Abstract
Although developed somewhat later, silicon-based cross-coupling has become a viable alternative to the more conventional Suzuki-Miyaura, Stille-Kosugi-Migita, and Negishi cross-coupling reactions because of its broad substrate scope, high stability of silicon-containing reagents, and low toxicity of waste streams. An empowering and yet underappreciated feature unique to silicon-based cross-coupling is the wide range of sequential processes available. In these processes, simple precursors are first converted to complex silicon-containing cross-coupling substrates, and the subsequent silicon-based cross-coupling reaction affords an even more highly functionalized product in a stereoselective fashion. In so doing, structurally simple and inexpensive starting materials are quickly transformed into value-added and densely substituted products. Therefore, sequential processes are often useful in constructing the carbon backbones of natural products. In this review, studies of sequential processes involving silicon-based cross-coupling are discussed. Additionally, the total syntheses that utilize these sequential processes are also presented.
Keywords: silicon, cross-coupling, palladium, homogeneous catalysis, sequential processes
1. Introduction
Since the earliest days of organic chemistry, the development of new and efficient methods for the construction of C-C bonds has always been a major focus of research. Since the 1970s, these aspirations have manifested themselves in the development of metal-catalyzed cross-coupling reactions with wide scope and broad generality. Compared to all the well-established variants of cross-coupling reactions, including Stille-Kosugi-Migita cross-coupling, Negishi cross-coupling, and Suzuki-Miyaura cross-coupling, silicon-based cross-coupling[i] has a number of important advantages. They include, (1) the stability of cross-coupling reagents, (2) the cost effectiveness of silane starting materials, and (3) the low environmental impact of the waste stream. As such, silicon-based cross-coupling has emerged as a viable alternative to the more conventional cross-coupling methods, and silicon-based cross-couplings has been showcased in a number of natural product syntheses.[ia] Another fundamental goal of organic chemistry is to transform inexpensive, readily available, and simple materials into value-added, structurally complex products. To achieve this goal, numerous cascade reactions or sequential processes have been developed.[ii] In general, such tandem process lead to more efficient introduction of molecular complexity (rings, stereocenters, functional groups) and overall streamline synthetic transformations. Silicon-based cross-coupling reactions are particularly well suited for implementations in tandem sequences because of the wide variety of methods available to selectively introduce an unsaturated silyl moiety or its equivalent with a high degree of functional group tolerance. These unsaturated silyl ethers or equivalents thereof can then undergo facile cross-coupling reactions with aryl halides and alkenyl halides, generating densely functionalized and highly substituted olefins with defined geometry. In some cases these two steps have to be carried out separately, whereas in others one-pot procedures have been developed, thus obviating the isolation of intermediates. This empowering feature is advantageous in the synthesis of complex molecules. Therefore, the development of sequential silylation/cross-coupling processes has been an area of active research. In this review, these sequential processes are discussed. In addition, the application these sequential reactions in total syntheses will also be briefly described.
2. Sequential Intermolecular Hydrosilylation/Cross-Coupling
Hydrosilylation is one of the most direct ways to introduce a silyl group and has been thoroughly studied.[iii] Therefore, the combination of hydrosilylation and cross-coupling is a logical starting point in developing sequential processes and an efficient one-pot hydrosilylation/cross-coupling protocol has been developed.[iv] Terminal alkynes are first subjected to a highly selective Markovnikov syn-hydrosilylation catalyzed by [(tBu3P)Pt(DVDS)],[v] in the presence of tetramethyldisiloxane, an inexpensive silylating agent. When the hydrosilylation reaction is complete, tetra-n-butylammonium fluoride (TBAF), Pd(dba)2 and an aryl iodide are charged into the same pot to afford olefin (E)-2 with high stereoselectivity, without the need to isolate the intermediate dialkenyldisiloxane 1 (Scheme 1). A complementary method involves a ruthenium-catalyzed, anti-Markovnikov hydrosilylation of terminal alkynes such as 3.[vi] The subsequent TBAF-promoted, palladium-catalyzed, cross-coupling of the resulting silane 4 with an aryl iodide provides access to 2-aryl-1-alkene 5 (Scheme 1).[vii]
Scheme 1.
Sequential intermolecular hydrosilylation/cross-coupling.[iv,vii] DVDS =1,3-divinyl-1,1,3,3-tetramethyldisiloxane, THF = tetrahydrofuran, dba = trans,trans-dibenzylideneacetone, TBAF = tetra-n-butylammonium fluoride, DMF = N,N-dimethylformamide, Bn = benzyl, Cp* = pentamethylcyclopentadienyl.
In a total synthesis (+)-NK-104, a hydrosilylation/cross-coupling sequence is employed to unite the biaryl and the alkenyl lactone fragments. Alkyne 6 first undergoes a platinum-catalyzed hydrosilylation with chlorodimethylsilane. The cross-coupling of the crude chlorosilane 7 and aryl iodide 8, catalyzed by [(π-allyl)PdCl]2 and promoted by TBAF, proceeds rapidly to afford styrene derivative 9 in good overall yield. This advanced intermediate is converted to (+)-NK-104 after a simple treatment with trifluoroacetic acid (Scheme 2).[viii]
Scheme 2.
Key steps in a synthesis of (+)-NK-104.[viii]
A recent synthesis of vitamin A nicely demonstrates the efficiency of the one-pot hydrosilylation/cross-coupling process (Scheme 3).[ix] Alkyne 10 and tetramethyldisiloxane are first stirred in the presence of [(tBu3P)Pt(DVDS)]. In the same pot, alkenyl iodide 12, TBAF, and Pd2(dba)3•CHCl3 are added after the completion of the hydrosilylation step, without isolating disiloxane 11. The desired pentaene, 13, is obtained in 74% yield, which is converted to vitamin A after a simple deprotection.
Scheme 3.

One-pot hydrosilylation/cross-coupling for the synthesis of vitamin A.[ix]
3. Sequential cis-Reduction/Cross-Coupling
An approach complementary to the syn-silylation has been reported, for the preparation of (Z)-alkenylsilanes, namely, the cis-reduction of alkynylsilanes. For example, alkynylsilane 14 can be converted to (Z)-alkenylsilane 15 via hydroalumination using diisobutylaluminum hydride (DIBAL), which is known to add to alkynes in a syn fashion.[x] (Z)-Alkenylsilane 15 is a competent substrate that undergoes a facile palladium-catalyzed cross-coupling promoted by TBAF with aryl as well as alkenyl iodides, to afford (Z)-2, Z-substituted styrenes and conjugated dienes, respectively (Scheme 4).[xi] Notably, the hydroxyl group on the cross-coupling partner is well tolerated.
Scheme 4.

