Abstract
Obesity causes sympathetic activation that promotes atherosclerosis, end-organ damage, and hypertension. Because high-fat induced weight gain in rats elevates plasma leptin at 1–3 days following onset of calorie dense diets, we hypothesized that diet-induced overfeeding will increase sympathetic activity within one week following onset of the regimen. To test this, we continuously measured sympathetic activity and blood pressure before and during the onset of diet-induced obesity using a high calorie cafeteria-style diet. Female Wistar rats, in which radiotelemeters had been implanted for continuous monitoring of lumbar sympathetic activity, mean arterial pressure, and heart rate, were randomly assigned to groups that received regular chow (control) or a cafeteria diet for a period of 15 days. This short-term cafeteria-feeding regimen caused modest but non-significant increases in body weight (P = 0.07) and a doubling of brown and white adipose tissue (P < 0.01). The increases in fat mass were accompanied by elevations in plasma leptin (P < 0.001) but no change in glucose. Overall heart rates and blood pressure were higher in cafeteria rats compared with controls (P < 0.05). Cafeteria diet-induced weight gain caused increases in lumbar sympathetic nerve activity that became significant by the 12th day of the diet (p < 0.001). These data show, for the first time, that the high-fat cafeteria-style diet stimulates sustained increases in lumbar sympathetic neural drive in rats.
Keywords: obesity, cafeteria diet, sympathetic nerve activity, leptin, blood pressure
INTRODUCTION
Accumulating evidence indicates that weight gain and obesity result in activation of the sympathetic nervous system. For example, obese hypertensive patients have increased muscle sympathetic nerve activity (SNA) compared with lean controls 1, and weight loss causes marked reductions in SNA 2. In addition, experimental animals and humans fed high fat diets show increases in renal and muscle SNA 3–7. This sympathetic over-activity, caused by obesity, may contribute to several complications that characterize this disease, including atherosclerosis, end-organ damage, left ventricular hypertrophy, and hypertension. In support of this, sympathetic activation causes growth promotion in vascular smooth muscle and myocardium 8, 9, stimulation of platelet number and aggregability 10, and mechanical injury of the vascular wall 8. Finally, increases in renal SNA may contribute to impaired pressure natriuresis that predisposes towards the development of hypertension 11.
Despite the importance of the link between obesity and sympathetic activation, the mechanisms responsible for the increases in SNA are not completely understood. For example, obesity-induced elevations in leptin, insulin, angiotensin II, adiponectin, and inflammation have been proposed as potential activators of sympathoexcitation 12. One way to better understand how weight gain increases SNA is to establish the time-course of sympathetic increases during the initial stages of diet-induced obesity. Prior and colleagues 6 demonstrated that 3 weeks of high fat feeding to rabbits caused increases in both leptin and renal SNA. However, it is unknown whether the SNA increased prior to its measurement at 3 weeks. Because high-fat feeding in rats significantly elevates abdominal fat mass by 3–7 days 13, 14 with corresponding increases in plasma leptin at 1–3 days following onset of the diets 15, 16, we hypothesized that diet-induced overfeeding will increase SNA within one week following onset of the regimen. To test this possibility, we continuously measured lumbar SNA, using telemetry, before and during the first 2 weeks of diet-induced obesity in freely moving rats. We selected female Wistar rats and the cafeteria diet because this combination produces rapid increases in both body weight and fat mass 17, 18.
METHODS
Animals
Female Wistar rats, weighing 200–225g, were purchased from Harlan Laboratories (Indianapolis, IN). Upon arrival, the rats were placed on regular rat chow (5001 Lab Diet, PMI Nutrition International, Brentwood, MO) and given water ad libitum for at least one week prior to entry into the protocol. They were housed individually in standard polycarbonate cages on pine-shaving bedding in a temperature controlled colony room illuminated on a 12:12 light-dark cycle. All procedures were performed in accordance with the Lehman College Institutional Animal Care and Use Committee and the National Institutes of Health guidelines for the care and use of experimental animals.
