Abstract
The nociceptive transmission under pathological chronic pain conditions involves transcriptional and/or translational alteration in spinal neurotransmitters and receptors expression, and modification of neuronal function. Studies indicate the involvement of MicroRNA (miRNA)-mediated transcriptional deregulation in pathophysiology of acute and chronic pain. In the present study, we tested the hypothesis that long-term cross-organ colonic hypersensitivity in neonatally zymosan-induced cystitis is due to miRNA-mediated posttranscriptional suppression of the developing spinal GABAergic system. Cystitis was produced by intravesicular injection of zymosan (1% in saline) into the bladder during postnatal (P) days P14 through P16 and spinal dorsal horns (L6-S1) were collected either on P60 (unchallenge groups) or on P30 following a zymosan re-challenge on P29 (re-challenge groups). miRNA arrays and Real-time reverse transcription polymerase chain reaction revealed significant, but differential, upregulation of mature miR-181a in the L6-S1 spinal dorsal horns from zymosan-treated rats compared with saline controls in both unchallenge and re-challenge groups. The target gene analysis demonstrated multiple complementary binding sites in miR-181a for GABAA receptor subunit GABAAα−1 gene with a miRSVR score of −1.83. Increase in miR-181a concomitantly resulted in significant downregulation of GABAAα−1 receptor subunit gene and protein expression in adult spinal cords from neonatal cystitis rats. Intrathecal administration of GABAA receptor agonist muscimol failed to attenuate viscero-motor response (VMR) to colon distension in neonatal cystitis rats, whereas, in adult zymosan-treated rats the drug produced significant decrease in VMR. These results support an integral role for miRNA-mediated transcriptional deregulation of GABAergic system in neonatal cystitis-induced chronic pelvic pain.
Keywords: Neonatal cystitis, Chronic pelvic pain, Micro RNA, Posttranscriptional deregulation, GABAA receptor, Colonic hypersensitivity
Introduction
Chronic pelvic pain is one of the most frequent disorders of female and estimated medical cost for the treatment is more than two billion dollar per year [28]. The etiology of chronic pelvic pain is still poorly understood. Several clinical findings suggest the involvement of a neural mechanism and pathophysiological cross-talk by which sensory and motor dysfunction of one internal organ can secondarily affect other pelvic organs or somatic structures [31;43;46]. Moreover, early-in-life exposure to noxious and/or inflammatory stimuli has been reported to enhance the vulnerability of the organism to subsequent pathological challenges in the adult life by producing long-lasting neuroanatomical and neurophysiological changes in the nociceptive system [1;17;25;37;45]. Randich et al., (2006) reported that bladder inflammation in neonate rat led to accentuated visceral-motor responses (VWR) to urinary bladder distension (UBD) when tested as adults [36]. In our recent study, neonatal cystitis in rats exhibited a hypersensitive response to colonic distension during their adulthood indicating a long-term effect and the development of overlapping chronic pelvic pain. This heightened response is maintained despite the absence of inflammation in bladder and colon tissues, and without long-lasting change in mechanotransduction properties of pelvic nerve afferent (PNA) fibers innervating the colon or bladder to visceral distension [29]. DeBerry et al (2010) reported that neonatal zymosan-induced bladder inflammation impaired the development and expression of spinal opioid system in adulthood, suggesting that the neonatal insult during development may permanently alter the central modulatory pathway which could be one of the causes of cross-organ (viscera-visceral and viscera-somatic) secondary hyperalgesia [14]. However, neuromolecular mechanism underlying this long-term sensitization has not been well defined.
The long-lasting nociceptive transmission under various pathological chronic pain conditions involve transcriptional and/or translational alteration in neurotransmitter and receptor expression as well as modification of neuronal function, morphology and synaptic connections [11;13;47;49]. Although it is largely unknown how such changes in gene expression induce chronic pain, recent evidence strongly suggests an important role for microRNAs (miRNAs, small non-coding RNAs) in the cellular plasticity underlying chronic pain. miRNAs are the major transcriptional regulators of gene expression and the involvement of miRNA-mediated gene regulation in pathophysiology of acute and chronic pain has recently been documented [2;3;18;35;40;41;44]. The involvement of miRNAs in inflammatory pain processing has also been established in an in vivo study using conditional Dicer (miRNA synthesizing enzymes) knockout Nav1.8 mice [48].
The present study was undertaken to test the hypothesis that long-term colonic hypersensitivity in neonatally zymosan-induced cystitis is due to miRNA-mediated posttranscriptional suppression of the developing spinal GABAergic system. We specifically carried out the identification of miRNAs and their target genes involved with GABAergic neurotransmission. We further examined the miRNA and GABAAα−1 receptor subunit gene interaction in lumbo-sacral (L6-S1, receiving inputs from both the urinary bladder and colon) spinal cord in adult rats using the rat model of neonatal cystitis-induced colonic hypersensitivity. We further examined the functional contributon of GABAA receptor agonist muscimol in neonatal vs adult cystitis rats.
Methods
Animal housing and handling
The study was performed on female Sprague–Dawley rats weighing 200-250 g. Time pregnant female rats were obtained from Harlan (Indianapolis, IN, USA) and maintained in separate cages. Rats were kept under controlled conditions with a 12 h light/dark schedule and had free access to both food and water ad libitum. Rat pups were born in our facility and only female pups were used in this study. The pups remained housed with their mothers until they were weaned at post natal (PN) day 21. For behavioral studies, 12h before recording, the animals were placed in a wire-bottom cage and access to food, was denied in order to empty the colon. The Institutional Animal Care and Use Committee at Medical College of Wisconsin approved all experimental protocols (MCW AUA 355). All experiments were performed according to the approved guidelines of the Institutional Animal Care and Use committee at the Medical College of Wisconsin and The International Association for the Study of Pain (IASP).
Induction of cystitis in neonatal (P14-P16) rats
Cystitis was produced by intravesicular injection of zymosan (1% in saline) into the bladder. Zymosan is a yeast cell wall derived carbohydrate known to induce local inflammatory process. Female pups (P14-P16) were lightly anesthetized with isoflurane (flow rate 2ml/min), the vaginal areas were swabbed clean with Betadine, and 100μl of zymosan or saline solution was injected into the bladder by inserting a shielded i.v. catheter (BD Insyte-N Autoguard, 24GA) transurethrally. The solution was left inside the bladder for 30 minutes and then the pups were returned to the cage. This procedure was repeated for three days. In adult rats, zymosan was injected in similar fashion for three days at P40-P43, but the volume of injection into the bladder was 200μl. The VMR studies were carried out at P60 for both neonate and adult zymosan-treated rats. For molecular studies, two different treatment protocols were used. In the first protocol, pups received either zymosan or saline on P14 through P16 and tissues were collected on P60 (unchallenge groups). In second protocol, a re-challenge with zymosan or saline was carried out on P29 (when the neurons were fully developed) following the neonatal treatment as mentioned in first protocol and tissues were collected on the next day at P30 (re-challenge groups).
miRNA array and bioinfomatic analysis
Total RNA was quantified on a NanoDrop ND-1000 spectrophotometer followed by RNA quality assessment on an Agilent 2100 bioanalyzer (Agilent, Palo Alto, CA, USA). RNA labeling was performed by the FlashTag Biotin RNA Labeling Kit for Affymetrix® GeneChip® miRNA Arrays (Genisphere, Hatfield, PA). Each Affymetrix® GeneChip® miRNA Arrays (Affymetrix, Santa Clara, CA) was hybridized with Flash Tag Biotin Labeled total RNA (500 ng) from experimental and control samples in 100μl hybridization cocktail. Target denaturation was performed at 99°C for 5 min and then 48°C for 5 min followed by hybridization for 18 hrs. Arrays were washed and stained using Genechip Fluidic Station 450, and hybridization signals were amplified using antibody amplification with goat IgG (Sigma-Aldrich) and anti-streptavidin biotinylated antibody (Vector Laboratories, Burlingame, CA). Chips were scanned on an Affymetrix Gene Chip Scanner 3000, using Command Console Software. MiRNA data were analyzed by Affymetrix MiRNA QC tool. Raw data were normalized and analyzed using GeneSpring software version 7.2 (Silico Genetics, Redwood, CA). Expression of each microRNA was normalized to average median. Background correction and normalization were carried out using Robust Multichip Average (RMA) with Genespring v 10.0 software (Agilent, Palo Alto, CA). We analyzed different algorithms to identify relevant targets for individual miRNAs using MiRanda, TargetScan, and Pictar databases. We initially focused on those targets for each miRNA associated with GABAergic neurotransmission such as GABAA receptor subunits, GABA synthesizing enzymes and GABA co-transporters.