Sequential cis-reduction/cross-coupling.[xi] DIBAL = diisobutylaluminum hydride.
The method of sequential cis-reduction/cross-coupling proved very useful for the synthesis of 11-cis-retinal reported recently by López and co-workers.[ix] Alkynylsilane 16 was reduced via hydrozirconation using Schwartz's reagent (Cp2ZrHCl).[xii] The cross-coupling of the resulting (Z)-alkenylsilane 17 and alkenyl iodide 12 proceeds in the presence of TBAF and Pd2(dba)3•CHCl3, to provide pentaene 18, the 11-cis-analogue of 13. The target molecule, 11-cis-retinal, is then obtained through a short sequence from 18 (Scheme 5).
Scheme 5.

Key steps in a total synthesis of 11-cis-retinal.[ix] THP = tetrahydropyranyl, Cp = cyclopentadienyl.
In the above reactions, DIBAL and Schwartz's reagent are employed for the stereoselective cis-reduction of alkynylsilanes. However, these reagents are hydrolytically sensitive and are incompatible with carbonyl groups. Thus, for application in complex molecule synthesis a milder reduction method is needed. Such is the case in a formal synthesis of (+)-fostriecin (CI-920), in which an advanced intermediate, alkynylsilane 19, bears a lactone and a hydroxyl group.[xiii] To obtain (Z)-alkenylsilane 20, diimide (generated in situ, using 2,4,6-triisopropylbenzenesulfonyl hydrazide (TPSH) and sodium bicarbonate) is used to reduce the triple bond in 19.[xiv] A late stage TBAF-promoted alkenyl-alkenyl cross-coupling is subsequently carried out using 20 and alkenyl iodide 21 under mild conditions, with a concomitant deprotection, to afford target molecule 22 (Scheme 6).[xv] It is noteworthy that in the synthesis, the silyl group is introduced in the first step and remained intact through twelve steps, including those involving strongly basic organolithium, organomagnesium, and organozinc reagents, as well as strongly Lewis acidic triethylsilyl triflate. This superior stability of silicon-containing species to harsh reagents is a hallmark of silicon-base cross-coupling.
Scheme 6.

Key steps in a formal synthesis of (+)-fostriecin (CI-920).[xiii] TBS = tert-butyldimethylsilyl, TES = triethylsilyl, TPSH = 2,4,6-triisopropylbenzenesulfonyl hydrazide.
4. Sequential Intramolecular Hydrosilylation/Cross-Coupling
While sequential, intermolecular hydrosilylation/cross-coupling can be used to efficiently synthesize disubstituted olefins, the intramolecular variants of this reaction have also been developed to access more complex structures. The intramolecular syn-hydrosilylation reaction of homopropargyloxyhydrosilane 23 with Speier's Catalyst (H2PtCl6•6H2O)[xvi] affords cyclic (E)-alkylidenylsilyl ether 25.[xvii] Interestingly, under ruthenium-catalysis, an anti-hydrosilylation takes place instead,[xviii] furnishing (Z)-alkylidenylsilyl ether 28 (isolated as a mixture of monomer and oligomers due to its strained nature).[xix] Both 25 and 28 undergo facile TBAF-promoted cross-coupling reactions with aryl iodides, resulting in trisubstituted (E)- and (Z)-homoallylic alcohols 26 and 29, respectively (Scheme 7).
Scheme 7.

Sequential intramolecular hydrosilylation/cross-coupling of homopropargyloxyhydrosilanes.[xviia,xix]
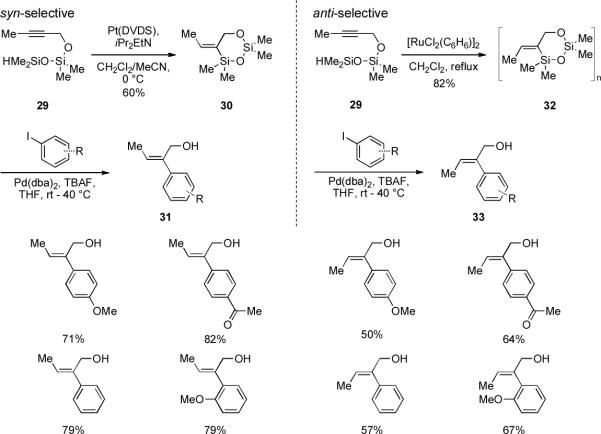
As mentioned above, five-membered cyclic silyl ethers obtained through the intramolecular hydrosilylation of homopropargyl alcohol derivatives contain a considerable amount of strain energy and can oligomerize. The analogous reaction of propargyl alcohol derivatives is even more difficult. An early study on the intramolecular hydrosilylation of propargyloxyhydrosilanes resulted only in polymerization.[xx] However, with a two-atom tether between the oxygen and the silicon atom, the intramolecular hydrosilylation of propargyloxysilyl ethers becomes feasible and the resulting six-membered cyclic disiloxane can be isolated and fully characterized. Propargyloxydisiloxane 29 is amenable to both syn- and anti-hydrosilylation under previously described conditions to afford (E)- and (Z)-alkylidenylsiloxanes 30 and 32, respectively. Highly substituted (E)- and (Z)-allylic alcohols 31 and 33 can then be obtained via TBAF-promoted cross-coupling with aryl iodides (Scheme 8).[xxi]
Scheme 8.
Sequential intramolecular hydrosilylation/cross-coupling of propargyloxydisiloxanes.[xxi]
5. Sequential Intramolecular Silylfunctionalization/Cross-Coupling
In the aforementioned examples, a silyl group and a hydrogen atom are added across triple bonds. Another layer of structural complexity can be introduced when the silyl group is incorporated in conjunction with another functional group. An illustration of this strategy is the sequential rhodium-catalyzed silylformylation/cross-coupling of homopropargyloxysilanes.[xxii] Homopropargyloxysilane 34 undergoes an intramolecular silylformylation in the presence of a bimetallic rhodium-cobalt catalyst under a carbon monoxide atmosphere,[xxiii] to form cyclic silyl ether 35 bearing an α,β-unsaturated aldehyde. The subsequent cross-coupling reaction of this sensitive substrate with aryl iodides, including heteroaryl iodides, is achieved with potassium fluoride and a copper co-catalyst, to furnish β-disubstituted-α,β-unsaturated aldehyde 36 exclusively to Z-configuration (Scheme 9). A similar sequential palladium-catalyzed silylcyanation/cross-coupling process has been reported.[xxiv] In this interesting reaction, homopropargyloxychlorosilane 37 and trimethylsilyl cyanide (TMSCN) are combined, and the homopropargyloxysilyl cyanide generated in situ is presumably the active species in the palladium-catalyzed silylcyanation. Cyclic silyl ether 38 can then undergo a potassium fluoride-promoted cross-coupling with iodobenzene, allyl bromide, benzyl bromide, as well as (E)-1-iodohexene, to afford tetrasubstituted (Z)-α,β-unsaturated nitriles 39.
Scheme 9.
Sequential intramolecular silylformylation/cross-coupling and sequential intramolecular silylcyanation/cross-coupling of homopropargyloxysilanes.[xxii,xxiv] TMSCN = trimethylsilyl cyanide, acac = acetylacetonate.
6. Sequential Silylcarbocyclization/Cross-Coupling
In a hydrosilylation reaction, the silicon atom and the hydrogen atom of a hydrosilane add across a double or triple bond, and in the cases of silylformylation and silylcyanation, the silicon atom and the carbon atom similarly add across the unsaturated unit. However, in silylcarbocyclization, the silicon atom and the hydrogen atom of a hydrosilane add across two unsaturated units of the substrate with a concomitant ring closure.[xxv] To achieve this transformation, benzyldimethylsilane and 1,6-enyne 40 are mixed in the presence of Rh4(CO)12. The resulting benzylsilylalkylidenecyclopentane 41 is subsequently treated with TBAF, and in the presence of an aryl iodide and Pd2(dba)3•CHCl3, a facile cross-coupling reaction takes place to afford benzylidenylcyclopentane 42 (Scheme 10).[xviib,xxvi]
Scheme 10.