Experimental Procedure
After one week of habituation, a telemetry-based recording electrode and blood pressure monitor (model TR46SP, Telemetry Research, Auckland, New Zealand) was placed on the lumbar sympathetic nerve and in the abdominal aorta, respectively, under isoflurane anesthesia (please see http://hyper.ahajournals.org for surgery and SNA recording details). After surgery, rats were housed individually and lumbar SNA and blood pressure were recorded continuously with a radiotelemetry system, consisting of the implanted device, a battery-charging device placed underneath the cage (model TR802, Telemetry Research), and a telemetry receiver for both blood pressure and SNA (model TR162, Telemetry Research) connected to a PowerLab data acquisition system (ADInstruments, Castle Hill, Australia) interfaced to a Macintosh computer. Heart Rate was calculated from the blood pressure pulse using the PowerLab system. During surgery for implantation of the radiotelemetry device (described above), a 0.5 ml fasting blood sample was taken, centrifuged, and the plasma was stored at −80° C.
The animals were allowed 4 days of post-surgery recovery, followed by a 2-day baseline period, during which blood pressure, heart rate, and lumbar SNA were recorded continuously. The rats were then randomly assigned to 2 experimental groups: a control group (n = 7) that received water and regular rat chow (5001 Lab Diet), and a cafeteria group (n = 7) that received, along with regular rat chow and water, vanilla wafers, crackers, buttered popcorn, cheetos, chocolate, salami, smoked fish, peanuts, shredded wheat, condensed milk (mixed 1:1 in water), and soda pop. To encourage over-consumption, 5 of the 9 solid items were randomly offered each day and the choices were alternated on a daily basis. In addition, these items were given in excess and were replaced every day. The control diet contained (%/weight) 23.0% protein, 48.7% carbohydrate, 4.5% lipid, and 23.8% other components without caloric value; the average composition of the cafeteria diet was (%/weight) 9.5% protein, 48.1% carbohydrate, 23.4% lipid, and 19% water and other materials. The control diet contained 4.0 mg sodium/g diet and the cafeteria diet averaged 5.2 mg sodium/g diet. Blood pressure, heart rate, and lumbar SNA were recorded continuously for 15 days on these dietary regimens.
At study completion, over-night fasted rats were anesthetized (isoflurane), a 0.5 ml blood sample was taken, and SNA as well as blood pressure signal quality were assessed (please see http://hyper.ahajournals.org). The gondal and peri-renal white adipose tissues (WAT), as well as the interscapular brown adipose tissues (IBAT) were excised, rinsed in saline, patted dry, and weighed. Plasma leptin was analyzed using an RIA kit with antibodies to authentic rat leptin (Millipore, Billerica, MA). Plasma glucose was determined using a GM9 autoanalyser (Analox Instruments, London, UK). The intra-assay and inter-assay coefficient of variation (CV) values for leptin were 4.3% and 6.0%, respectively, and for glucose, they were 0.6% and 3.0%, respectively.
Statistical analysis
All data were analyzed using appropriate single or repeated measures analysis of variance (ANOVA) and post-hoc comparisons were made using the Holm-Bonferroni method when the global F ratio was significant (GraphPad PRISM 5, Graphpad Software Inc., San Diego, CA). Data are presented as means ± SEM. Differences between groups were considered significant at the p < 0.05 level.
RESULTS
Effect of the Cafeteria Diet on Body Weight, adipose tissue, and hormones
Body weights did not differ between the groups before onset of the diets. During the 15-day feeding period, cafeteria-fed rats gained slightly more weight than controls (Table 1). ANOVA of these data revealed a significant effect for time (P < 0.0001) and a borderline significant group by repeated measures interaction (P = 0.07), indicating a tendency for more weight gain in cafeteria rats. Cafeteria rats also showed 2- to 3-fold greater WAT and IBAT weights than controls after 15 days on the diets (Table 1; P < 0.01).
Table 1.
Effect of cafeteria feeding on body weight, fat mass, and on blood levels of glucose and leptin
| Measure | Control Females | Cafeteria Females | ||
|---|---|---|---|---|
|
| ||||
| Day 1 | Day 17 | Day 1 | Day 17 | |
| Body weight (g) | 257.0 ± 8.1 | 284.3 ± 6.1 | 259.3 ± 9.7 | 305.7 ± 11.5 |
| Gonadal WAT (g) | --- | 1.0 ± 0.1 | --- | 2.2 ± 0.2* |
| Peri-renal WAT (g) | --- | 3.6 ± 0.9 | --- | 6.8 ± 0.6* |
| IBAT (g) | --- | 0.4 ± 0.02 | --- | 1.3 ± 0.2* |
| Plasma Glucose (mmol/L) | 8.4 ± 0.6 | 9.4 ± 0.5 | 9.1 ± 0.5 | 7.3 ± 0.6 |
| Plasma Leptin (ng/ml) | 0.87 ± 0.17 | 0.64 ± 0.15 | 1.03 ± 0.23 | 1.93 ± 0.19† |
All values are means ± SEM; WAT, white adipose tissue; IBAT, interscapular brown adipose tissue;
P < 0.01 compared with Control day 17;
P < 0.001 compared with Control day 17.
