M arrest and alterations in G2/M regulatory protein expression in HeLa cells. As shown in Figure 4 (panel C), both compounds caused phosphorylation of the phosphatase cdc25c.
The phosphorylation of cdc25c directly stimulates its phosphatase activity, and this is necessary to activate cdc2/cyclin B on entry into mitosis.20 In good agreement, we also observed at both 24 and 48 h of incubation, a dephosphorylation at Tyr15 of cdc2 kinase. The expression of cyclin B remained practically constant after 24 h of treatment with both compounds, followed at 48 h with compound 3e by a slight decrease in cyclin B expression.
3.7. Compounds 3e and 3k induced apoptosis
To evaluate the mode of cell death induced by compounds 3e and 3k, we performed a biparametric cytofluorimetric analysis using propidium iodide (PI) and annexin-V-FITC, which stain DNA and phosphatidylserine (PS) residues, respectively.
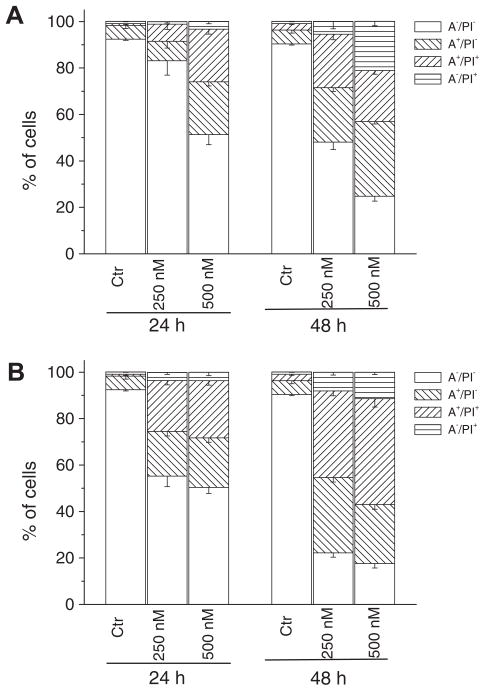
After treatment with the two compounds at either 250 or 500 nM for 24 or 48 h, HeLa cells were labeled with the two dyes, and the resulting red (PI) and green (FITC) fluorescence was monitored by flow cytometry. As shown in Figure 5 (panel A), 3k caused a significant induction of apoptotic cells (A+/PI− and A+/PI−) after 24 h, especially with the compound at 500 nM. The percentage of annexin-V positive cells then further increased at 48 h. Analogous behaviour but with a stronger effect was observed for 3e (Fig. 5, panel B). These data were supported by findings using the MTT assay. These findings prompted us to further investigate the apoptotic process after treatment of cells with 3e and 3k.
Figure 5.
Flow cytometric analysis of apoptotic cells after treatment of HeLa cells with 3k (panel A) and 3e (panel B) at the indicated concentrations after incubation for 24 or 48 h. The cells were harvested and labeled with annexin-V-FITC and PI and analyzed by flow cytometry. Data are represented as mean ± SEM of three independent experiments.
3.8. Effect of 3e and 3k on caspases activation
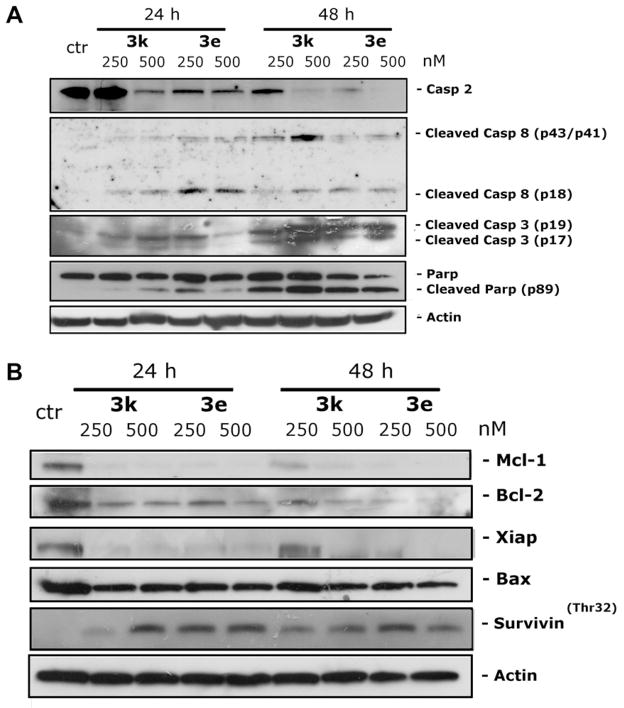
The activation of caspases plays a central role in apoptotic cell death. To determine which caspases were involved in cell death induced by compounds 3e and 3k, the expression of different caspases was measured by immunoblot analysis. We observed a clear activation of caspase-3, as well as cleavage of the caspase-3 substrate PARP, after 24 and 48 h exposures to the two compounds (Fig. 6, panel A). Of note, treatment with 3e and 3k did not induce activation of caspase-9, (data not shown), one of the major initiator caspases in the intrinsic (mitochondrial) apoptosis pathway. In particular, mitochondrial functions such as mitochondrial polarization and ROS production were only slightly affected by treatment with the two compounds (data not shown). Interestingly, we observed a clear caspase-8 and caspase-2 activation following treatment with compounds 3e and 3k (Fig. 6, panel A). The activation of caspase-2 is indicated by disappearance of the uncleaved protein in the western blot, while the activation of caspase-8 is indicated by appearance of the cleavage product. In this context, caspase-2 is a unique caspase with characteristics of both initiator and effector caspases.21 Recently, its key role in several apoptosis signaling cascades has emerged. In particular, caspase-2 has been implicated in the cell death induced by different antimitotic agents.22,23
Figure 6.
Effect of 3e and 3k on the expression of caspases (panel A) and Bcl-2 family members and IAP proteins (panel B). HeLa cells were treated with the indicated concentrations of compounds for 24 or 48 h. Then the cells were harvested and lysed for the detection of protein expression with specific antibodies by western blot analysis.