Sequential silylcarbocyclization/cross-coupling.[xxvia]
A process closely related to silylcarbocyclization is carbonylative silylcarbocyclization.[xxvii] In addition to the silylation and ring closure that occurs in a silylcarbocyclization reaction, a formyl group is introduced in a carbonylative silylcarbocyclization, which provides opportunity for further elaboration. To achieve a silylcarbocyclization, the enyne substrate 40 is mixed with a hydrosilane and the rhodium catalyst at elevated temperature and under elevated pressure of carbon monoxide in a pressurized vessel (a stainless steel autoclave or shaker).[xxvia] Under these conditions, aldehyde 43 is obtained in good yield (Scheme 11).
Scheme 11.

Silylcarbocyclization vs. carbonylative silylcarbocyclization.[xxvia]
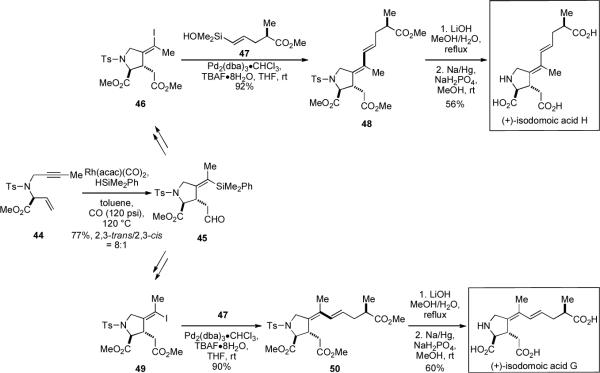
The ability to carry out a sequential carbonylative silylcarbocyclization/cross-coupling, in large part contributed to the success of recent total syntheses of isodomoic acids G and H.[xxviii] In the initial synthetic approach, enyne 44 is first subjected to a rhodium-catalyzed carbonylative silylcarbocyclization for the preparation of a highly substituted pyrrolidinylacetaldehyde related to 45. However, after an extensive survey of conditions, none of the key cross-coupling product 50 could be detected using this silicon donor and a 5-iodopentenoate acceptor related to 47. The failure to effect this cross-coupling leads to a reversal in the roles of the donor and the acceptor. Accordingly, aldehyde 45, synthesized by the carbonylative silylcarbocyclization of 44 with phenyldimethylsilane, is converted to either (Z)-alkenyl iodide 46 or (E)-alkenyl iodide 49 through a few straightforward transformations to set the stage for the key cross-coupling reaction. Interestingly, in this late-stage cross-coupling reaction of 46 and 49 with 47, the TBAF hydration level plays a critical role. When the TBAF is tri-, tetra- or hexahydrated, the conversion is only modest and the reaction stalls within 2 h. However, the reaction rate improved dramatically by employing TBAF•8H2O, resulting in the formation of conjugated dienes 48 and 50. Target molecules, (+)-isodomoic acid G and (+)-isodomoic acid H are obtained by simple deprotection steps from 50 and 48, respectively (Scheme 12).
Scheme 12.
Key steps in a total syntheses of isodomoic acids G and H.[xxviii]
7. Sequential Ring-Closing Metathesis/Cross-Coupling
Whereas the aforementioned intramolecular hydrosilylation, silylformylation, and silylcyanation reactions produce exo-alkylidenylsilyl ethers, silyl ethers bearing an endocyclic double bond can be prepared by the ring-closing metathesis (RCM)[xxix] of ω-alkenyl vinylsilyl ethers such as 51. For this transformation molybdenum-based Schrock's catalyst is required, presumably because of the hindered vinylsilyl group.[xxx] The resulting cyclic alkenylsilyl ether 52 undergoes facile cross-coupling reactions with aryl iodides under very mild conditions to furnish substituted alkenyl secondary homoallylic alcohols 53 with exclusive Z-configuration (Scheme 13).[xxxia,d]
Scheme 13.