Cafeteria diet feeding did not affect plasma glucose. In contrast, plasma leptin levels nearly doubled in cafeteria rats (P < 0.001) but did not change in control animals (Table 1).
Effect of the Cafeteria Diet on Lumbar SNA, MAP, and HR
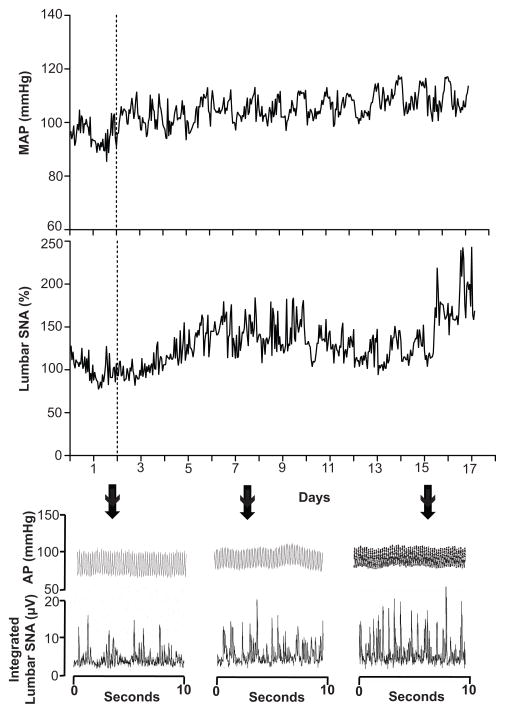
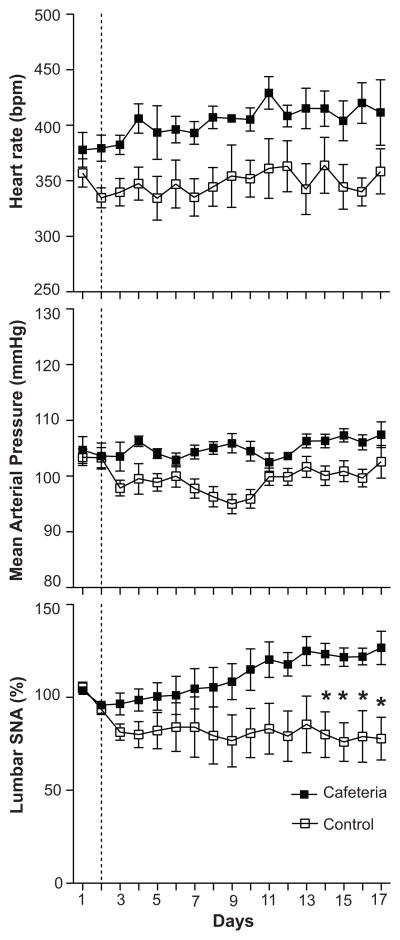
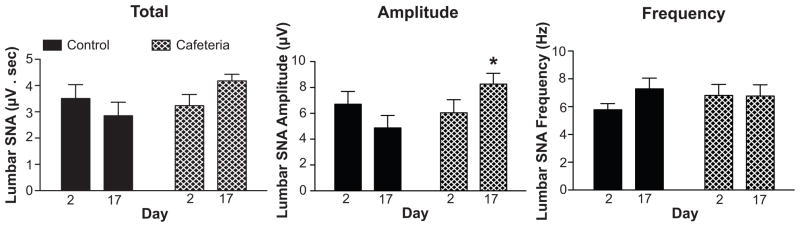
Representative lumbar SNA and MAP responses to the cafeteria diet in a single rat are shown in Figure 1. Analysis of the group data revealed that lumbar SNA, expressed as percentage change from baseline, decreased slightly in control rats during baseline, followed by unchanged SNA until the end of the study (Figure 2). In contrast, cafeteria rats displayed mild decreases during baseline, followed by increases in lumbar SNA that became significant by the 12th day on the diet. Because we used a post-surgery recovery period of 4 days, it is possible that the decreases in SNA in both groups during the first 2–3 days were due to after-surgery recovery. Nevertheless, the control and cafeteria rats received exactly the same post-surgery recovery period, and analysis of the raw SNA values during the 2-day baseline period failed to reveal significant differences between the 2 groups (please see http://hyper.ahajournals.org). ANOVA of the group data revealed a significant effect for group (P = 0.03) and a group by repeated measures interaction (P < 0.001), reflecting increasing SNA in the cafeteria group that became significant (P < 0.05) during the last 4 days of observation (Figure 2). Analysis of the integrated SNA signal, expressed as μV·sec, revealed a similar group by time interaction (P = 0.001), indicating increasing SNA in the cafeteria group (please see http://hyper.ahajournals.org). Further analyses comparing SNA at baseline (day 2) with the last day of observation (day 17) revealed that cafeteria feeding increased lumbar SNA primarily through increases in burst amplitude and not through changes in burst frequency (Figure 3). For both total lumbar SNA and amplitude of SNA bursts in Figure 3, there were significant group by time interactions (P = 0.02 and P = 0.006, respectively). Our data also indicated that cafeteria feeding increased lumbar SNA during the entire 24 hr period. In support of this, separate ANOVAs of the 12-hr day periods and 12-hr night periods revealed precisely the same findings (please see http://hyper.ahajournals.org). In addition, ANOVA of the rise in SNA from the day periods to the night periods showed no difference between the two groups (data not shown). A testing of nerve signal quality on the final day of the study showed blood pressure decreases to intravenous sodium nitroprusside (from 94.0 ± 5.4 mmHg to 76.8 ± 7.2 mmHg) and to intravenous hexamethonium bromide (to 55.0 ± 3.8 mmHg). Lumbar SNA responses to baroreceptor unloading with sodium nitroprusside (100% to 127.9 ± 6.7%) and to ganglionic blockade using hexamethonium (100% to 15.3 ± 3.1%) were appropriate and similar to previously published values (please see http://hyper.ahajournals.org) 19, 20.