3.9. Effect of 3e and 3k on BH3 protein and IAP expression
There is increasing evidence that regulation of the Bcl-2 family of proteins shares the signaling pathways induced by antimicrotubule compounds.20a,24 Several pro-apoptotic family proteins (e.g., Bax, Bid, Bim and Bak) promote the release of cytochrome c, whereas anti-apoptotic members (Bcl-2, Mcl-1) are capable of antagonizing the pro-apoptotic proteins and preventing the loss of mitochondrial membrane potential. Our results showed that Bcl-2 expression is strongly reduced after treatment with the two compounds (Fig. 6, panel B), while, in contrast, expression of Bax, a proapoptotic protein of the Bcl-2 family, was unchanged. Mcl-1 is an anti-apoptotic member of the Bcl-2 family, and recently it has been reported that sensitivity to antimitotic drugs is regulated by Mcl-1 levels.25,26 As shown in Figure 6 (panel B) we observed disappearance of the Mcl-1 band after 24 h treatments with both compounds.
Xiap and survivin are members of IAP family (Inhibitors of apoptosis protein), and, in general the IAP proteins function through direct interactions to inhibit the activity of several caspases, including caspase-3, caspase-7 and caspase-9, thereby inhibiting the processing and activation of the caspases.27 Our results ( Fig. 6) showed that expression of Xiap was almost eliminated after a 24 h treatment with either compound. Of note, survivin was phosphorylated on Thr32 upon treatment with the two compounds at both 24 and 48 h. This effect is consistent with cell cycle arrest in mitosis.28 In addition, since survivin phosphorylation on Thr32 may regulate apoptosis at cell division via an interaction with caspase-9,29 our results suggest that activation of caspase-2 may be a compensatory mechanism that leads to cell death.
4. Conclusions
In this manuscript we have reported a series of thiazole derivatives modified at their C2-position, which shared with previously published compounds 2a–e, the 3′,4′,5′-trimethoxyphenyl and aryl substituents at their C4- and C5-positions, respectively. The results showed that small changes at the 2-position of thiazole ring had a major impact on antiproliferative activity, with the new synthesized compounds 3a–o generally less active than the corresponding 2-aminothiazole analogues 2a–e. However, the most potent compound of this series, derivative 3e, displayed similar or more potent antiproliferative activity than CA-4, with IC50 values ranging from 1.7 to 38 nM against the tested cancer cell lines. Compound 3e was also the most active inhibitor of tubulin polymerization (IC50 = 0.89 μM) and strongly inhibited the binding of [3H]colchicine to tubulin (83% inhibition), with activities not greatly different from those of CA-4. For this latter derivative, a good correlation was observed between antiproliferative activity, inhibition of tubulin polymerization and inhibition of colchicine binding. Comparing compounds characterized by the same substituent at the C5-position of the thiazole ring, with the exception of MCF-7 and HT-29 cells, replacement of the 2-amino group with a N-methylamino, N,N-dimethylamino and methyl moieties produced a decrease of activity, with the potency decreasing in the following order: NH2 > NHCH3 > CH3 ≫ N(CH3)2. With the exception of 4′-trifluorophenyl derivative 2c, for derivatives 2a–b and 2d–e replacement of the amino group with a N-methylamino at the 2-position of the thiazole skeleton (compounds 3a–e) led to an increase in activity against MCF-7 cells. This effect was more evident for the 4′-tolyl analogue 3b, which was 4- to 30-fold more potent than derivatives 2a–b and 2d–e. The reduced antiproliferative activities of compounds 3f–j indicated that the presence of an additional methyl group on the N-methylamino moiety of analogues 3a–e was detrimental to activity. The 2-N-methylamino thiazole derivatives 3d–e retain the tubulin affinity of the parent 2-amino compounds 2d–e and CA-4. The greater antiproliferative activity of 2d–e as compared with 3d–e despite similar antitubulin effects suggests either differences in intracellular transport or, possibly, an additional cellular target for the former compounds.
The most active compounds strongly induced apoptosis, and this effect was followed by caspase activation. An interesting finding of this study was that, at least in HeLa cells, we did not observe a mitochondrial apoptotic pathway but instead the activation of caspase-8 and caspase-2 following treatment of cells with the most active compounds. In addition, the strong decrease of the antiapoptotic proteins of Bcl-2 and Mcl-1 along with reduction of Xiap enhanced the apoptotic pathway induced by this class of compounds.
5. Experimental section
5.1. Chemistry
5.1.1. Materials and methods
1H NMR spectra were recorded on a Varian VXR 200 spectrometer. Chemical shifts (δ) are given in ppm upfield, and the spectra were recorded in appropriate deuterated solvents, as indicated. Positive-ion electrospray ionization (ESI) mass spectra were recorded on a double-focusing Finnigan MAT 95 instrument with BE geometry. Melting points (mp) were determined on a Buchi– Tottoli apparatus and are uncorrected. All products reported showed 1H NMR spectra in agreement with the assigned structures. The purity of tested compounds was determined by combustion elemental analyses conducted by the Microanalytical Laboratory of the Chemistry Department of the University of Ferrara with a Yanagimoto MT-5 CHN recorder elemental analyzer. All tested compounds yielded data consistent with a purity of at least 95% as compared with the theoretical values. All reactions were carried out under an inert atmosphere of dry nitrogen, unless otherwise indicated. Standard syringe techniques were used for transferring dry solvents. Reaction courses and product mixtures were routinely monitored by TLC on silica gel (precoated F254 Merck plates), and compounds were visualized with aqueous KMnO4. Flash chromatography was performed using 230–400 mesh silica gel and the indicated solvent system. Organic solutions were dried over anhydrous Na2SO4. Arylboronic acids are commercially available and used as received. All chemicals and reagents were purchased from Aldrich (Sigma–Aldrich) or Lancaster (Alfa Aesar, Johnson Matthey Company).
5.2. General procedure (A) for the synthesis of compounds (6a) and (6c)
A mixture of 2-bromo-1-(3,4,5-trimethoxyphenyl)ethanone 4 (1.45 g, 5 mmol) and thioacetamide or N-methylthiourea (5.5 mmol) in anhydrous EtOH (20 mL) was heated to reflux for 1.5 h. After that, the solvent was removed in vacuo, and saturated aqueous NaHCO3 (5 mL) was added to make the mixture basic (pH 8–9). Then the mixture was extracted with CH2Cl2 (3 × 15 mL). The combined organic phases were washed with water (10 mL) and brine (10 mL), dried with anhydrous Na2SO4 and concentrated under vacuum.