Sequential RCM/cross-coupling.[xxxia,c]
In the above example, the cross-coupling proceeds in an intermolecular fashion. However, for the substrates that bear an alkenyl iodide moiety, the cross-coupling would proceed intramolecularly, and as a result of cross-coupling, a ring closure is achieved. Thus, vinylsilyl ethers 54 are subjected to molybdenum-catalyzed RCM as described above. Cyclic silyl ethers 55 obtained are then treated with TBAF and [(π-allyl)PdCl]2 under high dilution (to favor macrocyclization) and produce (Z,Z)-1,3-dienes, 56. Remarkably, despite the unfavorable entropic and enthalpic factors,[xxxii] even 12-membered rings bearing a cis,cis-1,3-diene unit have been constructed through this strategy (Scheme 14).[xxxia,b] Similarly, macrolactones bearing a cis,cis-1,3-diene, 59, can be prepared via this sequence. Alhtough the RCM of vinylsilyl ether 57 proceeds uneventfully under the conditions previously developed, the intramolecular cross-coupling of cyclic silyl ethers 58 requires some modification of parameters to prevent an undesired side-reaction (an intramolecular transesterification through the base-promoted attack of the secondary alcohol of lactone 59). Six waters of hydration are the optimal level for the TBAF employed, and DMF is the optimal solvent. The combination of these two parameters strikes a balance between moderating the fluoride basicity without severely attenuating its reactivity. Macrolactonizations with ring sizes as large as 14-members can be synthesized using this method.[xxxid]
Scheme 14.
Macrocyclization through sequential RCM/intramolecular cross-coupling.[xxxia,b,d]
From Scheme 14, it is apparent that the one of the synthetic applications of sequential RCM/intramolecular cross-coupling is the total synthesis of macrolide natural products and pharmaceutical agents. This synthetic approach offers a novel disconnection for the synthetic plan that differs from the conventional macrolactonization technologies (i.e. the activation of carboxylic acid or alcohol in the precursor).[xxxiii] This strategy has recently been illustrated in a model study toward the total synthesis of oximidine III, a benzo-fused, 12-membered macrolide bearing a 1,3-trans,cis-diene moiety (Scheme 15).[xxxib] To prepare the target macrolactone 62, vinylsilyl ether 60 is first treated with Schrock's catalyst to effect a RCM that results in the formation of a seven-membered cyclic silyl ether, 61, that bears an E-styryl iodide moiety. The macrocyclization is subsequently accomplished through a [(π-allyl)PdCl]2-catalyzed intramolecular cross-coupling promoted by TBAF•6H2O in DMF, as previously optimized.
Scheme 15.

A model study towards the total synthesis of oximidine III.[xxxib]
The strategy of sequential RCM/intramolecular cross-coupling is by no means limited to macrocyclic olefins and lactones, as it is also instrumental in a total synthesis of (+)-brasilenyne, a nine-membered cyclic ether.[xxxiv] Following the reaction conditions developed above an RCM reaction induced by Schrock's catalyst is carried out using vinylsilyl ether 63 to form the C(5)–C(6) double bond. The resulting cyclic alkenylsilyl ether 64 bears a silyloxy moiety at the proper position poised for macrocyclization. In the subsequent cross-coupling reaction, the six-membered siloxane ring is activated by TBAF, and in the presence of [(π-allyl)PdCl]2 the nine-membered ring of 64 is forged through the formation of the C(4)–C(5) bond. This sequential reaction allows for the rapid access to nine-membered cyclic ether 65, bearing a Z,Z-conjugated diene unit at the desired position. This intermediate is then easily elaborated to (+)-brasilenyne (Scheme 16).
Scheme 16.

The key steps in a total synthesis of (+)-brasilenyne.[xxxiv]
8. Sequential Enyne Coupling/Cross-Coupling
Whereas silylcarbocyclization and RCM unite two unsaturated functionalities intramolecularly, the ruthenium-catalyzed enyne coupling unites them intermolecularly.[xxxv] Combination of alkynylsilane 66 and an terminal alkene, such as 1-octene, in the presence of [CpRu(NCMe)3]PF6, 67 produces (1Z,4E)-alkenylsilane exclusively. Trisubstituted olefin 68 with a defined geometry is obtained as a result of the fluoride-promoted cross-coupling with aryl iodides (Scheme 17).[vii]
Scheme 17.

Sequential enyne coupling/cross-coupling.[vii]
More recently, the previously developed sequential enyne coupling/cross-coupling protocols has been extended to an interesting sequential enyne coupling/allylic cyclization/cross-coupling process.[xxxvi] Starting from an alkynylsilane 69 and homoallylic ether 70, the enyne coupling product 71 is obtained after the treatment with [CpRu(NCMe)3]PF6. Because 71 bears an electron-poor allylic ether and a hydroxyl group, it is poised to undergo an intramolecular allylic substitution reaction and this transformation is achieved by the addition of triethylamine and Pd2(dba)3•CHCl3. Finally, the allylic substitution product, silylalkylidenyltetrahydropyran 72, cross-couples with iodobenzene with TBAF and the same palladium(0) catalyst to afford 2-vinyl-4-(E)-benzylidenetetrahydropyran (73) (Scheme 18).
Scheme 18.
Sequential enyne coupling/allylic cyclization/cross-coupling.[xxxvi]
9. Sequential Cycloaddition/Cross-Coupling
In all of the above examples, only alkenyl-aryl and alkenyl-alkenyl cross-coupling reactions are involved. However, sequential cycloaddition/cross-coupling reactions offer opportunity to utilize heteroaryl-aryl as well as alkyl-aryl cross-coupling technologies.
Arylisoxazoles represent an important structural motif for COX-2 inhibitors such as Bextra.[xxxvii] Additionally, isoxazole-containing intermediates can be of great usefulness in natural product synthesis.[xxxviii] In a recent report, sequential [3 + 2] cycloaddition/cross-coupling has been developed as a convenient method to prepare 3,4,5-trisubstituted isoxazoles.[xxxix] First, the isoxazolylsilanol 76 is prepared by a dipolar [3 + 2] cycloaddition of a nitrile oxide generated in situ from chloro oxime 74, and alkynylsilyl ether 75,[xl] followed by hydrolysis of the silyl ether under acidic conditions. It is noteworthy that unlike all the examples discussed above, 76 undergoes cross-coupling with aryl iodides through the action of a Brønsted base, sodium tert-butoxide, to afford 3,4,5-trisubstituted isoxazoles 77 (Scheme 19).
Scheme 19.

Sequential [3 + 2] cycloaddition/cross-coupling.[xxxix]
The cross-coupling of alkylsilane derivatives has been a long standing problem, and reports on this topic are rare. Very recently, however, a Simmons-Smith cyclopropanation/cross-coupling sequence was demonstrated, that signaled good progress in the state of the art of the cross-coupling of alkylsilanes.[xli] In this report, di(tert-butoxy)alkenylsilanol 78 is first converted to di(tert-butoxy)cyclopropylsilanol 79 through Simmons-Smith cyclopropanation reaction.[xlii] Interestingly, when subjected to the TBAF-promoted cross-coupling conditions in the presence of (Ph3P)4Pd and an aryl bromide, 79 shows only a modest reactivity. However, the reactivity can be greatly enhanced by treating 79 with boron trifluoride to convert it to trifluorosilane 80 in situ,[xliii] prior to adding (Ph3P)4Pd and the aryl bromide cross-coupling partner. Consequently, trans-disubstituted cyclopropanes 81 are isolated in good yields (Scheme 20).
Scheme 20.