FIGURE 1.
Representative data showing hourly values for mean arterial pressure (MAP) and lumbar sympathetic nerve activity (SNA) in a single rat before (days 1–2) and during (days 3–17) presentation of the cafeteria diet. Bottom shows 10-second traces of arterial pressure (AP) integrated lumbar SNA on indicated days.
FIGURE 2.
Group data showing heart rate, mean arterial pressure (MAP), and lumbar sympathetic nerve activity (SNA) responses before (days 1–2) and during (days 3–17) control diet or cafeteria diets in rats. Values are means ± SEM. * P < 0.05, cafeteria rats vs. control rats.
FIGURE 3.
Lumbar SNA as total activity expressed as the area under the integrated SNA curves (μV·sec), as amplitude in μV, and as frequency in bursts per second from rats during baseline (day 2) and after 15 days of control or cafeteria diet feeding (day 17). Data are means ± SEM. *P < 0.05 for control day 17 vs. cafeteria day 17.
Twenty-four-hour MAP tended to decrease in controls contrasting with no change in cafeteria fed rats (Figure 2). ANOVA of these data revealed a significant effect for group (P = 0.002), reflecting overall higher levels in cafeteria rats, for time (P = 0.02), and a borderline significant group by time interaction (P = 0.09). Overall HRs were higher in cafeteria rats, as reflected by a significant effect for group (P = 0.03), but there was no interaction (Figure 2).
DISCUSSION
The major finding from this study is that short-term cafeteria-style feeding stimulated increases in directly recorded lumbar SNA from conscious rats. Furthermore, these radiotelemetry data revealed that lumbar SNA elevations in cafeteria rats were sustained throughout the 24-hr period, during both the day and night cycles. These SNA increases occurred with only modest elevations in body weight but with large increases in WAT, BAT, and plasma leptin.
Previous studies with rats have shown that high fat diets for 10–20 weeks stimulate increases in renal SNA 3, 5, 7. In humans, 10–12 weeks of overfeeding with Boost Plus resulted in modest weight gain and elevated muscle SNA 4. In a shorter-term study, 3 weeks of high fat feeding to rabbits also produced significant increases in renal SNA 6. The present study extended these findings by showing that sympathetic increases to overfeeding are significant by the 12th day on the diet and are sustained over each of the 24-hr periods. The elevation in lumbar SNA can be attributed to increased sympathetic burst amplitude (Figure 3), indicating an increasing number of recruited nerve fibers 21. This pattern of increased sympathetic burst amplitude without a change in burst frequency is similar to that in rabbits fed high fat and in obese humans 6, 22 but differs from humans given Boost Plus who exhibited increases in muscle SNA burst frequency 4.