5.2.1. Synthesis of 4-(3,4,5-trimethoxyphenyl)-N-methylthiazol-2-amine (6a)
Following general procedure (A), after work-up the orange oil residue was suspended in ethyl ether (20 mL) and stirred for 1 h at +4 °C. The solid was collected by filtration to give the title compound 6a as a white powder. Yield 56%, mp 123–125 °C. 1H NMR (CDCl3) δ: 2.99 (d, J = 5.2 Hz, 3H), 3.88 (s, 3H), 3.92 (s, 6H), 5.34 (bs, 1H), 6.64 (s, 1H), 7.03 (s, 2H). MS (ESI): [M]+ = 280.4.
5.2.2. Synthesis of 4-(3,4,5-trimethoxyphenyl)-2-methylthiazole (6c)
Following general procedure (A), after work-up the title compound 6c was obtained as an oil, which became a white solid at +4 °C. Yield 96%, mp 82–83 °C. 1H NMR (CDCl3) δ: 2.77 (s, 3H), 3.87 (s, 3H), 3.92 (s, 6H), 7.11 (s, 2H), 7.23 (s, 1H). MS (ESI): [M]+ = 265.5.
5.3. Synthesis of 4-(3,4,5-trimethoxyphenyl)-N,N-dimethylthiazol-2-amine (6b)
Piperidine (0.88 mL, 8.8 mmol) was added to a stirred solution of dithiocarbonic acid O-ethyl ester S-[2-oxo-2-(3,4,5-trimethoxyphenyl)-ethyl] ester 5 (1.32 g., 4 mmol) dissolved in ethanol (20 mL). The reaction mixture was stirred for 30 min at room temperature, and N, N′-dimethylcyanamide (0.32 mL, 4 mmol) was added. The solution was stirred at reflux for 4 h, after which ethanol was removed under reduced pressure and the residue portioned in a mixture of dichloromethane (15 mL) and a 3 N aqueous solution of HCl (30 mL). The aqueous acid phase was basified (pH 9) with sodium carbonate and washed with dichloromethane (3 × 15 mL). The combined organic phase was washed with brine (10 mL) and dried over sodium sulfate. After concentration under reduced pressure, the residue was triturated with petroleum ether (10 mL), and this furnished the final compound as a brown solid. Yield: 62%, mp 80–81 °C. 1H NMR (CDCl3) δ: 3.15 (s, 6H), 3.86 (s, 3H), 3.92 (s, 6H), 6.62 (s, 1H), 7.07 (s, 2H). MS (ESI): [M]+ = 294.4.
5.4. Synthesis of N-(4-(3,4,5-trimethoxyphenyl)thiazol-2-yl)-N-methylacetamide (6d)
Sodium acetate (250 mg, 3 mmol) was added to a stirred solution of 6a (840 mg, 3 mmol) in acetic anhydride (30 mL). The mixture was refluxed for 18 h, the solvent evaporated in vacuo and the residue portioned in a mixture of dichloromethane (25 mL) and water (10 mL). The organic extract was washed with brine (10 mL), dried over Na2SO4 and concentrated at reduced pressure. The crude product suspended in petroleum ether (15 mL) and stirred for 1 h, furnished the title compound 6d as a brownish solid. Yield: 89%, mp 197–198 °C. 1H NMR (CDCl3) δ: 2.44 (s, 3H), 3.82 (s, 3H), 3.88 (s, 3H), 3.94 (s, 6H), 7.10 (s, 1H), 7.12 (s, 2H). MS (ESI): [M]+ = 322.5.
5.5. General procedure (B) for the synthesis of compounds (7a–c)
A solution of the appropriate thiazole 6b–d (2 mmol) in anhydrous chloroform (10 mL) was cooled to 0 °C and treated with N-bromosuccinimide (392 mg, 2.2 mmol) under nitrogen. The reaction was allowed to warm to room temperature and then stirred for 4 h. After quenching with saturated Na2S2O3 (5 mL), the resulting mixture was diluted with dichloromethane (10 mL). The organic phase was washed with water (5 mL) and brine (5 mL), dried (MgSO4) and evaporated.
5.5.1. Synthesis of N-(5-bromo-4-(3,4,5-trimethoxyphenyl)thiazol-2-yl)-N-methylacetamide (7a)
Following general procedure (B), after work-up the solid residue was stirred in ethyl ether (10 mL) for 30 min. After filtration, the title compound 7a was isolated as a cream coloured solid. Yield 86%, mp 177–178 °C. 1H NMR (CDCl3) δ: 2.43 (s, 3H), 3.74 (s, 3H), 3.86 (s, 3H), 3.92 (s, 6H), 7.22 (s, 2H). MS (ESI): [M]+ = 400.3, [M+2]+ = 402.5.
5.5.2. Synthesis of 5-bromo-4-(3,4,5-trimethoxyphenyl)-N,N-dimethylthiazol-2-amine (7b)
Following general procedure (B), after work-up the residue was purified by column chromatography, using ethyl acetate/petroleum ether 6–4 v/v as eluent, to afford 7b as a white solid. Yield 68%, mp 96–97 °C. 1H NMR (CDCl3) δ: 3.10 (s, 3H), 3.11 (s, 3H), 3.87 (s, 3H), 3.91 (s, 6H), 7.16 (s, 2H). MS (ESI): [M]+ = 372.2, [M+2]+ = 374.3.
5.5.3. Synthesis of 5-bromo-4-(3,4,5-trimethoxyphenyl)-2-methylthiazole (7c)
Following general procedure (B), after work-up the residue was purified by column chromatography, using ethyl acetate/petroleum ether 3–7 v/v as eluent, to afford a colorless oil, which, when triturated with hexane, furnished 7c as a white solid. Yield 74%, mp 98–99 °C. 1H NMR (CDCl3) δ: 2.70 (s, 3H), 3.89 (s, 3H), 3.93 (s, 6H), 7.15 (s, 2H). MS (ESI): [M]+ = 343.1, [M+2]+ = 345.1.