Sequential Simmons-Smith cyclopropanation/cross-coupling.[xli]
10. Sequential Larock Indole Synthesis/Cross-Coupling
The cross-coupling of heterocyclic silanols have benefited greatly from the use of alkali metal conjugate bases. Heteroaromatic compounds, including benzofuranyl-,[xliv] pyrrolyl-, furanyl-, thienyl-, and indolylsilanols undergo cross-coupling as preformed silanolate salts or by in situ generation with Brønsted bases.[xlv] These cross-coupling reagents possess the unique advantages of high stability and high reactivity compared to their boronic acid (prone to protodeborylation and homodimerization)[xlvi] as well as organostannane (harsh reaction conditions required)[xlvii] analogues. From the foregoing discussion, it is apparent that sequential processes involving silicon-based cross-coupling can be very useful in efficiently synthesizing differentially substituted heteroaromatic compounds. Building upon the previous efforts and in response to the need of a more efficient method to prepare 2,3-disubstituted indoles, a sequential Larock indole synthesis/cross-coupling process had been developed (Scheme 21).[xlviii] 2,3-Disubtituted indoles can be found in a number of therapeutic agents of serious diseases such as developmental disorders, stroke, and hypercholesterolemia.[xlix]
Scheme 21.

Sequential Larock indole synthesis/cross-coupling.[xlviii] OAc = acetate, S-Phos = 2-dicyclohexylphosphino-2',6'-dimethoxybiphenyl, RuPhos = 2-dicyclohexylphosphino-2',6'-diisopropoxybiphenyl.
In this sequential process, the indolylsilane formation is brought about via a palladium-catalyzed reaction of protected 2-iodoaniline 82 and alkynylsilyl ether 83. This combination results in the exclusive production of the 2-indoylsilyl ether.[l] Silanol 84 is isolated after an acidic hydrolysis of the resulting indoylsilyl ether. The cross-coupling of 84 is achieved via its sodium salt, and through a judicious choice of Buchwald-type ligands, namely S-Phos[li] and RuPhos,[lii] aryl chlorides can be used as coupling partners in this reaction. The possibility of employing aryl chlorides represents an empowering advantage of this sequential protocol, since aryl chlorides are more readily available and cost effective than their bromide or iodide analogues. Finally, 2,3-disubstituted indoles 85 are obtained in good yield (Scheme 21).
11. Conclusion and Outlook
As evident from the examples discussed in this brief review, organosilanes with a broad range of structural features can be generated from a large number of reactions ranging from the hydrosilylation to complex transformations such as silylcarbocyclization, enyne coupling, or Larock indole synthesis. Most importantly, more structural complexity can be generated when these processes are parlayed with a cross-coupling reaction. The possibilities for extension of this strategy are limited only by the imagination of synthetic chemists, and in the near future, it is expected many more sequential processes involving silicon-based cross-coupling will be reported. It is also expected that many of sequential processes will find great use in forging key C-C bonds in the total synthesis of complex natural products.
Acknowledgements
We are grateful to the National Institutes of Health for generous financial support (GM63167).
Biographies