Although it is unclear how increased adiposity elevates lumbar SNA, several lines of evidence point to the hormone leptin. We observed a near doubling of plasma leptin after only 15 days of cafeteria feeding, which agrees with others reporting increasing leptin after only 7 days on a high fat diet 23. Supporting a sympathoexcitatory role for leptin, both intravenous and intracerebroventricular leptin administration have been shown to increase directly recorded brown adipose tissue, renal, and lumbar SNA 24, 25. Passage of leptin through the blood brain barrier allows concentrations of cerebrospinal fluid leptin to closely follow changes in blood levels of the hormone 26. Once in the central nervous system, leptin may act in the ventromedial hypothalamus (VMH) and arcuate nucleus (ARC) to increase SNA. In support of this, microinjection of leptin into these two areas increases SNA, whereas lesions of the VMH and ARC or ablation of leptin receptors in the ARC abolishes increases in SNA to intravenous leptin 27–29.
Even though cafeteria diet feeding stimulated chronic increases in lumbar SNA, the regimen did not clearly elevate MAP compared with controls. It is likely that a longer period of overfeeding is necessary to stimulate increases in blood pressure since most 30, 31 but not all 32 cafeteria diet studies demonstrated elevations in blood pressure after 8–20 weeks on the diets. As with SNA increases, blood pressure elevations may be secondary to increases in circulating leptin, since both intravenous and intracerebroventricular administration of leptin to rats have been shown to produce slowly developing increases in arterial pressure 33, 34. In support of a pro-hypertensive role for leptin, deletion of leptin receptors in the arcuate nucleus of mice abolished both sympathoexcitatory responses to intracerebroventricular leptin and increases in blood pressure to 20 weeks of diet-induced obesity 27.
There were a number of limitations to the present study. First, our sample size was relatively small due to the difficulty in obtaining long-term SNA recordings. A second limitation was that we included only female Wistar rats. Previous studies have shown that cafeteria feeding stimulates smaller decreases in adiponectin and resistin in female Wistar rats compared with males 17, and smaller increases in blood pressure in females 35. Therefore, sympathetic increases to the cafeteria diet may also be different in males, and our finding should not be extrapolated beyond the population studied. Third, we did not measure food or calorie intake in the present study. Nevertheless, several previous experiments using similar cafeteria diets over the same time period as in our experiment documented 30–40% increases in daily energy intake compared with rats on regular chow 36, 37. In addition, the elevations in body weight, WAT, and IBAT observed in the current study were very similar to those, over identical time periods, in the same published studies 36, 37. A final limitation was that we recorded SNA only from the lumbar nerve, which sends sympathetic signals to muscle and skin vasculature in the hindlimb of the rat. We therefore cannot extrapolate the present SNA findings to other vascular beds, although others have reported increases in renal and muscle SNA following high-fat diet-induced obesity 3, 5–7.
Supplementary Material
Perspectives.
By using telemetry-based 24-hr recordings of sympathetic nerve activity in freely moving rats, the current work established that feeding the high-calorie cafeteria diet produces increases in both fat mass and lumbar SNA. The present data therefore provide further key evidence that diet-induced increases in adiposity activates chronic increases in sympathetic nerve activity, indicating that the current obesity epidemic may also result in an epidemic of sympathetic over-activation. The mechanism of this early rise in sympathetic activity is likely to involve rapid increases in plasma leptin levels, although other hormones may play a role in later stages of the sympathoexcitation. A determination of whether diet-induced elevations in sympathetic activity can be abolished, such as by eliminating the actions of leptin in the hypothalamus, will require further research.
Novelty and Significance.
What is new?
To our knowledge, these are the first telemetry-based 24-hr recordings of sympathetic nerve activity in freely moving rats.
The high-calorie cafeteria-style diet stimulated immediate increases in lumbar sympathetic nerve activity that became significant by the 12th day on the diet.
The increases in sympathetic activity, caused by the cafeteria diet, were sustained over the entirety of the 24-hr periods.
What is relevant?
The increases in sympathetic activity that accompany weight gain are thought to be a primary mechanism through which obesity results in hypertension.
Summary
The cafeteria diet stimulated rapid increases in fat mass and plasma leptin levels in rats.