Validation of microRNA array results by Real-time quatitative RT-PCR
Real-time quantitative reverse transcriptase polymerase chain reaction was performed to validate the results obtained by miRNA arrays. Total RNA (10-50 ng) from each sample (n=5/group) was reverse-transcribed to cDNA using Taqman MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and miRNA specific primers (Applied Biosystems). The reference internal control used was U87. All reactions were run in triplicate. A no-template control was used as a negative control. The PCR reaction was carried out in an icycler MyIQ machine (BioRad). The relative quantity of each miRNA was determined as 2−ΔCT where ΔCT= (CTmiRNA−CTU87).
Transfection study using GABAAα−1 3′UTR reporter constructs
To confirm that the regulation of GABAAα−1 subunit receptor expression is mediated through targeting of its 3′-UTR by miR 181a, we used vector pEZX-MTO1 carrying miRNA binding sequences of 3′UTR of GABAAα−1 gene (GeneCopoeia, Rockville, MD) in combination with Luciferase reporter assay (Promega). This vector has dual reporters including one for regulatory detection (firefly) and another for internal control and signal normalization (Renilla). HEK293 cells were co-transfected with plasmid carrying 3′ UTR of GABAAα−1 and either Pre-miR-181a (Pre-miR (miRNA precursor), 50 nM, Ambion, Austin, TX,) or Pre-miR negative control # 1 (50 no). These Pre-miR-miRNA precursor molecules are small, chemically modified double stranded RNA molecules designed to mimic endogenous mature miRNAs. The transfection was carried out in serum free medium using 0.15% lipofectamine according to the manufacturer's protocol (Invitrogen). The dual luciferase activity of the transfected cells were measured using microtiter plate luminometer (Dynex). The results were expressed as normalized firefly luciferase activity against Renilla luciferase activity. Three individual transfections were carried out and the results were expressed as percentage reduction of luciferase activity in Pre-miR-181a transfected cells in relation to 100% activity for cells transfected with Pre-miR negative control.
Transfection study using neuronally-differentiated mouse P19 embryonic cells
To establish an in vitro system for further examination of GABAAα−1 regulation by miR-181a, we differentiated mouse P19 embryonic carcinoma cells (ATCC, CRL-1825) into neuronal-like cells using retinoic acid according to a protocol adapted from Jones-Villeneuve et al [21]. The P19 cells (2×105 cell/ml) were transfected 48 hours after retinoic acid treatment with either miR-181a (Ambion # AM 17100; Pre-miR-miRNA precursor) or negative control 1 (Ambion # AM 17110; Pre miR control) at 50 nM concentration using 0.15% lipofectamine and allowed to grow in CO2 incubator. Cells were harvested 7 days and 10 days after transfection for immunohistochemistry (IHC) and RNA extraction respectively.
GABAAα−1 gene expression profile in transfected cells by quatitative Real-time PCR
Total cellular RNA was isolated from differentiated P19 cells transfected with miR-181a and miR control using RNA STAT-60 reagent (TEL-TEST B, Inc., Friendswood, TX). The reverse transcription was carried out with 1μg total RNA using iScript cDNA synthesis kit (Bio-Rad, Richmond, CA) in iCycler (Bio-Rad). Thereafter, Real-time PCR was performed using iQ SYBR Green Supermix (Bio-Rad), 5 pmole forward and reverse primers, and 2μl cDNA from each tissue sample as template in a total of 25μl of the reaction mixture using CFX96 Real Time System (Bio-Rad). The PCR cycles used were 3 min at 95°C followed by 45 cycles of 10 s at 95°C and 30 s at 57°C. The primers used for GABAAα−1 were (sense): 5′-CGATTTT-GCTGACGCTGTTA- 3′ and (antisense) 5′- GTGGTTCCAGAAAA-GCCAAA-3′. Primers for reference gene ribosomal RNA were (sense): 5′ GACCATAAACGAT-GCCGACT 3′ and (antisense); 5′ GTGAGGTTTCCCGTGTTGAG3′ (IDT, Coralville, IA). The CT values for reference gene ribosomal RNA were highly reproducible between samples and between PCR reactions. The reactions with CT values <35 were considered as specific amplification and reactions with CT values >35 were not included in the study. A no template control was run as a negative control. Three sets of transfections were carried out and cDNA preparations from three sets of experiments were run in Real-time PCR. Results were expressed as relative mRNA expression in terms of CT values in relation to the amount of reference gene mRNA expression using formula = 2−(CTtarget − CTrRNA).
GABAAα−1 protein expression profile in transfected cells by immunocytochemistry
P19 cells were grown on cover slips in 16-well plates. Cells were rinsed with PBS once, fixed with methanol for 8 min at −20° C and then washed with PBS for 20 min. The cells were then blocked with the blocking buffer (2% normal goat serum, 1% BSA, 0.1% Triton-X-100, 0.05% Tween 20, 0.05% sodium azide, 0.01M PBS pH 7.2) for 1 hour at room temperature. The cover slips were then placed on the slides with para-film on top, cell side up. Twenty five microliter of GABAAα−1 (1:250; Alomone labs, Jerusalem, Israel) was added to the cover slips and incubated in a wet chamber overnight at 4° C. Cells were washed with PBS thrice for 10 min each and incubated with Alexa Fluor 568 goat anti-rabbit IgG (1:4000, Invitrogen, Carlasbad, CA) at room temperature for 2 hours. The cells were washed with PBS four times for 15 min each, mounted using Fluormount-G (Southern Biotech, Birmingham, AL) and observed under a fluorescent microscope (Olympus BX40). We measured the intensity of GABAAα−1 expression in P19 cells transfected with Pre-miR-181a and compared with cells transfected with Pre-miR negative control. Slides were examined under a fluorescence microscope (Nikon Eclipse 50i) using narrow band cubes for Alexa 568 (DM 568, excitation filter 540–560, barrier filter 575–645 nm). Images were captured with a Spot II high-resolution digital camera (Diagnostic Instruments, Sterling Heights, MI) and processed with the Adobe Photoshop program. To maintain the consistency of image capturing we used the same time of exposure, gain, and gamma adjustment for the control and experimental samples. Images were then opened in Image J program (NIH, Bethesda, MD) and the free hand tool was used to trace the outline of each cell. Using these settings, we measured the intensity of staining for individual cells (5 to 10 cells per coverslip). Three sets of transfection was carried out and the mean intensity of staining was compared between the cells transfected with Pre-miR-181a and Pre-miR negative control. The intensity of staining was compared between the groups using student t test and presented as mean± SD using student t test. Values with p<0.05 were considered to be significant.
GABAAα−1 gene expression in spinal dorsal horn neurons (L6-S1) by Real-Time quatitative RT-PCR
Total RNA was extracted from spinal dorsal horn samples both unchallenge (P60) and re-challenge (P30) groups of animals using Total RNA extraction from Bio-Rad (#732-6830). Same methods of reverse transcription and Real-time PCR were carried out as described in earlier section for neuronally differentiated P19 cells.
GABAAα−1 protein expression in spinal dorsal horn neurons (L6-S1) by westernblot analysis
Spinal dorsal horns samples both from unchallenge (P60) and re-challenge (P30) groups were processed and tissue extracts were prepared following the method described in our previous publications [5-7]. In brief, approximately 25 μg of spinal tissue extracts were electrophoresed on 8% SDS-PAGE and transferred onto nitrocellulose membrane. After transfer, the membrane was blocked with 5% nonfat milk and then probed with rabbit anti-GABAAα−1 (1:1000; Alomone labs). The antigen-antibody reaction was visualized using horseradish peroxidase-conjugated goat anti-rabbit antibody (1;5000, Jackson Immununo-Research, West Grove, PA) and an enhanced chemiluminescent detection system (Thermo Scientific). The relative changes in the intensity of GABAAα−1 subunit expression in various samples were normalized against the intensity of housekeeping gene β-actin for the same tissue sample by using alpha imager 3400 software. The relative intensity of staining was compared between the groups using student t test and presented as mean± SD. Values with p<0.05 were considered to be significant.