Scott E. Denmark was born in Lynbrook, New York on 17 June 1953. He obtained an S.B. degree from MIT in 1975 (working with Richard H. Holm and Daniel S. Kemp) and his D. Sc. Tech. (under the direction of Albert Eschenmoser) from the ETH Zürich in 1980. That same year he began his career at the University of Illinois. He was promoted to associate professor in 1986 and to full professor in 1987, and since 1991 he has been the Reynold C. Fuson Professor of Chemistry. His research interests include the invention of new synthetic reactions, exploratory organoelement chemistry, and the origin of stereocontrol in fundamental carbon-carbon bond forming processes. Professor Denmark is currently the Editor in Chief of Organic Reactions and edited Volume 85 of Organic Syntheses. He served for six years as an Associate Editor of Organic Letters and for nine years as Editor of Topics in Stereochemistry. He is a Fellow of the Royal Society of Chemistry and was selected as an ACS Fellow in the inaugural year, 2009.
Jack Hung-Chang Liu was born in Taipei, Taiwan in 1979. He obtained a Hon. B. Sc. Degree at University of Toronto in 2002 (Working with Robert A. Batey and Mark Lautens). He then joined the research group of Scott E. Denmark at University of Illinois at Urbana-Champaign, focusing on the development and application of silicon-based cross-coupling. After obtaining his Ph. D. degree in 2009, he moved to University of California, Berkeley for post-doctoral research under F. Dean Toste.
References
- [i].For reviews on silicon-based coupling, see: Denmark SE, Liu JH-C. Angew. Chem. Int. Ed. 2010;49:2978–2986. doi: 10.1002/anie.200905657.; Denmark SE. J. Org. Chem. 2009;74:2915–2927. doi: 10.1021/jo900032x.; Denmark SE, Regens CS. Acc. Chem. Res. 2008;41:1486–1499. doi: 10.1021/ar800037p.; Denmark SE, Baird JD. Chem. Eur. J. 2006;12:4953–4963.; Nakao Y, Sahoo AK, Imanaka H, Yada A, Hiyama T. Pur. Appl. Chem. 2006;78:435–440.; Tsuji J. Palladium Reagents and Catalysts: New Perspectives for the 21st Century. Wiley; Wessex: 2004. pp. 339–348.; Denmark SE, Sweis RF. In: Metal Catalyzed Cross-Coupling Reactions. Second, Completely Revised and Enlarged Edition. de Meijere A, Diederich F, editors. Vol. 1. Wiley-VCH; Weinheim: 2004. Chapter 4.; Spivey AC, Gripton CJG, Hannah JP. Curr. Org. Synth. 2004;1:211–226.; Denmark SE, Ober MH. Aldrichim. Acta. 2003;36:75–85.; Denmark SE, Sweis RF. Acc. Chem. Res. 2002;35:835–846. doi: 10.1021/ar020001r.; Denmark SE, Sweis RF. Chem. Pharm. Bull. 2002;50:1531–1541. doi: 10.1248/cpb.50.1531.; Hiyama T, Shirakawa E. Top. Curr. Chem. 2002;219:61–85.; DeShong P, Handy CJ, Mowery ME. Pure Appl. Chem. 2000;72:1655–1658.; Hiyama T. In: Metal Catalyzed Cross-Coupling Reactions. Diederich F, Stang PJ, editors. Wiley-VCH; Weinheim: 1998. Chapter 10.; Hiyama T, Hatanaka Y. Pure Appl. Chem. 1994;66:1471–1478.; Hatanaka Y, Hiyama T. Synlett. 1991:845–853.; Hatanaka Y, Hiyama T. J. Syn. Org. Chem. Jpn. 1990;48:834–843..
- [ii].For leading reviews, see: Grondal C, Jeanty M, Enders D. Nature Chem. 2010;2:167–178. doi: 10.1038/nchem.539.; Fustero S, Sánchez-Roselló M, del Pozo C. Pure Appl. Chem. 2010;82:669–677.; Zhou J. Chem. Asian J. 2010;5:422–434. doi: 10.1002/asia.200900458.; Poulin J, Grisé-Bard CM, Barriault L. Chem. Soc. Rev. 2009;38:3092–3101. doi: 10.1039/b819798a.; Kirsch SF. Synthesis. 2008:3183–3204.; Yu X, Wang W. Org. Biomol. Chem. 2008;6:2037–2046. doi: 10.1039/b800245m.; Chapman CJ, Frost CG. Synthesis. 2007:1–21.; Patil NT, Yamamoto Y. Synlett. 2007:1994–2005.; Padwa A, Bur SK. Tetrahedron. 2007;63:5341–5378. doi: 10.1016/j.tet.2007.03.158.; Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem. Int. Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872.; Topics in Organometallic Chemistry. 2006;19(special issue: Metal Catalyzed Cascade Reactions); Pellissier H. Tetrahedron. 2006;62:2143–2173.; Tietze LF, Brasche G, Gericke K. Domino Reactions in Organic Synthesis. Wiley-VCH; Weinheim: 2006. ; Tietze LF. Chem. Rev. 1996;96:115–136. doi: 10.1021/cr950027e.; Denmark SE, Thorarensen A. Chem. Rev. 1996;96:137–166. doi: 10.1021/cr940277f.; Ho T-L. Tandem Organic Reactions. Wiley; New York: 1992. .
- [iii].For reviews on hydrosilylation, see: Roy AK. Adv. Organomet. Chem. 2008;55:1–59.; Trost BM, Ball ZT. Synthesis. 2005:853–887.; Ojima I, Li Z, Zhu J. In: The Chemistry of Organic Silicon Compounds. Rappoport Z, Apeloig Y, editors. Vol. 2. Wiley; Chichester, UK: 1998. pp. 1687–1792..
- [iv].Denmark SE, Wang Z. Org. Lett. 2001;3:1073–1076. doi: 10.1021/ol0156751. [DOI] [PubMed] [Google Scholar]
- [v].Chandra G, Lo PY, Hitchcock PB, Lappert MF. Organometallics. 1987;6:191–192. [Google Scholar]
- [vi].Trost BM, Ball ZT. J. Am. Chem. Soc. 2001;123:12726–12727. doi: 10.1021/ja0121033. [DOI] [PubMed] [Google Scholar]
- [vii].a) Trost BM, Ball ZT. J. Am. Chem. Soc. 2005;127:17644–17655. doi: 10.1021/ja0528580. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Trost BM, Machacek MR, Ball ZT. Org. Lett. 2003;5:1895–1898. doi: 10.1021/ol034463w. [DOI] [PubMed] [Google Scholar]
- [viii].a) Takahashi K, Minami T, Ohara Y, Hiyama T. Bull. Chem. Soc. Jpn. 1995;68:2649–2656. [Google Scholar]; b) Takahishi K, Minami T, Ohara Y, Hiyama T. Tetrahedron Lett. 1993;34:8263–8266. [Google Scholar]