5.6. General procedure (C) for the synthesis of compounds 3k–o and 8a–e
A stirred suspension of 5-bromo-4-(3,4,5-trimethoxyphenyl)-thiazole derivative 7a or 7c (0.5 mmol) and the appropriate phenylboronic acid (1 mmol) in dioxane (6 mL containing 3 drops of water) was degassed under a stream of nitrogen for 10 min, then treated with [1,1′-bis(diphenylphosphino)ferrocene] dichloropalladium (II) methylene chloride complex (41 mg, 0.05 mmol) and cesium fluoride (190 mg, 1.25 mmol). The reaction mixture was heated under nitrogen at 45 °C for 30 min, then at 65 °C for 5 h. The reaction mixture was cooled to ambient temperature, diluted with CH2Cl2 (10 mL), filtered through a pad of celite and evaporated in vacuo. The residue was dissolved with CH2Cl2 (15 mL), and the resultant solution was washed sequentially with water (5 mL) and brine (5 mL). The organic layer was dried and evaporated, and the residue was purified by flash chromatography on silica gel.
5.6.1. 4-(3,4,5-Trimethoxyphenyl)-2-methyl-5-(naphthalen-2-yl)thiazole (3k)
Following general procedure (C), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 6:4 (v:v) as eluent furnished 3k as a yellow oil. Yield 71%. 1H NMR (CDCl3) δ: 2.78 (s, 3H), 3.58 (s, 6H), 3.82 (s, 3H), 6.77 (s, 2H), 7.34 (d, J = 8.8 Hz, 1H), 7.44 (m, 2H), 7.79 (m, 3H), 7.88 (s, 1H). MS (ESI): [M]+ = 391.3. Anal. (C23H21NO3S): C, H, N.
5.6.2. 4-(3,4,5-Trimethoxyphenyl)-2-methyl-5-p-tolylthiazole (3l)
Following general procedure (C), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 4:6 (v:v) as eluent, furnished 3l as a yellow oil. Yield 71%. 1H NMR (CDCl3) δ: 2.34 (s, 3H), 2.77 (s, 3H), 3.66 (s, 6H), 3.72 (s, 3H), 6.70 (s, 2H), 7.10 (d, J = 8.2 Hz, 2H), 7.23 (d, J = 8.2 Hz, 2H). MS (ESI): [M]+ = 355.5. Anal. (C20H21NO3S): C, H, N.
5.6.3. 5-(4-(Trifluoromethyl)phenyl)-4-(3,4,5-trimethoxyphenyl)-2-methylthiazole (3m)
Following general procedure (C), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 4:6 (v:v) as eluent, furnished 3m as a colorless oil. Yield 66%. 1H NMR (CDCl3) δ: 2.77 (s, 3H), 3.66 (s, 6H), 3.84 (s, 3H), 6.67 (s, 2H), 7.49 (d, J = 8.4 Hz, 2H), 7.57 (d, J = 8.8 Hz, 2H). MS (ESI): [M]+ = 409.2. Anal. (C20H18F3NO3S): C, H, N.
5.6.4. 4-(3,4,5-Trimethoxyphenyl)-5-(4-methoxyphenyl)-2-methylthiazole (3n)
Following general procedure (C), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 6:4 (v:v) as eluent, furnished 3n as a colorless oil. Yield 68%. 1H NMR (CDCl3) δ: 2.73 (s, 3H), 3.68 (s, 6H), 3.81 (s, 3H), 3.83(s, 3H), 6.74 (s, 2H), 6.86 (d, J = 8.8 Hz, 2H), 7.26 (d, J = 8.8 Hz, 2H). MS (ESI): [M]+ = 371.3. Anal. (C20H21NO4S): C, H, N.
5.6.5. 5-(4-Ethoxyphenyl)-4-(3,4,5-trimethoxyphenyl)-2-methylthiazole (3o)
Following general procedure (C), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 4:6 (v:v) as eluent, furnished 3o as a yellow oil. Yield 79%. 1H NMR (CDCl3) δ: 1.41 (t, J = 6.8 Hz, 3H), 2.73 (s, 3H), 3.68 (s, 6H), 3.83 (s, 3H), 4.04 (q, J = 6.8 Hz, 2H), 6.75 (s, 2H), 6.84 (d, J = 8.8 Hz, 2H), 7.26 (d, J = 8.8 Hz, 2H). MS (ESI): [M]+ = 385.5. Anal. (C21H23NO4S): C, H, N.
5.6.6. N-(4-(3,4,5-Trimethoxyphenyl)-5-(naphthalen-2-yl)thiazol-2-yl)-N-methylacetamide (8a)
Following general procedure (C), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 4:6 (v:v) as eluent, furnished the 8a precursor as a colorless oil. Yield 82%. 1H NMR (CDCl3) δ: 2.46 (s, 3H), 3.58 (s, 6H), 3.70 (s, 3H), 3.81 (s, 3H), 6.82 (s, 2H), 7.46 (m, 3H), 7.78 (m, 3H), 7.93 (s, 1H). MS (ESI): [M]+ = 448.5.
5.6.7. N-(4-(3,4,5-Trimethoxyphenyl)-5-p-tolylthiazol-2-yl)-N-methylacetamide (8b)
Following general procedure (C), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 4:6 (v:v) as eluent, furnished 8b as a yellow oil. Yield 71%. 1H NMR (CDCl3) δ: 2.34 (s, 3H), 2.44 (s, 3H), 3.67 (s, 6H), 3.78 (s, 3H), 3.84 (s, 3H), 6.78 (s, 2H), 7.16 (d, J = 8.2 Hz, 2H), 7.28 (d, J = 8.2 Hz, 2H). MS (ESI): [M]+ = 412.5.
5.6.8. N-(5-(4-(Trifluoromethyl)phenyl)-4-(3,4,5-trimethoxyphenyl) thiazol-2-yl)-N-methylacetamide (8c)
Following general procedure (C), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 4:6 (v:v) as eluent, furnished 8c as a colorless oil. Yield 67%. 1H NMR (CDCl3) δ: 2.46 (s, 3H), 3.70 (s, 6H), 3.75 (s, 3H), 3.84 (s, 3H), 6.71 (s, 2H), 7.49 (d, J = 8.4 Hz, 2H), 7.58 (d, J = 8.4 Hz, 2H). MS (ESI): [M]+ = 466.5.