Immunostaining of GABAAα−1 in spinal dorsal horn neurons (L6-S1)
Rats in unchallenge groups were deeply anesthetized with pentobarbital sodium (50 mg/kg ip) on P60 and chests were opened by midsternal incision. Animals were perfused transcardially with cold phosphate buffer solution followed by 4% paraformaldehyde in 0.1 M PBS, pH 7.4 and L6-S1 spinal cords were collected and incubated in 4% Para-formaldehyde overnight at 4°C followed by incubating in 20% sucrose in 0.1 M PBS for 24 hour. Thereafter, the tissues were embedded in HistoPrep (Fisher Scientific, Pittsburgh, PA), and serial sections of 20-μm thickness were taken on a cryostat. The immunostaining was carried out according to the method described previously [4]. In brief, nonspecific sites were blocked by incubating the sections in PBS containing 10% normal goat serum (NGS), 0.5% Triton X-100, and 0.1% sodium azide for 60 min at room temperature. This was followed by incubation with primary antibody rabbit anti-GABAAα−1(1:250; Alomone Labs) overnight at 4°C. The secondary antibody used was Alexa 568-conjugated goat anti-rabbit antibody (1:2,000, Molecular Probes (Invitrogen) and incubation was carried for 2 hours at room temperature. The specificity of the primary antibodies was assessed by preincubating the antibody for 2 hours at room temperature with the immunizing peptides (Alomone, 10 μM) prior to the application to the sections. Three animals each from zymosan-treated and saline-treated animals were used for quantifying the intensity of staining. We selected one section per animal from both groups and the image at 20× magnification was opened under image J program (NIH, Bethesda, MD). The freehand tool was used to trace the outline of 10 randomly selected cells and the intensity of staining was determined. The average intensity of staining from 3 sections per group was determined. The mean intensity of staining from zymosan-treated rats were compared with saline-treated controls using student t test and presented as mean±SD and values with p<0.05 were considered to be significant.
In situ hybridization (ISH) and immunohistochemical (IHC) staining of spinal cords (L6-S1) for dual expression of miR 181a and GABAAα−1
Spinal cords (SC-L6S1) were collected from rats on P60 following zymosan/saline treatment at P14-P16 without perfusion and fixed overnight at 4°C in 4% paraformaldehyde with gentle shaking. The tissues were then washed with PBST (1× PBS + 0.1% Tween-20) and equilibrated in 100% methanol (3×20 min) and stored at −20°C overnight followed by rehydration using PBST containing decreasing percentage of methanol. The tissues were then subjecte to proteinase K digestion (1μg/ml) (Qiagen, #19131) for 15 min to increase the penetration of probe. The tissues were then postfixed in 4% PFA for 30 min at RT. This was followed by prehybridization in microRNA ISH hybridization buffer (Exiqon, #90000) for 2 hrs at 55°C and incubated with miRCURY LNA detection probe hsa-mir-181a (Exiqon, # 18066-15) at 55°C overnight. The LNA-scrambled DIG double labeled miRNA probe (5′-GTGTAACACGT-CTATACGCCCA-3′) was used as negative control. After several washes with prehybridization buffer containing various percentages of 2XSSC and finally with 100% PBST, tissues were incubated in ISH blocking buffer (2 mg/ml BSA + 1.2% sheep serum + PBST) and immunohistochemistry (IHC) blocking buffer (0.1M PBS + 0.25% Triton-X-100) with 10% normal goat serum at RT for 1 hour and further incubated in sheep anti-digoxigenin-AP conjugated Fabs (1:2000, Roche, Indianapolis,IN,) and GABAAα−1 (1:100, Alomone labs) in IHC blocking solution with 1% normal goat serum for 2 days at 4°C. Tissues were washed with PBST and equilibrated in alkaline phosphatase buffer (NTMT) (0.1M Tris-HCl, pH 9.5, 50mM MgCl2, 100mM NaCl, 0.1% Tween-20) and stained with the substrate, NBT/BCIP solution (Roche) under dark with gentle shaking at RT for 2 days. The staining was stopped by incubating the tissue with stop solution (PBS+1mM EDTA) for 10min at RT, washed with PBST and fixed with 4% PFA overnight. The tissue was washed with PBST and cryoprotected in increasing concentration of sucrose and incubated overnight in 30% sucrose at 4°C overnight. The tissues were then embedded in embedding medium (Tissue Tek, Hatfield, PA) and 10μm sections were cut using a cryostat (Microm HM 505N). The sections were washed with PBST and incubated with 1:100 goat anti-rabbit-AP (Sigma) in IHC blocking buffer with 1% normal goat serum overnight at 4°C. The sections were then washed with PBST and equilibrated with NTMT and incubated with Fast red (Roche) in 0.1M Tris-HCl, pH 8.8 for 36 hours at 4°C. The staining was stopped with stop solution, washed with PBST and and rehydrated. The slides were mounted using DPX mountant (Sigma) and images were taken using Zeiss Axioplan 2 imaging system. The relative quantification of miR 181a expression in L6-S1 SC from saline and zymosan-treated rats is analyzed using Image J program (NIH, Bethesda). The images are converted into grey scale and the intensity of expression in the dorsal horns (as indicated by red boundaries in figure 4D) were selected using a region of interest (ROI). The mean intensity of staining was measured from three sections from each animal (n=3/group). The non-specific background staining was measured in similar way from the dorsal column region for each section, and the net intensity was calculated by subtracting the background intensity from the intensity of miR 181a staining in the dorsal horn regions. The mean intensity of staining from zymosan-treated rats were compared with saline-treated controls using student t test.
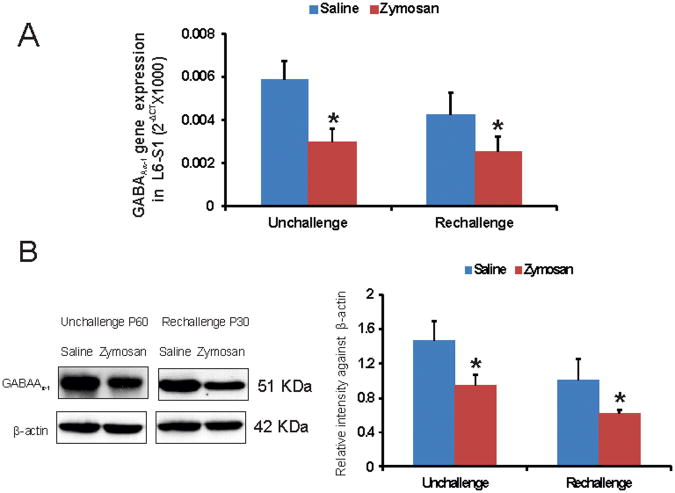
Figure 4.
A: Real-time RT-PCR analysis of differentially expressed GABAAα−1 gene in spinal dorsal horns (L6-S1) from rats with neonatal cystitis. Two different treatment protocols were followed and tissues were examined on P60 from unchallenge protocol and on P30 from re-challenge protocol. The ordinate value 2−ΔCT corresponds to the mRNA expression relative to reference gene ribosomal RNA (rRNA) and represented as mean ± SD (n=5/group). B: westernblot analysis of GABAAα−1 protein spinal dorsal horn samples (L6-S1) from rats with neonatal cystitis. The intensity of GABAAα−1 immunoreactivity for different tissues was normalized against the intensity of β-actin expression for the same tissue. Results were expressed as means ± SD (n=5/group). Asterisks indicate significant difference at p value of <0.05 (*).
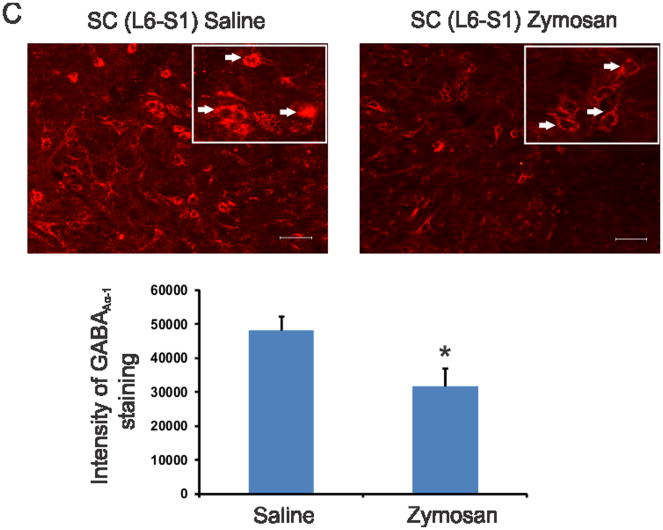
Figure 4C. GABAAα−1 immunostaining of differentially expressed GABAAα−1 gene in spinal dorsal horns (L6-S1) from rats with neonatal cystitis. The tissues were examined on P60 following zymosan/saline treatment at P14-P16. The scale bar is 50 μm. The intensity of staining for 10 individual cells from each group was determined and presented as means±SD. Asterisk indicate significant difference at p value of <0.001 (*).