- [ix].Montenegro J, Bergueiro J, Saá C, López S. Org. Lett. 2009;11:141–144. doi: 10.1021/ol802551a. [DOI] [PubMed] [Google Scholar]
- [x].Matsumoto K, Takeyama Y, Miura K, Oshima K, Utimoto K. Bull. Chem. Soc. Jpn. 1995;68:250–261. [Google Scholar]
- [xi].Denmark S, Choi JY. J. Am. Chem. Soc. 1999;121:5821–5822. [Google Scholar]
- [xii].For a reliable procedure of preparation, see: Buchwald SL, LaMaire SJ, Nielsen RB, Watson BT, King SM. Tetrahedron Lett. 1987;38:3895–3898.; Buchwald SL, LaMaire SJ, Nielsen RB, Watson BT, King SM. Org. Synth., Coll. Vol. 1998;9:162–165. For a recent comprehensive review on hydrozirconation, see: P. Wipf, Top. Organomet. Chem. 2005, 8, 1–25..
- [xiii].Trost BM, Frederiksen MU, Papillon JPN, Harrington PE, Shin S, Shireman BT. J. Am. Chem. Soc. 2005;127:3666–3667. doi: 10.1021/ja042435i. [DOI] [PubMed] [Google Scholar]
- [xiv].Cusack NJ, Reese CB, Risius AC, Roozepeikar B. Tetrahedron. 1976;32:2157–2162. [Google Scholar]
- [xv].Boger DL, Ichikawa S, Zhong W. J. Am. Chem. Soc. 2001;123:4161–4167. doi: 10.1021/ja010195q. [DOI] [PubMed] [Google Scholar]
- [xvi].Saam JC, Speier JL. J. Am. Chem. Soc. 1958;80:4101–4106. [Google Scholar]
- [xvii].a) Denmark SE, Pan W. Org. Lett. 2001;3:61–64. doi: 10.1021/ol006769y. [DOI] [PubMed] [Google Scholar]; b) Tamao K, Kobayashi K, Ito Y. Tetrahedron Lett. 1989;30:6051–6054. [Google Scholar]
- [xviii].For recent examples of intermolecular anti-hydrosilylations: Menozzi C, Dalko PI, Cossy J. J. Org. Chem. 2005;70:10717–10719. doi: 10.1021/jo051637+.; Katayama H, Taniguchi K, Kobayashi M, Sagawa T, Minami T, Ozawa F. J. Organomet. Chem. 2002;645:192–200.; Kawanami Y, Sonoda Y, Mori T, Yamamoto K. Org. Lett. 2002;4:2825–2827. doi: 10.1021/ol026089q.; Trost BM, Ball ZT, Jöge T. J. Am. Chem. Soc. 2002;124:7922–7923. doi: 10.1021/ja026457l.; e) Ref. 6; Na Y, Chang S. Org. Lett. 2000;2:1887–1889. doi: 10.1021/ol0059697.; Mori A, Takahisa E, Kajiro H, Hirabayashi K, Nishihara Y, Hiyama T. Chem. Lett. 1998:443–444..
- [xix].Denmark SE, Pan W. Org. Lett. 2002;4:4163–4166. doi: 10.1021/ol026933c. [DOI] [PubMed] [Google Scholar]
- [xx].Tamao K, Maeda K, Tanaka T, Ito Y. Tetrahedron Lett. 1988;29:6955–6956. [Google Scholar]
- [xxi].Denmark SE, Pan W. Org. Lett. 2003;5:1119–1122. doi: 10.1021/ol0342002. [DOI] [PubMed] [Google Scholar]
- [xxii].Denmark SE, Kobayashi T. J. Org. Chem. 2003;68:5153–5159. doi: 10.1021/jo034064e. [DOI] [PubMed] [Google Scholar]
- [xxiii].a) Ojima I, Vidal E, Tzamarioudaki M, Matsuda I. J. Am. Chem. Soc. 1995;117:6797–6798. [Google Scholar]; b) Monteil F, Matsuda I, Alper H. J. Am. Chem. Soc. 1995;117:4419–4420. [Google Scholar]
- [xxiv].Suginome M, Kinugasa H, Ito Y. Tetrahedron Lett. 1994;35:8635–8638. [Google Scholar]
- [xxv].For reviews on silylcarbocyclization, see: Widenhoefer RA, Bender CF. In: Comprehensive Organometallic Chemistry III. Crabtree RS, Mingos DMP, editors. Elsevier; Amsterdam: 2007. p. 11.11.; Varchi G, Ojima I. Curr. Org. Chem. 2006;10:1341–1362.; Widenhoefer RA. Acc. Chem. Res. 2003;35:905–913. doi: 10.1021/ar010040n.; Ojima I, Moralee AC, Vassar VC. Top. Catal. 2002;19:89–99.; Tamao K, Kobayashi K, Ito Y. Synlett. 1992:539–546..
- [xxvi].a) Denmark SE, Liu JH-C. J. Am. Chem. Soc. 2007;129:3737–3744. doi: 10.1021/ja067854p. [DOI] [PubMed] [Google Scholar]; b) Tamao K, Kobayashi K, Ito Y. J. Am. Chem. Soc. 1989;111:6478–6480. [Google Scholar]
- [xxvii].a) Ojima I, Vu AT, Lee S-Y, McCullagh JV, Moralee AC, Fujiwara M, Hoang TH. J. Am. Chem. Soc. 2002;124:9164–9174. doi: 10.1021/ja0258982. [DOI] [PubMed] [Google Scholar]; b) Maerten E, Delerue H, Queste M, Nowicki A, Suisse I, Agbossou-Niedercorn F. Tetrahedron: Asymm. 2004;15:3019–3022. [Google Scholar]
- [xxviii].Denmark SE, Liu JH-C, Muhuhi JM. J. Am. Chem. Soc. 2009;131:14188–14189. doi: 10.1021/ja9063475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [xxix].For recent reviews on ring-closing metathesis, see: Monfette S, Fogg DE. Chem. Rev. 2009;109:3783–3816. doi: 10.1021/cr800541y.; Hoveyda AH, Zhugralin AR. Nature. 2007;450:243–251. doi: 10.1038/nature06351.; Majumdar KC, Rahaman H, Roy B. Curr. Org. Chem. 2007;11:1339–1365.; Deiters A, Martin SF. Chem. Rev. 2004;104:2199–2238. doi: 10.1021/cr0200872.; McReynolds MD, Dougherty JM, Hanson PR. Chem. Rev. 2004;104:2239–2258. doi: 10.1021/cr020109k.; Yeol S-Y, Chang S. In: Handbook of Metathesis. Grubbs RH, editor. Vol. II. Wiley-VCH; Weinheim: 2004. pp. 5–127.; Trnka TM, Grubbs RH. Acc. Chem. Res. 2001;34:18–29. doi: 10.1021/ar000114f.; Fürstner A. Angew. Chem. Int. Ed. 2000;39:3012–3043. A. Fürstner, Angew. Chem. 2000, 112, 3140–3172..
- [xxx].Schrock RR, Murdzek JS, Bazan GC, Robbins J, DiMare M, O'Regan M. J. Am. Chem. Soc. 1990;112:3875–3886.. For reviews on Schrock's catalyst, see: Schrock RR. Chem. Rev. 2009;109:3211–3226. doi: 10.1021/cr800502p.; Schrock RR. J. Mol. Catal. A: Chem. 2004;213:21–30.; Schrock RR. In: Handbook of Metathesis. Grubbs RH, editor. Vol. I. Wiley-VCH; Weinheim: 2004. pp. 8–32.; Schrock RR, Hoveyda AH. Angew. Chem. Int. Ed. 2003;42:4592–4633. doi: 10.1002/anie.200300576. R. R. Schrock, Hoveyda, A. H. Angew. Chem. 2003, 115, 4740–4782.; Schrock RR. Tetrahedron. 1999;55:8141–8153.; Schrock RR. Top. Organomet. Chem. 1999;1:1–36..