5.6.9. N-(4-(3,4,5-Trimethoxyphenyl)-5-(4-methoxyphenyl) thiazol-2-yl)-N-methylacetamide (8d)
Following general procedure (C), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 4:6 (v:v) as eluent, furnished 8d as a yellow oil. Yield: 69% yield. 1H NMR (CDCl3) δ: 2.44 (s, 3H), 3.68 (s, 6H), 3.78 (s, 3H), 3.81 (s, 3H); 3.84 (s, 3H), 6.80 (s, 2H), 6.87 (d, J = 8.8 Hz, 2H), 7.31 (d, J = 8.8 Hz, 2H). MS (ESI): [M]+ = 428.6.
5.6.10. N-(5-(4-Ethoxyphenyl)-4-(3,4,5-trimethoxyphenyl) thiazol-2-yl)-N-methylacetamide (8e)
Following general procedure (C), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 4:6 (v:v) as eluent, furnished 8e as a colorless oil. Yield 72%. 1H NMR (CDCl3) δ: 1.41 (t, J = 6.8 Hz, 3H), 2.44 (s, 3H), 3.68 (s, 6H), 3.78 (s, 3H), 3.83 (s, 3H); 4.05 (q, J = 6.8 Hz, 2H), 6.81 (s, 2H), 6.86 (d, J = 8.6 Hz, 2H), 7.29 (d, J = 8.6 Hz, 2H). MS (ESI): [M]+ = 442.5.
5.7. General procedure (D) for the synthesis of compounds (3a–e)
A mixture of N-acetyl thiazole derivative 8a–e (0.5 mmol), 1 N aqueous NaOH (0.55 mL, 0.55 mmol) and EtOH (8 mL) was refluxed for 1 h, and the solvent was removed by evaporation. The residue was dissolved in a mixture of water (5 mL) and dichloromethane (10 mL). The organic phase was washed with brine (5 mL), dried (Na2SO4) and concentrated in vacuo. Ethyl ether (5 mL) was added to the residue, and the resulting precipitate was collected by filtration to furnish the title compound as a solid.
5.7.1. 4-(3,4,5-Trimethoxyphenyl)-N-methyl-5-(naphthalen-2-yl)thiazol-2-amine (3a)
Following general procedure (D), after work-up 3a was isolated as a white solid. Yield 81%, mp 194–195 °C. 1H NMR (CDCl3) δ: 3.03 (d, J = 4.8 Hz, 3H), 3.59 (s, 6H), 3.83 (s, 3H), 5.25 (bs, 1H), 6.76 (s, 2H), 7.34 (d, J = 6.8 Hz, 1H), 7.47 (m, 2H), 7.72 (m, 3H), 7.84 (s, 1H). MS (ESI): [M]+ = 406.7. Anal. (C23H22N2O3S): C, H, N.
5.7.2. 4-(3,4,5-Trimethoxyphenyl)-N-methyl-5-p-tolylthiazol-2-amine (3b)
Following general procedure (D), after work-up 3b was isolated as a yellow solid. Yield 82%, mp 189–190 °C. 1H NMR (CDCl3) δ: 2.32 (s, 3H), 2.98 (d, J = 5.2 Hz, 3H), 3.67 (s, 6H), 3.82 (s, 3H), 5.22 (bs, 1H), 6.72 (s, 2H), 7.09 (d, J = 8.0 Hz, 2H), 7.20 (d, J = 8.0 Hz, 2H). MS (ESI): [M]+ = 370.5. Anal. (C20H22N2O3S): C, H, N.
5.7.3. 5-(4-(Trifluoromethyl)phenyl)-4-(3,4,5-trimethoxyphenyl)-N-methylthiazol-2-amine (3c)
Following general procedure (D), after work-up 3c was isolated as a white solid. Yield 78%, mp 183–184 °C. 1H NMR (CDCl3) δ: 4.03 (d, J = 5.2 Hz, 3H), 3.67 (s, 6H), 3.84 (s, 3H), 5.23 (bs, 1H), 6.69 (s, 2H), 7.37 (d, J = 8.8 Hz, 2H), 7.52 (d, J = 8.8 Hz, 2H). MS (ESI): [M]+ = 424.9. Anal. (C20H19F3N2O3S): C, H, N.
5.7.4. 4-(3,4,5-Trimethoxyphenyl)-5-(4-methoxyphenyl)-N-methylthiazol-2-amine (3d)
Following general procedure (D), after work-up 3d was isolated as a brown solid. Yield 84%, mp 174–176 °C. 1H NMR (CDCl3) δ: 2.99 (d, J = 4.4 Hz, 3H), 3.67 (s, 6H), 3.79 (s, 3H), 3.82 (s, 3H), 5.20 (bs, 1H), 6.73 (s, 2H), 6.83 (d, J = 8.8 Hz, 2H), 7.23 (d, J = 8.8 Hz, 2H). MS (ESI): [M]+ = 386.6. Anal. (C20H22N2O4S): C, H, N.
5.7.5. 5-(4-Ethoxyphenyl)-4-(3,4,5-trimethoxyphenyl)-N-methylthiazol-2-amine (3e)
Following general procedure (D), after work-up 3e was isolated as a white solid. Yield 82%, mp 136–137 °C. 1H NMR (CDCl3) δ: 1.40 (t, J = 7.0 Hz, 3H), 2.99 (d, J = 5.2 Hz, 3H), 3.67 (s, 6H), 3.82 (s, 3H), 4.03 (q, J = 7.0 Hz, 2H), 5.20 (bs, 1H), 6.73 (s, 2H), 6.82 (d, J = 8.8 Hz, 2H), 7.21 (d, J = 8.8 Hz, 2H). MS (ESI): [M]+ = 400.7. Anal. (C21H24N2O4S): C, H, N.