The increases in fat mass and leptin were accompanied by rapid elevations in lumbar sympathetic nerve activity, measured by telemetry.
Despite the increase in sympathetic activity, 15 days of cafeteria feeding did not clearly affect arterial blood pressure or heart rate.
Acknowledgments
The authors would like to thank the kind assistance of Yim Dam and the New York Obesity Research Center, St. Luke’s/Roosevelt Hospital Center, New York, NY, for performing the insulin, leptin, and glucose assays.
Sources of Funding: This work was supported in part by NIH award 1SC1DK083062.
Footnotes
Conflict of Interest: None
References
- 1.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 2.Grassi G, Seravalle G, Columbo M, Bolla G, Lanfranchi A, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflexin obese normotensive humans. Circulation Research. 1998;97:2037–2042. doi: 10.1161/01.cir.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 3.Barnes MJ, Lapanowski K, Conley A, Rafols JA, Jen KLC, Dunbar JC. High fat feeding is associated with increased blood pressure sympathetic nerve activity and hypothalamic mu opioid receptors. Brain Research Bulletin. 2003;61:511–519. doi: 10.1016/s0361-9230(03)00188-6. [DOI] [PubMed] [Google Scholar]
- 4.Gentile CL, Orr JS, Davy BM, Davy KP. Modest weight gain is associated with sympathetic neural activation in non-obese humans. Am J Physiol. 2007;292:R1834–R1838. doi: 10.1152/ajpregu.00876.2006. [DOI] [PubMed] [Google Scholar]
- 5.Iwashita S, Tanida M, Terui N, Ootsuka Y, Shu M, Kang D, Suzuki M. Direct measurement of renal sympathetic nervous activity in high-fat diet-related hypertensive rats. Life Sciences. 2002;71:537–546. doi: 10.1016/s0024-3205(02)01707-1. [DOI] [PubMed] [Google Scholar]
- 6.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55:862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 7.Tanida M, Iwashita S, Terui N, Ootsuka Y, Shu M, Kang D, Suzuki M. Effect of peripheral administration of leptin on the renal sympathetic nerve activity in high-fat diet-related hypertensive rats. Life Sciences. 2006;78:1149–1154. doi: 10.1016/j.lfs.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 8.Julius S. The evidence for a pathophysiologic significance of the sympathetic overactivity in hypertension. Clin Exper Hypertens. 1996;18 (3&4):305–321. doi: 10.3109/10641969609088965. [DOI] [PubMed] [Google Scholar]
- 9.Mancia G, Di Rienzo M, Parati G, Grassi G. Sympathetic activity, blood pressure variability and end organ damage in hypertension. J Hum Hypertens. 1997;11 (Suppl 1):S3–S8. [PubMed] [Google Scholar]
- 10.Sloan JA, Hooper M, Izzo JLJ. Effects of circulating norepinephrine on platelets, leukocyte and rbc counts by alpha1-adrenergic stimulation. Am J Cardiol. 1989;63:1140–1142. doi: 10.1016/0002-9149(89)90096-9. [DOI] [PubMed] [Google Scholar]
- 11.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–897. doi: 10.1161/01.hyp.25.4.893. [DOI] [PubMed] [Google Scholar]
- 12.Dorresteijn JAN, Visseren FLJ, Spiering W. Mechanisms linking obesity to hypertension. Obesity Reviews. 2012;13:17–26. doi: 10.1111/j.1467-789X.2011.00914.x. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier M-S, Favier R, Lavoie J-M. Time course of the development of non-alcoholic hepatic steatosis in response to high-fat diet-induced obesity in rats. Br J Nutr. 2006;95:273–281. doi: 10.1079/bjn20051635. [DOI] [PubMed] [Google Scholar]
- 14.Rizkalla SW, Mandenoff A, Betoulle D, Boillot J, Apfelhaum M. Decreased insulin binding to adipocytes precedes both hyperinsulinemia and decreased insulin binding to erythrocytes in cafeteria-fed rats. Int J Obes. 1987;11:493–505. [PubMed] [Google Scholar]
- 15.Levin BE, Dunn-Meynell AA, Ricci MR, Cummings DE. Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am J Physiol. 2003;285:E949–E957. doi: 10.1152/ajpendo.00186.2003. [DOI] [PubMed] [Google Scholar]
- 16.Ziotopoulou M, Mantzoros CS, Hileman SM, Flier JS. Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. Am J Physiol. 2000;279:E838–E845. doi: 10.1152/ajpendo.2000.279.4.E838. [DOI] [PubMed] [Google Scholar]