- [xxxi].a) Denmark SE, Yang S-M. Tetrahedron. 2004;60:9695–9708. [Google Scholar]; b) Denmark SE, Yang S-M. J. Am. Chem. Soc. 2002;124:2102–2103. doi: 10.1021/ja0178158. [DOI] [PubMed] [Google Scholar]; c) Denmark SE, Yang S-M. Org. Lett. 2001;3:1749–1752. doi: 10.1021/ol015950j. [DOI] [PubMed] [Google Scholar]; d) Denmark SE, Muhuhi JM. J. Am. Chem. Soc. 2010;132 doi: 10.1021/ja1047363. doi: 10.1021/ja1047363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [xxxii].a) Illuminati G, Mandolini L. Acc. Chem. Res. 1981;14:95–102. [Google Scholar]; b) Liebman JF, Greenberg A. Chem. Rev. 1976;76:311–365. [Google Scholar]
- [xxxiii].For reviews on macrolactonization, see: Sun C-L, Li B-J, Shi Z-J. In: Handbook of Cyclization Reactions. S., editor. Vol. 2. Wiley-VCH; Weinheim: 2010. pp. 1055–1097.; Parenty A, Moreau X, Campagne J-M. Chem. Rev. 2006;106:911–939. doi: 10.1021/cr0301402..
- [xxxiv].a) Denmark SE, Yang S-M. In: Strategies and Tactics in Organic Synthesis. Harmata MA, editor. Vol. 6. Elsevier; Amsterdam: 2005. Chapt. 4. [Google Scholar]; b) Denmark SE, Yang S-M. J. Am. Chem. Soc. 2004;126:12432–12440. doi: 10.1021/ja0466863. [DOI] [PubMed] [Google Scholar]; c) Denmark SE, Yang S-M. J. Am. Chem. Soc. 2002;124:15196–15197. doi: 10.1021/ja028936q. [DOI] [PubMed] [Google Scholar]
- [xxxv].a) Trost BM, Shen HC, Pinkerton AB. Chem. Eur. J. 2002;8:2341–2349. doi: 10.1002/1521-3765(20020517)8:10<2341::AID-CHEM2341>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]; b) Trost BM, Pinkerton AB, Toste FD, Sperrle M. J. Am. Chem. Soc. 2001;123:12504–12509. doi: 10.1021/ja012009m. [DOI] [PubMed] [Google Scholar]; c) Trost BM, Surivet J-P, Toste FD. J. Am. Chem. Soc. 2001;123:2897–2898. doi: 10.1021/ja003870p. [DOI] [PubMed] [Google Scholar]; d) Trost BM, Machacek M, Schnaderbeck MJ. Org. Lett. 2000;2:1761–1764. doi: 10.1021/ol0059504. [DOI] [PubMed] [Google Scholar]; e) Trost BM, Toste FD. Tetrahedron Lett. 1999;40:7739–7743. [Google Scholar]
- [xxxvi].Trost BM, Machacek MR, Faulk BD. J. Am. Chem. Soc. 2006;128:6745–6754. doi: 10.1021/ja060812g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [xxxvii].Talley JJ, Brown DL, Carter JS, Graneto MJ, Koboldt CM, Masferrer JL, Perkins WE, Rogers RS, Shaffer AF, Zhang YY, Zweifel BS, Seibert K. J. Med. Chem. 2000;43:775–777. doi: 10.1021/jm990577v. [DOI] [PubMed] [Google Scholar]
- [xxxviii].a) Baraldi PG, Barco A, Benetti S, Pollini GP, Simoni D. Synthesis. 1987:857–869. [Google Scholar]; b) Kotyatkina AI, Zhabinsky VN, Khripach VA. Russ. Chem. Rev. 2001;70:641–653. [Google Scholar]
- [xxxix].Denmark SE, Kallemeyn JM. J. Org. Chem. 2005;70:2839–2842. doi: 10.1021/jo047755z. [DOI] [PubMed] [Google Scholar]
- [xl].For a review on 1,3-dipolar cycloaddition reaction, see: Jaeger V, Colinas PA. In: Chemistry of Heterocyclic Compounds. Padwa A, Pearson WH, editors. Vol. 59. Wiley; Hoboken: 2002. pp. 361–472..
- [xli].Beaulieu L-PB, Delvos LB, Charette AB. Org. Lett. 2010;12:1348–1351. doi: 10.1021/ol1002863. [DOI] [PubMed] [Google Scholar]
- [xlii].a) Hirabayashi K, Mori A, Hiyama T. Tetrahedron Lett. 1997;38:461–464. [Google Scholar]; b) Hirabayashi K, Takahisa E, Nishihara Y, Mori A, Hiyama T. Bull. Chem. Soc. Jpn. 1998;71:2409–2417. [Google Scholar]
- [xliii].Carré F, Corriu RJP, Kpoton A, Poirier M, Royo G, Young JC, Belin C. J. Organomet. Chem. 1994;470:43–57. [Google Scholar]
- [xliv].Denmark SE, Smith RC, Chang W-TT, Muhuhi JM. J. Am. Chem. Soc. 2009;131:3104–3118. doi: 10.1021/ja8091449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [xlv].a) Denmark SE, Baird JD, Regens CS. J. Org. Chem. 2008;73:1440–1445. doi: 10.1021/jo7023784. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Denmark SE, Baird JD. Org. Lett. 2006;8:793–795. doi: 10.1021/ol053165r. [DOI] [PubMed] [Google Scholar]; c) Denmark SE, Baird JD. Org. Lett. 2004;6:3649–3652. doi: 10.1021/ol048328a. [DOI] [PubMed] [Google Scholar]
- [xlvi].Johnson CN, Stemp G, Anand N, Stephen SC, Gallagher T. Synlett. 1998:1025–1027. [Google Scholar]
- [xlvii].Labadie SS, Teng E. J. Org. Chem. 1994;59:4250–4253. [Google Scholar]
- [xlviii].Denmark SE, Baird JD. Tetrahedron. 2009;65:3120–3129. doi: 10.1016/j.tet.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [xlix].a) Walsh TF, Toupence RB, Ujjainwalla F, Young JR, Goulet MT. Tetrahedron. 2001;57:5233–5241. [Google Scholar]; b) Watson TJN, Horgan SW, Shah RS, Farr RA, Schnettler RA, Nevill CR, Weiberth FJ, Huber EW, Baron BM, Webster ME, Mishra RJ, Harrison BL, Nyce PL, Rand CL, Gorlaski CT. Org. Process Res. Dev. 2000;4:477–487. [Google Scholar]; c) Jacotot B, Banga JD, Pfister P, Mehra M. Br. J. Clin. Pharmacol. 1994;38:257–263. doi: 10.1111/j.1365-2125.1994.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [l].Larock RC, Yum EK, Refvik MD. J. Org. Chem. 1998;63:7652–7662. [Google Scholar]
- [li].a) Walker SD, Barder TE, Martinelli JR, Buchwald SL. Angew. Chem. Int. Ed. 2004;43:1871–1876. doi: 10.1002/anie.200353615. [DOI] [PubMed] [Google Scholar]; b) Barder TE, Walker SD, Martinelli JR, Buchwald SL. J. Am. Chem. Soc. 2005;127:4685–4696. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
- [lii].Milne JE, Buchwald SL. J. Am. Chem. Soc. 2004;126:13028–13032. doi: 10.1021/ja0474493. [DOI] [PubMed] [Google Scholar]