5.8. General procedure (E) for the synthesis of compounds 3f–j
To a stirred suspension of 7b (0.5 mmol) and the appropriate phenylboronic acid (1 mmol) in toluene (7 mL) was added [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium (II) methylene chloride complex (41 mg, 0.05 mmol) and cesium fluoride (190 mg, 1.25 mmol). The reaction mixture was heated under nitrogen at 55 °C for 45 min, then at 75 °C for 20 h. The reaction mixture was cooled to ambient temperature, diluted with CH2Cl2 (10 mL), filtered through a pad of celite and evaporated in vacuo. The residue was dissolved with CH2Cl2 (15 mL), and the resultant solution was washed sequentially with water (5 mL) and brine (5 mL). The organic layer was dried and evaporated, and the residue was purified by flash chromatography on silica gel.
5.8.1. 4-(3,4,5-Trimethoxyphenyl)-N,N-dimethyl-5-(naphthalen-3-yl)thiazol-2-amine (3f)
Following general procedure E, the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 2.5:7.5 (v:v) as eluent, furnished 3f as a yellow oil. Yield 63%. 1H NMR (CDCl3) δ: 3.18 (s, 6H), 3.58 (s, 6H), 3.82 (s, 3H), 6.80 (s, 2H), 7.34 (d, J = 8.8 Hz, 1H), 7.42 (m, 2H), 7.76 (m, 3H), 7.88 (s, 1H). MS (ESI): [M]+ = 420.6. Anal. (C24H24N2O3S): C, H, N.
5.8.2. 4-(3,4,5-Trimethoxyphenyl)-N,N-dimethyl-5-p-tolylthiazol-2-amine (3g)
Following general procedure (E), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 3:7 (v:v) as eluent, furnished 3g as a brown solid. Yield 67%, mp 77–78 °C. 1H NMR (CDCl3) δ: 2.32 (s, 3H), 3.14 (s, 3H), 3.15 (s, 3H), 3.66 (s, 6H), 3.82 (s, 3H), 6.76 (s, 2H), 7.10 (d, J = 8.0 Hz, 2H), 7.18 (d, J = 8.0 Hz, 2H). MS (ESI): [M]+ = 384.5. Anal. (C21H24N2O3S): C, H, N.
5.8.3. 5-(4-(Trifluoromethyl)phenyl)-4-(3,4,5-trimethoxyphenyl)- N,N-dimethylthiazol-2-amine (3h)
Following general procedure (E), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 4:6 (v:v) as eluent, furnished 3h as an orange oil. Yield 68%. 1H NMR (CDCl3) δ: 3.17 (s, 6H), 3.68 (s, 6H), 3.83 (s, 3H), 6.69 (s, 2H), 7.37 (d, J = 8.2 Hz, 2H), 7.50 (d, J = 8.2 Hz, 2H). MS (ESI): [M]+ = 438.6. Anal. (C21H21F3N2O3S): C, H, N.
5.8.4. 4-(3,4,5-Trimethoxyphenyl)-5-(4-methoxyphenyl)-N,N-dimethylthiazol-2-amine (3i)
Following general procedure (E), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 3:7 (v:v) as eluent, furnished 3i as a cream colored solid. Yield 72%, mp 140–142 °C. 1H NMR (CDCl3) δ: 3.14 (s, 6H), 3.67 (s, 6H), 3.79 (s, 3H), 3.81 (s, 3H), 6.76 (s, 2H), 6.82 (d, J = 8.8 Hz, 2H), 7.22 (d, J = 8.8 Hz, 2H). MS (ESI): [M]+ = 400.6. Anal. (C21H24N2O4S): C, H, N.
5.8.5. 5-(4-Ethoxyphenyl)-4-(3,4,5-trimethoxyphenyl)-N,N-dimethylthiazol- 2-amine (3j)
Following general procedure (E), the crude residue, purified by flash chromatography using ethyl acetate/petroleum ether 2.5:7.5 (v:v) as eluent, furnished 3j as a red oil. Yield 73%. 1H NMR (CDCl3) δ: 1.38 (t, J = 7.0 Hz, 3H), 3.14 (s, 6H), 3.67 (s, 6H), 3.81 (s, 3H), 4.02 (q, J = 7.0 Hz, 2H), 6.77 (s, 2H), 6.81 (d, J = 8.8 Hz, 2H), 7.22 (d, J = 8.8 Hz, 2H). MS (ESI): [M]+ = 414.6. Anal. (C22H26N2O4S): C, H, N.
5.9. Biology experiments
5.9.1. Antiproliferative assays
Human T-leukemia (Jurkat) and human promyelocytic leukemia (HL-60) cells were grown in RPMI-1640 medium, (Gibco, Milano, Italy). Breast adenocarcinoma (MCF-7), human non-small cell lung carcinoma (A549), human cervix carcinoma (HeLa) and human colon adenocarcinoma (HT-29) cells were grown in DMEM medium (Gibco, Milano, Italy). Both media were supplemented with 115 units/mL of penicillin G (Gibco, Milano, Italy), 115 μg/mL of streptomycin (Invitrogen, Milano, Italy) and 10% fetal bovine serum (Invitrogen, Milano, Italy). All these cell lines were purchased by ATCC. LoVoDoxo Cells are a doxorubicin resistant subclone of LoVo cells15 and were grown in complete Ham’s F12 medium supplemented with doxorubicin (0.1 μg/mL). CEMVbl-100 cells are a multidrug-resistant line selected against vinblastine.16 LoVoDoxo and CEMVbl-100 were a kind gift of Dr. G. Arancia (Istituto Superiore di Sanità, Rome, Italy) A549-T12 cells are a non-small cell lung carcinoma line exhibiting resistance to taxol17 were kindly donated by Professor I. Castaglioulo (University of Padova). They were grown in complete DMEM medium supplemented with taxol (12 nM). Stock solutions (10 mM) of the different compounds were obtained by dissolving them in DMSO. Individual wells of a 96-well tissue culture microtiter plate were inoculated with 100 μL of complete medium containing 8 × 103 cells. The plates were incubated at 37 °C in a humidified 5% CO2 incubator for 18 h prior to the experiments. After medium removal, 100 μL of fresh medium containing the test compound at different concentrations was added to each well and incubated at 37 °C for 72 h. The percentage of DMSO in the medium never exceeded 0.25%. This was also the maximum DMSO concentration in all cell-based assays described below. Cell viability was assayed by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide test as previously described.30 The IC50 was defined as the compound concentration required to inhibit cell proliferation by 50%, in comparison with cells treated with the maximum amount of DMSO (0.25%) and considered as 100% viability.