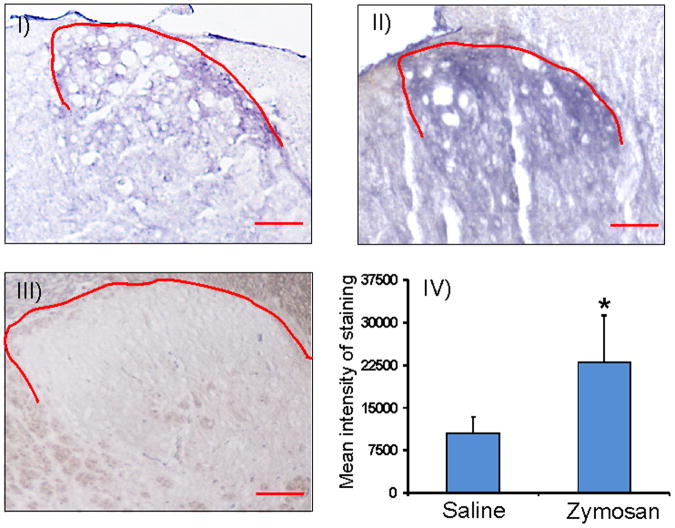
Figure 4D: miR 181a staining of spinal cord (L6) tissues from rats with neonatal cystitis using in situ hybridization protocol. The tissues were examined on P60 following zymosa or saline treatment at P14-P16. The scale bar is 20 μm. The red line indicates the area of spinal dorsal horns exhibiting miR 181a expression in saline (I), zymosan (II) treated animals. The scrambled miRNA used as negative control shows no significant staining in spinal dorsal horn lamina from saline treated rats (III). The mean intensity of miR 181a expression was calculated by subtracting the mean background staining from the staining in the selected regions fromr saline and zymosan-treated rats. Results were expressed as mean±SD, n=3/group (IV). Asterisk indicates significant difference at p value of <0.05 (*).
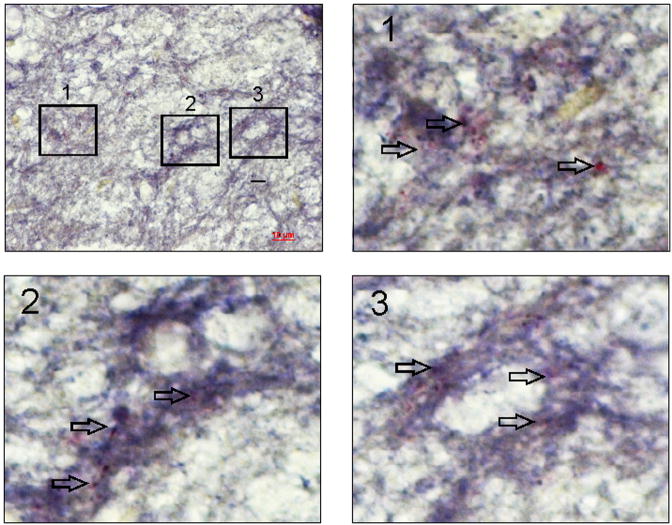
Figure 4E: An example of dual in situ hybridization and immunohistochemistry in spinal cord (L6) from zymosan-treated rat to demonstrate coexpression of miR 181a and its target protein GABAAα−1 in the dorsal horn neurons. The scale bar is 10 μm. The arrows in enlarged images 1, 2 & 3 demonstrate colocalization of miR 181a (purple) and GABAAα−1 (red) mostly on the cell membrane.
Surgery for electromyography (EMG) recording and lumbosacral (LS) intrathecal (i.t.) catheter implantation
Rats were anesthetized with sodium pentobarbital (45-50 mg/kg, i.p.). A pair of teflon-coated electrode (Cooner Wire, Chatsworth, CA) was implanted into the external oblique muscle of the abdomen and externalized through the neck for electromyography (EMG) recordings. At the same time a catheter (PE-10, ∼5.5-6cm) was inserted through the dura overlying the atlanto-occipital junction into the spinal sub-arachnoid space and guided until the tip of catheter lay in the lumbo-sacral (LS) segment of the spinal cord. Rats received analgesic (Carprofen, 5mg/kg/day, i.m. for 3 days) and antibiotic (Enrofloxacin, 2.5mg/kg/day, i.m. for 3 days) post-operatively. Following the surgery rats were housed separately and allowed to recover for at least 3 days prior to further interventions. Seventy-two hours following the surgery, rats were put inside the plexiglass restraining tubes two times a day for two hours (1 hour/session) in order to acclimatize them to experimental conditions. The increase in EMG activity to colorectal distension (CRD) was used as an objective measure of visceral sensation and referred as viscero-motor response (VMR). Isobaric (constant pressure) distention (10, 20, 30, 40, and 60 mmHg) was delivered for duration of 30 second with a 180 second inter-stimulus time interval. For intrathecal (i.t.) drug administration through the chronically implanted catheter the volume of injection was restricted to 5μl followed by 10μl of saline flush. The effect of drug was on VMR was tested 10 minutes following injection. Statistical analysis for VMR data was performed using SigmaStat (V2.03, SPSS Inc, Chicago, IL). All results are expressed as mean ± SEM. Values with p<0.05 were considered to be significant. The EMG activity to colon distension was measured as area under the curve and student t test was used to calculate the significance of the differences. A p value of <0.05 was considered significant. The EMG activity was normalized to maximum response at distending pressure of 60mmHg. In neonatal (P14-P16) zymosan-treated rats the values were compared and normalized to maximum response observed in neonatal saline-treated rats. For adult (P43-P46) zymosan-treated rats, a baseline VMR was recorded before starting zymosan treatment. The VMRs were repeated three days after zymosan treatment and following intrathecal injection of GABAa agonist muscimol.
Results
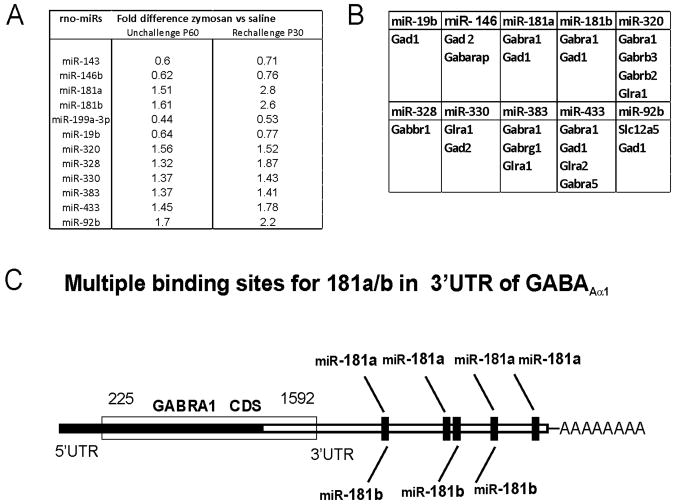
For miRNA microarray assay, Affymetrix miRNA GeneChips were used. These GeneChips contained miRNA probes from 71 different species including rat, human and mouse designed on the basis of version (v.11) of the Sanger miRBase (Sanger institute, Cambridge). We examined miRNA expression in the lumbosacral (L6-S1) spinal dorsal horn samples on P60 from rats that received either intravesical zymosan or saline during postnatal P14 through P16. Clustering of miRNAs based on the expression data was classified in two distinct groups, one with down-regulated miRNAs and other was up-regulated ones. The volcano plot analysis of the arrays indicated a differential miRNA expression pattern with 4 showing downregulation and 8 upregulation in the neonatal zymosan-treated samples compared to controls (fold differences ≤ 1.5, n=3/group, p<0.05, fig.1A). This result prompted us to design another treatment protocol, where rats received either zymosan or saline re-challenge on P29 (after complete neuronal maturation) following treatment at P14-16 and spinal cord tissues were collected on P30 for miRNA analysis. The reason for miRNA analysis in second set of animals was to examine whether zymosan rechallenge immediately after neuronal maturation could result in a higher fold difference of miRNA expression in the spinal cord between zymosan- and saline-treated rats. We examined the expression patterns of the same set of miRNAs that exhibited differential expression in our first treatment (unchallenge) protocol. The fold differences in signal intensities of the selected miRNAs between the zymosan-treated and saline-treated samples in the re-challenge protocol were ≤ 3.0 (p<0.05, n=3/group, fig. 1A). Importantly, the pattern of miRNA expression was comparable between the two protocols. However, in re-challenge protocol at P30, miRNAs such as miR 181a exhibited higher fold differences i.e. 2.8 between the zymosan and saline-treated groups in comparison with 1.5 fold difference between groups on P60 from unchallenged protocol (fig. 1A).