- 17.Ribot J, Rodriguez AM, Rodriguez E, Palou A. Adiponectin and resistin response in the onset of obesity in male and female rats. Obesity. 2008;16:723–730. doi: 10.1038/oby.2008.113. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez AM, Quevedo-Coli S, Roca P, Palou A. Sex-dependent dietary obesity, induction of ucps, and leptin expression in rat adipose tissues. Obes Res. 2001;9:579–588. doi: 10.1038/oby.2001.75. [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Duanmu Z, Scislo T, Dunbar JC. The co-existence of insulin-mediated decreased mean arterial pressure and increased sympathetic nerve activity is not mediated by baroreceptor reflex and differentially by hypoglycemia. Clin Exper Hypertens. 1998;20(2):165–183. doi: 10.3109/10641969809053213. [DOI] [PubMed] [Google Scholar]
- 20.Scislo TJ, Augustyniak AR, O’Leary SD. Differential arterial baroreflex regulation of renal, lumbar, and adrenal sympathetic nerve activity in the rat. Am J Physiol. 1998;44:R995–R1002. doi: 10.1152/ajpregu.1998.275.4.R995. [DOI] [PubMed] [Google Scholar]
- 21.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 22.Lambert E, Stranicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50:862–868. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- 23.Cruciani-Guglielmacci C, Vincent-Lamon M, Rouch C, Orosco M, Ktorza A, Magnan C. Early changes in insulin secretion and action induced by high-fat diet are related to a decreased sympathetic tone. Am J Physiol. 2004;288:E148–E154. doi: 10.1152/ajpendo.00225.2004. [DOI] [PubMed] [Google Scholar]
- 24.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumber and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- 25.Haynes WG, Morgan AD, Walsh SA, Mark AL, Sivitz WI. Receptor mediated regional sympathetic nerve activition by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin in the human hypothalamus. J Clin Endocrinol Metab. 2007;92:1129–1136. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 27.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circulation Research. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh AJ, Fontes MAP, Killinger S, Pawlak DB, Polson JW, Dampney RAL. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42:488–493. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- 29.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49(part 2):647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 30.Coatmellec-Taglioni G, Dausse J-P, Ribiere C, Giudicelli Y. Hypertension in cafeteria-fed rats: Alterations in renal alpha2-adrenoceptor subtypes. Am J Hypertens. 2000;13:529–534. doi: 10.1016/s0895-7061(99)00234-4. [DOI] [PubMed] [Google Scholar]
- 31.Howitt L, Sandow SL, Grayson TH, Ellis EZ, Morris MJ, Murphy TV. Differential effects of diet-induced obesity on bkca b1-subunit expression and functional in rat skeletal muscle arterioles and small cerebral arteries. Am J Physiol. 2011;301:H29–H40. doi: 10.1152/ajpheart.00134.2011. [DOI] [PubMed] [Google Scholar]
- 32.Sishi B, Loos B, Ellis B, Smith W, du Toit EF, Engelbrecht AM. Diet-induced obesity alters signalling pathways and induce atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Experimental Physiology. 2010;96.2:179–193. doi: 10.1113/expphysiol.2010.054189. [DOI] [PubMed] [Google Scholar]
- 33.Correia MLG, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Leptin acts in the central nervous sytem to produce dose dependent changes in arterial pressure. Hypertension. 2001;37:936–942. doi: 10.1161/01.hyp.37.3.936. [DOI] [PubMed] [Google Scholar]
- 34.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 35.Plut C, Ribiere C, Giudicelli Y, Dausse J-P. Hypothalamic leptin receptor and signaling molecule expressions in cafeteria diet-fed rats. J Pharm Exper Ther. 2003;307:544–549. doi: 10.1124/jpet.103.054726. [DOI] [PubMed] [Google Scholar]
- 36.Chaves VE, Frasson D, Martins-Santos MES, Navagantes LCC, Galban VD, Garofalo MAR, Kettelhut IC, Migliorini RH. Fatty acid synthesis and generation of glycerol-3-phosphate in brown adipose tissue from rats fed a cafetaria diet. Can J Physiol Pharmacol. 2008;86:416–423. doi: 10.1139/y08-052. [DOI] [PubMed] [Google Scholar]
- 37.Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to a high-fat diet. Obesity. 2011;19:1109–1117. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.