5.9.2. Effects on tubulin polymerization and on colchicine binding to tubulin
To evaluate the effect of the compounds on tubulin assembly in vitro,18 varying concentrations of compounds were preincubated with 10 μM bovine brain tubulin in glutamate buffer at 30 °C for 15 min and then cooled to 0 °C. After addition of 0.4 mM GTP, the mixtures were transferred to 0 °C cuvettes in a recording spectrophotometer and warmed to 30 °C. Tubulin assembly was followed turbidimetrically at 350 nm. The IC50 was defined as the compound concentration that inhibited the extent of assembly by 50% after a 20 min incubation. The capacity of the test compounds to inhibit colchicine binding to tubulin was measured as described,19 except that the reaction mixtures contained 1 μM tubulin, 5 μM [3H]colchicine and 5 μM test compound.
5.9.3. Molecular modeling
All molecular modeling studies were performed on a MacPro dual 2.66 GHz Xeon running Ubuntu 10. The tubulin structure was downloaded from the PDB data bank (http://www.rcsb.org/— PDB code:1SA0).31 Hydrogen atoms were added to the protein, using the Protonate3D function of molecular operating environment (MOE).32 Ligand structures were built with MOE and minimized using the MMFF94x forcefield until a RMSD gradient of 0.05 kcal mol−1 Å−1 was reached. The docking simulations were performed using plants.33 Molecular dynamics was performed with the Gromacs 4.534 with the Amber99 force field. The structure was solvated using TIP3P water molecules, providing a minimum of 9.0 Å of water between the protein surface and any periodic box edge. The system was neutralized, minimized and then a position restrain dynamics simulation was carried out for 150 ps. The production simulation was conducted for 2 ns at 298 K using a NPT environment. Inhibitors were parameterized by Antechamber of AmberTool 1.5.35 Trajectories analysis were carried out by VMD.35
5.9.4. Flow cytometric analysis of cell cycle distribution
For flow cytometric analysis of DNA content, 5 × 105 HeLa or Jurkat cells in exponential growth were treated with different concentrations of the test compounds for 24 or 48 h. After incubation, the cells were collected, centrifuged and fixed with ice-cold ethanol (70%). The cells were treated with lysis buffer containing RNAse A and 0.1% Triton X-100 and stained with PI. Samples were analyzed on a Cytomic FC500 flow cytometer (Beckman Coulter). DNA histograms were analyzed using MultiCycle® for Windows (Phoenix Flow Systems).
5.9.5. Annexin-V assay
Surface exposure of PS on apoptotic cells was measured by flow cytometry with a Coulter Cytomics FC500 (Beckman Coulter) by adding annexin-V-FITC to cells according to the manufacturer’s instructions (Annexin-V Fluos, Roche Diagnostic). Simultaneously, the cells were stained with PI.
5.9.6. Western blot analysis
HeLa cells were incubated in the presence of test compounds and, after different times, were collected, centrifuged and washed two times with ice cold phosphate-buffered saline (PBS). The pellet was resuspended in lysis buffer. After the cells were lysed on ice for 30 min, lysates were centrifuged at 15000 ×g at 4 °C for 10 min. The protein concentration in the supernatant was determined using BCA protein assay reagents (Pierce, Italy). Equal amounts of protein (20 μg) were resolved using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) (7.5–15% acrylamide gels) and transferred to a PVDF Hybond-p membrane (GE Healthcare). Membranes were blocked with I-block (Tropix), the membrane being gently rotated overnight at 4 °C. Membranes were incubated with primary antibodies against, Bcl-2, Mcl-1, Xiap, cleaved caspase-8, procaspase-2, PARP, cdc25C, Bax, survivin Thr34 and cdc2 (Tyr 15) (all rabbit, Cell Signalling, Milano, Italy), cyclin B1 (mouse, BD, Milano, Italy) cleaved caspase-3 (mouse, Alexis) or β-actin (mouse, Sigma–Aldrich, Milano, Italy) for 2 h at room temperature. Membranes were next incubated with peroxidase-labeled goat anti-rabbit IgG (Sigma–Aldrich, Milano, Italy) or peroxidase-labeled goat anti-mouse IgG (Sigma–Aldrich) for 60 min. All membranes were visualized using ECL Advance (GE Healthcare) and exposed to Hyperfilm MP (GE Healthcare). To ensure equal protein loading, each membrane was stripped and reprobed with anti-β-actin antibody.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Alberto Casolari for excellent technical assistance.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2012.10.001.
Contributor Information
Romeo Romagnoli, Email: rmr@unife.it.
Pier Giovanni Baraldi, Email: baraldi@unife.it.
Giampietro Viola, Email: giampietro.viola1@unipd.it.