Figure 1.
Differentially expressed miRNAs in the spinal (L6-S1) dorsal horns following neonatal zymosan-induced cystitis. A: miRNAs that demonstrate up-regulation and down-regulation using two different zymosan treatment protocols. The data was analyzed using GeneSpring v11 software and unpaired Mann-whiney Test to identify differentially expressed miRNAs (n=3/group, p<0.05 vs saline controls). B: differentially expressed miRNAs with 3′UTR binding sites for various genes associated with spinal GABAergic system. C: schematic representation of 3′UTR of GABAAα−1 gene demonstrating multiple complementary binding sites of miR-181a and miR-181b.
The miRNA targets were predicted based on three independent miRNA target prediction database; miRBase, miRDB and TargetScan, that utilizes the miRanda, MirTarget2 and TargetScan algorithms. Since we are interested in studying the effect of neonatal cystitis on the development of inhibitory neurotransmission system in spinal cord, we looked for target genes involved with GABA receptor subunits, GABA synthesizing enzymes Gad1 and Gad 2, chloride ion co-transporter (Slc12a5, KCC2), GABA transporter (VGAT) and also glycine receptor alpha 1 and 2. Among the upregulated miRNAs, 6 demonstrated 3′UTR binding sites for GABAA−1, one of the predominant subunits of GABAA receptor in the central nervous system (fig. 1B). The majority of upregulated miRNAs also contain complementary binding sites for genes associated with the synthesis of GABA such as Gad 1, Gad 2, glycenergic receptors and Cl- ion co-transporter KCC2, whereas, only two of the downregulated miRNAs exhibited target sites for Gad1, Gad2 and GABAA receptor subunit (fig. 1B). We subsequently looked for the mRNA target prediction based on mirSVR scores provided at microrna.org. Among the selected miRNAs, miR-181a (−1.82) and miR-181b (−1.31) showed the strongest mirSVR scores for GABAA−1 receptor subunit with multiple complementary binding sites at 3′UTR region (fig. 1C). Based on our miRNA array results, we selected miR-181a to study further its involvement in GABAA−1 receptor subunit downregulation and long-term spinal sensitization in rats with neonatal-induced cystitis.
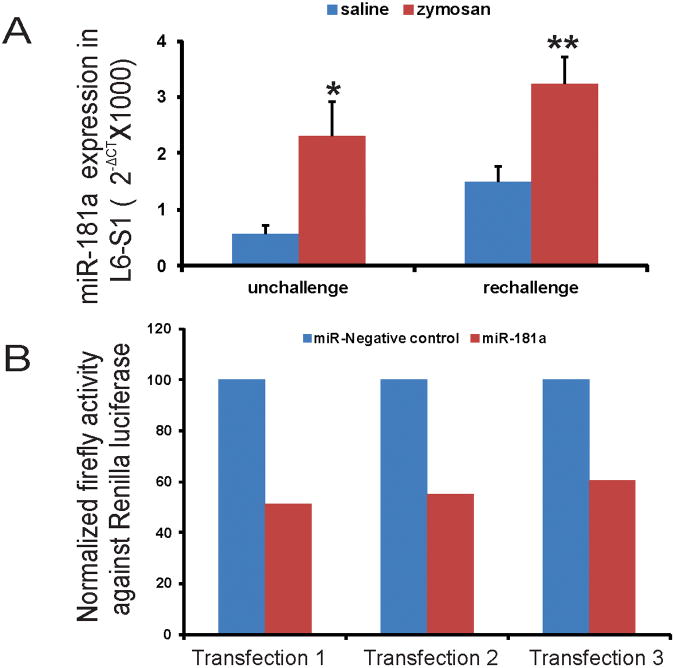
To validate microarray results, we performed subsequent quantitative RT-PCR for miR-181a in spinal dorsal horn samples (L6-S1) from both zymosan unchallenge and re-challenge groups of animals. The relative expression (2−ΔCT) of miR-181a in zymosan-treated samples were significantly higher compared with saline-treated controls both in unchallenge (p<0.01) and re-challenge (p<0.001) groups of animals (fig. 2A). Therefore, quantitative PCR data for miR-181a expression validates the result obtained in our miRNA arrays.
Figure 2.
A: Real-time RT-PCR analysis of differentially expressed miR-181a in spinal dorsal horns (L6-S1) from rats with neonatal cystitis. Two different treatment protocols were followed and tissues were examined on P60 from unchallenge protocol and on P30 from re-challenge protocol. The ordinate value 2−ΔCT corresponds to the miRNA expression relative to endogenous control U87 and represented as mean ±SD (n=4/group). Asterisks indicate significant difference at p value of <0.01(*) and <0.001(**). B: GABAAα−1 3′UTR interaction with miR-181a in HEK293. The percentage reduction in normalized firefly activity against Renilla luciferase activity for cells transfected with Pre-miR-181a was evaluated by defining the normalized firefly luciferase activity for cells transfected with Pre- miR- negative control as 100%. The data represented the normalized luciferase activity of three different sets of transfections.
In order to study whether 3′UTR of GABAAα−1 is a target site for miR-181a, we used pEZ-MT01 reporter vector carrying 3′UTR region of the receptor gene and carried out an in vitro transfection and dual luciferase reporter assay. The HEK293 cells were co-transfected either with pEZ-MT01 plasmid and Pre-mir181a or pEZ-MT01 and Pre-miR negative control (fig. 2B). The HEK293 cells co-transfected with plasmid carrying GABAAα−1 3′ UTR and miR-181a exhibited a significant reduction in firefly luciferase activity compared with cells co-transfected with the same plasmid and miR negative controls. The percentage reduction in firefly luciferase activity in miR-181a transfected cells in three different transfections was between 40-50% in relation to 100% activity defined for the cells transfected with miR-negative control (fig. 2B). This in vitro study, therefore suggests a direct post-transcriptional regulatory control of miR-181a on GABAAα−1 receptor gene expression.
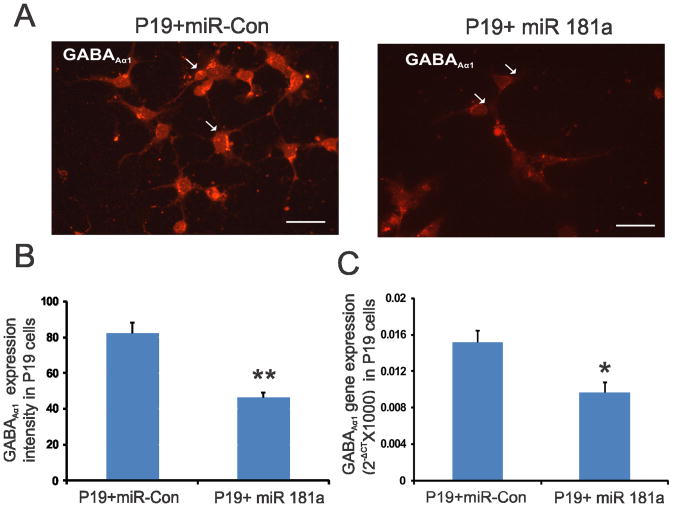
To established further the direct involvement of miR-181a in GABAAα−1 receptor subunit expression in neuronal cells, we transfected neuronally differentiated mouse P19 cells either with Pre-miR-181a or Pre-miR negative control 1. The GABAA−1 subunit immunostaining was significantly reduced in P19 cells transfected with Pre-miR-181a compared with Pre miR-negative control 1 (figs. 3A & 3B, p<0.001). In Real-time PCR, Pre miR-181a transfection resulted in significant reduction in GABAAα-1 gene expression compared with cells transfected with Pre-miR negative control 1 (fig. 3C, p<0.01). Based on this result we proposed that miR-181a negatively regulates the expression of GABAA−1 receptor in neuronal cells.