References and notes
- 1.(a) Honore S, Pasquier E, Braguer D. Cell Mol Life Sci. 2005;62:3039. doi: 10.1007/s00018-005-5330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Amos LA. Org Biomol Chem. 2004;2:2153. doi: 10.1039/b403634d. [DOI] [PubMed] [Google Scholar]
- 2.(a) Dumontet C, Jordan MA. Nat Rev Drug Disc. 2010;9:790. doi: 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Risinger AL, Giles FJ, Mooberry SL. Cancer Treat Rev. 2008;35:255. doi: 10.1016/j.ctrv.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hearn BR, Shaw SJ, Myles DC. Compr Med Chem II. 2007;7:81. [Google Scholar]
- 3.(a) Yue QX, Liu X, Guo DA. Planta Med. 2010;76:1037. doi: 10.1055/s-0030-1250073. [DOI] [PubMed] [Google Scholar]; (b) Kingston DG. J Nat Prod. 2009;72:507. doi: 10.1021/np800568j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Experentia. 1989;45:209. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]
- 5.Lin CM, Ho HH, Pettit GR, Hamel E. Biochemistry. 1989;28:6984. doi: 10.1021/bi00443a031. [DOI] [PubMed] [Google Scholar]
- 6.McGown AT, Fox BW. Cancer Chemother Pharmacol. 1990;26:79. doi: 10.1007/BF02940301. [DOI] [PubMed] [Google Scholar]
- 7.Pettit GR, Temple C, Jr, Narayanan VL, Varma R, Boyd MR, Rener GA, Bansal N. Anti-Cancer Drug Des. 1995;10:299. [PubMed] [Google Scholar]
- 8.(a) Rustin GJ, Shreeves G, Nathan PD, Gaya A, Ganesan TS, Wang D, Boxall J, Poupard L, Chaplin DJ, Stratford MRL, Balkissoon J, Zweifel M. Br J Cancer. 2010;102:1355. doi: 10.1038/sj.bjc.6605650. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Siemann DW, Chaplin DJ, Walike PA. Expert Opin Investig Drugs. 2009;18:189. doi: 10.1517/13543780802691068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA. J Med Chem. 2006;49:3033. doi: 10.1021/jm0512903. [DOI] [PubMed] [Google Scholar]
- 10.(a) Nam NH. Curr Med Chem. 2003;10:1697. doi: 10.2174/0929867033457151. [DOI] [PubMed] [Google Scholar]; (b) Cushman M, Nagarathnam D, Gopal D, He HM, Lin CM, Hamel E. J Med Chem. 1992;35:2293. doi: 10.1021/jm00090a021. [DOI] [PubMed] [Google Scholar]; (c) Hatanaka T, Fujita K, Ohsumi K, Nakagawa R, Fukuda Y, Nihei Y, Suga Y, Akiyama Y, Tsuji T. Bioorg Med Chem Lett. 1998;8:3371. doi: 10.1016/s0960-894x(98)00622-2. [DOI] [PubMed] [Google Scholar]
- 11.(a) Chaudari A, Pandeya SN, Kumar P, Sharma PP, Gupta S, Soni N, Verma KK, Bhardwaj G. Mini-Rev Med Chem. 2007;12:1186. doi: 10.2174/138955707782795647. [DOI] [PubMed] [Google Scholar]; (b) Hsieh HP, Liou JP, Mahindroo N. Curr Pharm Des. 2005;11:1655. doi: 10.2174/1381612053764751. [DOI] [PubMed] [Google Scholar]
- 12.Romagnoli R, Baraldi PG, Brancale A, Ricci A, Hamel E, Bortolozzi R, Basso G, Viola G. J Med Chem. 2011;54:5144. doi: 10.1021/jm200392p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown MD, Gillon DW, Meakins GD, Whitham GH. J Chem Soc, Perkin Trans I. 1985:1623. [Google Scholar]
- 14.Dall’Acqua F, Linardi MA, Maggi F, Nicoletti M, Petitto V, Innocenti G, Basso G, Viola G. Bioorg Med Chem. 2011;19:5876. doi: 10.1016/j.bmc.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Toffoli G, Viel A, Tuimoto I, Bisconti G, Rossi G, Baoiocchi M. Br J Cancer. 1991;63:51. doi: 10.1038/bjc.1991.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupuis M, Flego M, Molinari A, Cianfriglia M. HIV Med. 2003;4:338. doi: 10.1046/j.1468-1293.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- 17.Martello LA, Verdier-Pinard P, Shen HJ, He L, Torres K, Orr GA, Horwitz SB. Cancer Res. 2003;63:448. [PubMed] [Google Scholar]
- 18.Hamel E. Cell Biochem Biophys. 2003;38:1. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
- 19.Verdier-Pinard P, Lai JY, Yoo HD, Yu J, Marquez B, Nagle DG, Nambu M, White JD, Falck JR, Gerwick WH, Day BW, Hamel E. Mol Pharmacol. 1998;53:62. doi: 10.1124/mol.53.1.62. [DOI] [PubMed] [Google Scholar]
- 20.(a) Mollinedo F, Gajate C. Apoptosis. 2003;8:413. doi: 10.1023/a:1025513106330. [DOI] [PubMed] [Google Scholar]; (b) Clarke PR, Allan LA. Trends Cell Biol. 2009;19:89. doi: 10.1016/j.tcb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Vakifahmetoglu-Norberg H, Zhivotovsky B. Trends Cell Biol. 2010;20:150. doi: 10.1016/j.tcb.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Mhaidat NM, Wang Y, Kiejda KA, Zang XD, Hersey P. Mol Cancer Ther. 2007;6:752. doi: 10.1158/1535-7163.MCT-06-0564. [DOI] [PubMed] [Google Scholar]
- 23.Ho LH, Read SH, Dorstyn L, Lambrusco L, Kumar S. Oncogene. 2008;27:3393. doi: 10.1038/sj.onc.1211005. [DOI] [PubMed] [Google Scholar]
- 24.Bhalla KN. Oncogene. 2003;22:9075. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 25.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, et al. Nature. 2011;471:110. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 26.Matson DR, Stukenberg PT. Mol Interv. 2011;11:141. doi: 10.1124/mi.11.2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altieri DC. Biochem J. 2010;430:199. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Oncogene. 2004;23:2825. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, Altieri DC. Proc Natl Acad Sci USA. 2000;9:13103. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viola G, Fortunato E, Cecconet L, Del Giudice L, Dall’Acqua F, Basso G. Toxicol Appl Pharm. 2008;227:84. doi: 10.1016/j.taap.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Ravelli RBG, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Nature. 2004;428:198. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 32.Molecular Operating Environment (MOE 2010) Chemical Computing Group, Inc; Montreal, Quebec, Canada: [accessed Sep 2012]. http://www.chemcomp.com. [Google Scholar]
- 33.Korb O, Stützle T, Exner TE. LNCS. 2006;4150:247. [Google Scholar]
- 34.Case DA, Cheatham TE, III, Darden T, Gohlke H, Luo R, Merz KM, Jr, Onufriev A, Simmerling C, Wang C, Woods R. J Comput Chem. 2005;26:1668. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphrey W, Dalke A, Schulten K. J Mol Graph. 1996;14:33. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.