Figure 3.
A: GABAAα−1 immunostaining of neuronally differentiated P19 cells transfected with Pre-miR-181a and Pre-miR-negative control. B: the intensity of staining for 10 individual cells from each group was determined and presented as means±SD. The scale bar is 50 μm. C: Real-time RT-PCR analysis of GABAAα−1 gene expression in neuronally transfected P19 cells transfected with miR-181a. Three sets of transfections were carried out and GABAAα−1 gene expression relative to reference gene ribosomal RNA was presented using formula 2− (CTGABAAα−1 − CTrRNA). Asterisks indicate significant difference at p value of <0.01(*) and <0.001(**).
We further examined the expression profile of GABAAα−1 gene in spinal dorsal horns (L6-S1) from both unchallenge and re-challenge groups of animals. In unchallenge group, neonatally zymosan treatment demonstrated a long-term downregulation of GABAAα−1 mRNA in adult spinal cord at P60 that was significantly lower than in saline-treated controls (p<0.05, fig. 4A). A similar downregulation of spinal GABAA−1 mRNA was observed in zymosan re-challenge group compared to saline-treated controls at P30 (p<0.05, fig. 4A). In westernblots, zymosan-treated animals from both unchallenge and re-challenge groups demonstrated significant downregulation of GABAAα−1 protein expression in the spinal dorsal horn neurons (L6-S1) compared to their respective saline controls (p<0.05, fig. 4B). We also examined the expression pattern of GABAAα−1 receptor subunit in lumbosacral spinal dorsal horn neurons from P60 rats that had received either zymosan or saline at P14-P16. The immunostaining of individual cells exhibited a distinct difference in the level of expression with saline-treated groups exhibiting a strong expression both in the cytoplasm and the cell membrane (arrows, fig. 4C), whereas a significantly low expression with staining mainly within the cell membrane was observed in spinal neurons from rats treated with zymosan (p<0.001, fig. 4C). In in situ hybridization, miR 181a expression is prominent in the dorsal horn region (from lamina I through IV) of the L6 spinal cord (fig. 4D). The expression is predominantly observed around the cell and often in punctate fashion. Moreover, in zymosan-treated animals there is a significant increase in miR 181a expression (fig. 4DII) compared to saline-treated controls (fig. 4DI). The mean intensity of miR 181a expression in the zymosan-treated rats was almost a fold higher compared with saline-treated rats (p<0.05 vs saline-controls, fig. 4DIV). The specificity of miR 181a expression was confirmed with no significant staining using a scrambled miR probe (fig. 4DIII). The dual in situ hybridization for miR 181a (purple staining) and immunohistochemistry for GABAAα−1 (red staining) in spinal cord tissues demonstrated distinct colocalization of miR 181a with target protein (fig. 4E).
Effect GABAA receptor agonist muscimol on viscero-motor response (VMR)
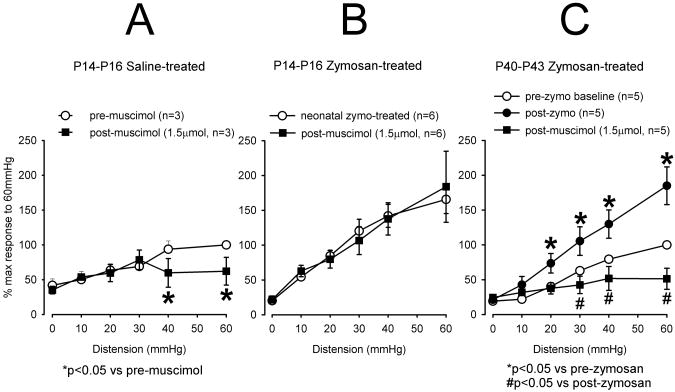
Since molecular and IHC data indicated downregulation of GABAAα−1 receptor subunits, we tested the efficacy of GABAA agonist muscimol in attenuation of VMRs of neonate zymosan-treated and adult zymosan-treated rats. To test the efficacy of GABAA receptor agonist to attenuate the VMR in neonatally zymosan-treated rats and to compare that with neonatal saline-treated and also with adult zymosan-treated rats that were not subjected to neonatal zymosan treatment, we injected (i.t.) muscimol. In neonatal saline-treated rats, a bolus dose of muscimol (1.5μmol in 5μl) significantly (p<0.05 vs pre-zymosan) reduced the VMR at distending pressure ≥ 40mmHg (fig 5A). After a few trials in naïve rats this dose of the drug was carefully selected that did not produce any muscle paralysis and postural imbalance. The same dose of muscimol was ineffective to attenuate VMR in neonatal zymosan-treated rats (fig 5B). In adult rats (P40-P43), intravesical zymosan injections for 3 days produced significant (p<0.05 vs pre-zymosan) increase in VMR to CRD when tested 24 hours following the last injection. Unlike neonatal cystitis group, in adult rats, muscimol produced significant (p<0.05 vs post-zymosan) attenuation of VMR (fig 5C).
Figure 5.
The summary data of VMRs following intrathecal (i.t.) injection of GABAA receptor agonist muscimol (1.5μmol in 5μl). A: In neonatal saline-treated rats, muscimol significantly (*p<0.05 vs pre-zymosan) decreased VMR at distending pressure ≥ 40mmHg. B: Neonatal zymosan-treated rats exhibited greater VMR compared to saline-treated rats. However, muscimol failed to attenuate VMR in these rats. C: In adult rats 3 days of intravesical zymosan produced a significant (*p<0.05 vs pre-zymosan) increase in VMR. Muscimol significantly (#p<0.05 vs post-zymosan) attenuated VMR in these rats.
Discussion
miRNAs have been implicated as the regulators of neuronal development and synaptic plasticity both in vivo and in vitro [22;23;27;34]. Our data support the existence of miRNA-mediated transcriptional deregulation of GABAergic neurotransmission in adult spinal cord following neonatal cystitis. We observed upregulation of several miRNAs carrying target sites for genes associated with GABAA receptor subunits, GABA synthesizing enzymes and K+,Cl− co-transporter (KCC2). Among the selected miRNAs, miR-181a and miR-181b carry several 3′UTR complementary binding sites for GABAAα−1 receptor subunit with mirSVR score of −1.82 and −1.32, respectively. In quantitative real time PCR, a significant increase in spinal miR-181a was observed in neonatal cystitis rats compared with saline-treated controls both in zymosan re- challenge and non-challenge groups of rats. Increase in miR-181a concomitantly resulted in transcriptional as well as translational repression of GABAA receptor expression with a significant downregulation of GABAAα−1 receptor subunit expression both at the gene and protein levels in adult spinal cord from neonatal cystitis rats compared with saline-treated rats. We demonstrated using in situ hybridization that neonatal zymosan treatment resulted in distinct upregulation of miR 181a expression in the spinal dorsal horns compared with saline-treated controls. In dual in situ hybridization and immunohistochemistry study, we further confirmed the coexpression of miR-181a and GABAAα−1 on spinal dorsal horn cells emphasizing a possible regulatory effect of miR 181a on GABAAα−1 expression. In our behavioral study, intrathecal administration of GABAA receptor agonist muscimol failed to show inhibitory effect on VMR responses to CRD in neonatal zymosan-treated, whereas in neonatal saline-treated and adult zymosan-treated rats it produced a significant inhibition of VMRs. To our knowledge, this is the first report documenting that in neonatal zymosan-induced cystitis GABAA receptor agonist are less effective to produce visceral analgesia. The behavioral visceral pain assessment result indicates that there is GABAA receptor downregulation in the LS segment of the spinal cord following the development of cystitis in early stage of life. It is very likely that miRNA-mediated downregulation of GABAA receptors results in loss of inhibitory tone in the spinal cord and subsequent unmasking of excitatory pathways to manifest long-lasting visceral hypersensitivity.
Although the increase in expression of spinal miR-181a was apparently not robust (1.5 to 3-folds) in unchallenge and re-challenge groups of animals, this moderate increase may have important functional consequences for the sensitized spinal neurons as evident in our molecular and behavioral studies. Most importantly, we observed a similar expression pattern in our quantitative Taqman Real-time PCR confirming the results of miRNA arrays. In accordance with the present result, several recent reports also suggest that even small changes in miRNA expression may have biological relevance due to their simultaneous regulatory effect on multiple genes [2;10;35]. Not much information is available on the relationship between endogenous miRNA expression levels and the extent of target mRNA expression. However, experiments using in vitro cell culture systems with reporter gene suggest that a certain threshold of miRNA level is needed to achieve target gene suppression [8]. In line with these findings, our in vitro study demonstrated a significant reduction in firefly luciferase activity in HEK cell co-transfected with miR-181a and plasmid carrying 3′UTR of GABAAα−1 indicating a direct interaction between miR-181a and its target gene. We further demonstrated transcriptional regulation of GABAAα−1 by miR-181 in neuronally differentiated P19 cells, with significant downregulation of GABAAα−1 receptor subunit expression in cells transfected with miR-181a. Therefore, both our in vivo and in vitro studies clearly indicate the involvement of miR-181a mediated transcriptional deregulation of spinal GABAAα−1 receptor subunit expression in rats with early-life cystitis. Besides miR-181a, five other miRNAs with binding sites for GABAA−1 3′UTR region also demonstrated upregulation in our miRNA arrays of spinal dorsal horn samples and bio-informatic analysis reveals that several of these miRNAs also carry regulatory sites for GABA synthesizing enzymes, α-5, β-2, β-3 subunits of GABAA receptor and glycenergic receptor subunit α-1. Therefore, an overall spinal disinhibition in neonatal cystitis rats could be due to miRNA-mediated posttranscriptional deregulation of multiple genes associated with GABAergic and other inhibitory neurotransmission.
The expression pattern of miRNAs in the nociceptive system demonstrated temporal and spatial specificity, and a marked stimulus-dependent pattern of regulation [2;3;24;35;48]. Over the last few years, several studies documented the expression pattern of hundreds of miRNAs and in some cases identified their targets. However, very few data are available on the involvement of miRNA expression in the nociceptive system. Bai et al., (2007) reported significant downregulation of miR-10a, -29a, -98, -99a, -124a, -134, and -183 in ipsilateral trigeminal ganglia following CFA-induced inflammatory muscle pain in rats [3]. These authors suggest that the decrease in miRNAs allows an upregulation of pain-related proteins and concomitant development of inflammation and allodynia. Involvement of miRNAs in inflammatory pain processing has also been established in an in vivo study using conditional Dicer (miRNA synthesizing enzymes) knockout Nav1.8 mice [48]. Similarly, in a rat model of traumatic spinal cord contusion injury (SCI), differential expression of more than 250 miRNAs have been reported at 4 hours, 1 day, or 7 days after the surgery [26]. Subsequent bioinformatic analysis in this study identified target genes that play important roles in the pathogenesis of SCI by altering inflammatory process, oxidation and apoptosis in neurons. In a rat model of peripheral nerve injury (L5, spinal nerve ligation, SNL), a significant downregulation of miRNAs of the 183 family (miR-96, miR-182, miR-183) was observed in ipsilateral DRG neurons [2]. Besides expressional changes, it has been suggested that miRNA activity is also altered after nerve injury as indicated by their translocation to the cellular periphery and colocalization with a marker for stress granules (TIA-1). Although several studies documented differential expression of miRNAs in various experimental models of inflammation and neuropathic pain, the functional implication of these observations has not been studied systematically. Since miRNAs regulate protein synthesis through sequence-guided complementary binding with target mRNA blocking the transcription and translation process, the miR-181a expression that remains upregulated even six weeks after neonatal visceral painful stimulation may represent one of the earliest events underlying a permanent phenotypic switch in GABAa receptor subunit expression in the developing spinal neurons and long-lasting sensitization of pain signaling pathway. Our miRNA arrays for both unchallenge and re-challenge protocols demonstrated significant upregulation of miR-92b expression in zymosan-treated rats compared to saline controls. Since miR-92b carries complementary binding sites for 3′UTRs of co transporter KCC2 and GABA vesicular transporter, (VGAT) genes, it will be interesting to study further the involvement of transcriptional deregulation of KCC2 and VGAT in early-life induced cystitis. It has recently been shown that in rats with diabetic neuropathic pain, a decrease in expression of KCC2 accounts for the action of GABA from inhibitory to excitatory spinal neurotransmission [20;32;33]. Therefore, in addition to the lack of inhibitory GABAergic tone, it is also possible that early life visceral inflammation may also involve miRNA- mediated transcriptional deregulation of ion co-transporter such as potassium chloride co-transporter (KCC2) and GABA transporter VGAT.
There is evidence that the heightened pain sensitivity due to repeated noxious stimulus in infants also alters the cerebral pain processing and greater pain-related brain activation in primary somato-sensory, insula and anterior cingulate cortex (ACC) of school-aged children [12;19;45]. Similarly, in experimental animals, noxious events during the period of neural network construction and learning processes can result in functional and behavioral alterations of adult pain processing [16;17;38]. In our recent study, repeated acid infusion in the esophagus of P7-P14 rats resulted in long-lasting and enhanced expression of NMDA receptor subunits and postsynaptic density protein (PSD95) in ACC when tested at adult age of P60 [5]. In addition to this work, we have also documented in several recent reports that the neonatal noxious stimulus in visceral and somatic structures produces long-term visceral and somatic hyperalgesia in rats [29;30;42]. Moreover, this long-lasting visceral hypersensitivity could be due to neuroplasticity in gastrointestinal system, specifically increased branching of primary afferents at both peripheral and central locations, increased spontaneous and evoked activity of dorsal horn neurons, and alteration in the balance between descending inhibitory and excitatory systems [14;39]. Recent evidence also suggest an imbalance in the spinal nociceptive and anti-nociceptive systems, where neonatal cystitis exhibited a higher bladder content of calcitonin gene-related peptide (CGRP) and substance P (SP) and a subsequent impairment of the central opioid-inhibitory mechanism [14;15]. Similarly, our previous report of an increase in NMDA receptor NR1 subunit expression in spinal dorsal horn neurons in rats with neonatal cystitis and the present findings of transcriptional reregulation of spinal GABAA receptor subunits clearly indicate that both excitatory and inhibitory neurotransmissions are involved in long-lasting sensitization of visceral sensory system [29].
The significance of miRNAs in pain research is emerging in recent studies. In patients with chronic bladder pain syndrome, an increase in miR-449b and 500 in the bladder tissue has been linked with a downregulation of neurokinin receptors [40]. Similarly, alterations in miRNA expression have been reported in the endometrium of women with painful endometriosis in the early secretory phase [9]. Our present findings also strongly suggest that miRNAs play an important role in the regulation of gene expression in developing nociceptive system associated with pain. Future investigations on functional studies and electrophysiological characterization of neurons will be needed to elucidate how miRNAs are involved in development and maintenance of chronic pelvic pain and this may help further in characterizing miRNAs as potential and specific targets to relieve pain.
Acknowledgments
We would like to thank Dr. Kalpana Ghoshal at the Ohio state university for helpful discussions. We thank Dr. Chang Zoon Chun for help with in situ hybridization and Dr. Jeffery Shadley for transfection studies. The study has been supported by NIH Grant R56DK089493 awarded to Banani Banerjee and Jyoti N. Sengupta.
Footnotes
Conflicts of interest statement: The authors declare no financial or other conflicts of interest related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 2.Aldrich BT, Frakes EP, Kasuya J, Hammond DL, Kitamoto T. Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience. 2009;164:711–723. doi: 10.1016/j.neuroscience.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai G, Ambalavanar R, Wei D, Dessem D. Downregulation of selective microRNAs in trigeminal ganglion neurons following inflammatory muscle pain. Mol Pain. 2007;3:15. doi: 10.1186/1744-8069-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee B, Medda BK, Lazarova Z, Bansal N, Shaker R, Sengupta JN. Effect of reflux-induced inflammation on transient receptor potential vanilloid one (TRPV1) expression in primary sensory neurons innervating the oesophagus of rats. Neurogastroenterol Motil. 2007;19:681–691. doi: 10.1111/j.1365-2982.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee B, Medda BK, Schmidt J, Lang IM, Sengupta JN, Shaker R. Neuronal Plasticity in the Cingulate Cortex of Rats Following Esophageal Acid Exposure in Early Life. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee B, Medda BK, Schmidt J, Zheng Y, Zhang Z, Shaker R, Sengupta JN. Altered expression of P2X3 in vagal and spinal afferents following es ophagitis in rats. Histochem Cell Biol. 2009;132:585–597. doi: 10.1007/s00418-009-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee B, Medda BK, Zheng Y, Miller H, Miranda A, Sengupta JN, Shaker R. Alterations in N-methyl-D-aspartate receptor subunits in primary sensory neurons following acid-induced esophagitis in cats. Am J Physiol Gastrointest Liver Physiol. 2009;296:G66–G77. doi: 10.1152/ajpgi.90419.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown BD, Gentner B, Cantore A, Colleoni S, Amendola M, Zingale A, Baccarini A, Lazzari G, Galli C, Naldini L. Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nat Biotechnol. 2007;25:1457–1467. doi: 10.1038/nbt1372. [DOI] [PubMed] [Google Scholar]
- 9.Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, Giudice LC. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod. 2009;15:625–631. doi: 10.1093/molehr/gap068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 11.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, Saizou C, Lapillonne A, Granier M, Durand P, Lenclen R, Coursol A, Hubert P, de Saint BL, Boelle PY, Annequin D, Cimerman P, Anand KJ, Breart G. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 13.Costigan M, Woolf CJ. Pain: molecular mechanisms. J Pain. 2000;1:35–44. doi: 10.1054/jpai.2000.9818. [DOI] [PubMed] [Google Scholar]
- 14.DeBerry J, Ness TJ, Robbins MT, Randich A. Inflammation-induced enhancement of the visceromotor reflex to urinary bladder distention: modulation by endogenous opioids and the effects of early-in-life experience with bladder inflammation. J Pain. 2007;8:914–923. doi: 10.1016/j.jpain.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeBerry J, Randich A, Shaffer AD, Robbins MT, Ness TJ. Neonatal bladder inflammation produces functional changes and alters neuropeptide content in bladders of adult female rats. J Pain. 2010;11:247–255. doi: 10.1016/j.jpain.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 17.Hathway GJ, Koch S, Low L, Fitzgerald M. The changing balance of brainstem-spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol. 2009;587:2927–2935. doi: 10.1113/jphysiol.2008.168013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opiod tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci. 2010;30:10251–10258. doi: 10.1523/JNEUROSCI.2419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hohmeister J, Kroll A, Wollgarten-Hadamek I, Zohsel K, Demirakca S, Flor H, Hermann C. Cerebral processing of pain in school-aged children with neonatal nociceptive input: an exploratory fMRI study. Pain. 2010;150:257–267. doi: 10.1016/j.pain.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Jolivalt CG, Lee CA, Ramos KM, Calcutt NA. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain. 2008;140:48–57. doi: 10.1016/j.pain.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones-Villeneuve EM, McBurney MW, Rogers KA, Kalnins VI. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982;94:253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kocerha J, Kauppinen S, Wahlestedt C. microRNAs in CNS disorders. Neuromolecular Med. 2009;11:162–172. doi: 10.1007/s12017-009-8066-1. [DOI] [PubMed] [Google Scholar]
- 23.Konecna A, Heraud JE, Schoderboeck L, Raposo AA, Kiebler MA. What are the roles of microRNAs at the mammalian synapse? Neurosci Lett. 2009;466:63–68. doi: 10.1016/j.neulet.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 24.Kusuda R, Cadetti F, Ravanelli MI, Sousa TA, Zanon S, De Lucca FL, Lucas G. Differential expression of microRNAs in mouse pain models. Mol Pain. 2011;7:17. doi: 10.1186/1744-8069-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neanatal colon pain. Brain Res. 2003;971:73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- 26.Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol. 2009;219:424–429. doi: 10.1016/j.expneurol.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madathil SK, Nelson PT, Saatman KE, Wilfred BR. MicroRNAs in CNS injury: potential roles and therapeutic implications. Bioessays. 2011;33:21–26. doi: 10.1002/bies.201000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321–327. doi: 10.1016/0029-7844(95)00458-0. [DOI] [PubMed] [Google Scholar]
- 29.Miranda A, Mickle A, Schmidt J, Zhang Z, Shaker R, Banerjee B, Sengupta JN. Neonatal cystitis-induced colonic hypersensitivity in adult rats: a model of viscero-visceral convergence. Neurogastroenterol Motil. 2011;23:683–e281. doi: 10.1111/j.1365-2982.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miranda A, Peles S, Shaker R, Rudolph C, Sengupta JN. Neonatal nociceptive somatic stimulation differentially modifies the activity of spinal neurons in rats and results in altered somatic and visceral sensation. J Physiol. 2006;572:775–787. doi: 10.1113/jphysiol.2006.108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monga AK, Marrero JM, Stanton SL, Lemieux MC, Maxwell JD. Is there an irritable bladder in the irritable bowel syndrome? Br J Obstet Gynaecol. 1997;104:1409–1412. doi: 10.1111/j.1471-0528.1997.tb11013.x. [DOI] [PubMed] [Google Scholar]
- 32.Morgado C, Pereira-Terra P, Cruz CD, Tavares I. Minocycline completely reverses mechanical hyperalgesia in diabetic rats through microglia-induced changes in the expression of the potassium chloride co-transporter 2 (KCC2) at the spinal cord. Diabetes Obes Metab. 2011;13:150–159. doi: 10.1111/j.1463-1326.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 33.Morgado C, Pinto-Ribeiro F, Tavares I. Diabetes affects the expression of GABA and potassium chloride cotransporter in the spinal cord: a study in streptozotocin diabetic rats. Neurosci Lett. 2008;438:102–106. doi: 10.1016/j.neulet.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 34.Muddashetty R, Bassell GJ. A boost in microRNAs shapes up the neuron. EMBO J. 2009;28:617–618. doi: 10.1038/emboj.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poh KW, Yeo JF, Ong WY. MicroRNA changes in the mouse prefrontal cortex after inflammatory pain. Eur J Pain. 2011 doi: 10.1016/j.ejpain.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7:469–479. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- 37.Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–631. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez F V, Burkhard FC, Kessler TM, Kuhn A, Draeger A, Monastyrskaya K. MicroRNAs may mediate the down-regulation of neurokinin-1 receptor in chronic bladder pain syndrome. Am J Pathol. 2010;176:288–303. doi: 10.2353/ajpath.2010.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Simon FM, Zhang XX, Loh HH, Law PY, Rodriguez RE. Morphine regulates dopaminergic neuron differentiation via miR-133b. Mol Pharmacol. 2010;78:935–942. doi: 10.1124/mol.110.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith C, Nordstrom E, Sengupta JN, Miranda A. Neonatal gastric suctioning results in chronic visceral and somatic hyperalgesia: role of corticotropin releasin factor. Neurogastroenterol Motil. 2007;19:692–699. doi: 10.1111/j.1365-2982.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- 43.Terruzzi V, Magatti F, Quadri G, Tenore C, Minoli G, Belloni C. Bladder dysfunction and irritable bowel syndrome. Am J Gastroenterol. 1992;87:1231–1232. [PubMed] [Google Scholar]
- 44.von SD, Agostino MJ, Murray BS, Li Y, Reddy PS, Chen J, Choe SE, Strassle BW, Li C, Bates B, Zhang L, Hu H, Kotnis S, Bingham B, Liu W, Whiteside GT, Samad TA, Kennedy JD, Ajit SK. Dynamic changes in the microRNA expression profile reveal multiple regulatory mechanisms in the spinal nerve ligation model of neuropathic pain. PLoS One. 2011;6:e17670. doi: 10.1371/journal.pone.0017670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009;141:79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Whorwell PJ, Lupton EW, Erduran D, Wilson K. Bladder smooth muscle dysfunction in patients with irritable bowel syndrome. Gut. 1986;27:1014–1017. doi: 10.1136/gut.27.9.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 48.Zhao J, Lee MC, Momin A, Cendan CM, Shepherd ST, Baker MD, Asante C, Bee L, Bethry A, Perkins JR, Nassar MA, Abrahamsen B, Dickenson A, Cobb BS, Merkenschlager M, Wood JN. Small RNAs control sodium channel expression, nociceptor excitability, and pain thresholds. J Neurosci. 2010;30:10860–10871. doi: 10.1523/JNEUROSCI.1980-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]