Abstract
Ataxia telangiectasia mutated (ATM) kinase is critical in sensing and repairing DNA double-stranded breaks (DSBs) such as those induced by temozolomide (TMZ). ATM deficiency increases TMZ sensitivity, which suggests that ATM inhibitors may be effective TMZ sensitizing agents. In this study, the TMZ sensitizing effects of 2 ATM specific inhibitors were studied in established and xenograft-derived glioblastoma (GBM) lines that are inherently sensitive to TMZ and derivative TMZ-resistant lines. In parental U251 and U87 glioma lines, the addition of KU-55933 to TMZ significantly increased cell killing compared to TMZ alone [U251 survival: 0.004 ± 0.0015 vs. 0.08 ± 0.01 (p<0.001), respectively, and U87 survival: 0.02 ± 0.005 vs. 0.04 ± 0.002 (p<0.001), respectively] and also elevated the fraction of cells arrested in G2/ M [U251 G2/ M fraction: 61.8 ± 1.1% vs. 35 ± 0.8% (p<0.001), respectively, and U87 G2/ M fraction 25 ± 0.2% vs.18.6 ± 0.4% (p<0.001), respectively]. In contrast, KU-55933 did not sensitize the resistant lines to TMZ, and neither TMZ alone or combined with KU-55933 induced a G2/ M arrest. While KU-55933 did not enhance TMZ induced Chk1/ Chk2 activation, it increased TMZ-induced residual γ-H2AX foci in the parental cells but not in the TMZ resistant cells. Similar sensitization was observed with either KU-55933 or CP-466722 combined with TMZ in GBM12 xenograft line but not in GBM12TMZ, which is resistant to TMZ due to MGMT overexpression. These findings are consistent with a model where ATM inhibition suppresses the repair of TMZ-induced DSBs in inherently TMZ-sensitive tumor lines, which suggests an ATM inhibitor potentially could be deployed with an improvement in the therapeutic window when combined with TMZ.
Keywords: Temozolomide, Glioblastoma, DNA Repair, ATM inhibitor
INTRODUCTION
Integration of small molecule DNA repair inhibitors into GBM therapy has the potential to enhance the efficacy of temozolomide (TMZ) and improve the outcome of GBM treatment [1]. The key cytotoxic DNA lesion induced by TMZ is O6-methylguanine, which is removed specifically by O6-methylguanine methyltransferase (MGMT)[2]. Disruption of MGMT-mediated repair ultimately can lead to stalled replication forks that degenerate into DNA double strand breaks (DSBs). These DSBs trigger a damage response mediated by ATM and the ATM and Rad3-related kinase (ATR) protein kinases phosphatidylinositol 3’ kinase related kinases (PIKK)[3]. These kinases initiate cell cycle arrest through effects on Chk1 and Chk2 and facilitate the assembly and activation of DNA repair complexes to restore DNA integrity. Consistent with a critical role in DNA repair, ATM inactivation is associated with increased sensitivity to ionizing radiation and other DSB-inducing agents [4]. Following TMZ treatment, ATM modulates the repair of secondary DSBs, and ATM deficiency is associated with increased sensitivity to TMZ [3]. KU-55933 is a specific ATM inhibitor and a potent sensitizing agent when combined with radiation. The specificity of this compound for ATM was established by counter-screening it against other members of the PIKK family which demonstrated a 100-fold differential in selectivity towards ATM kinase activity. KU-55933 specifically inhibited ATM-mediated DNA repair events [4] and also sensitized patient xenograft derived stem-like neurospheres to TMZ. Given the potential role for ATM in modulating the repair of secondary DSBs induced by TMZ, we tested the hypothesis that ATM inhibitors would enhance the efficacy TMZ in inherently TMZ-sensitive glioma cell lines in which TMZ treatment will result in DNA double-strand breaks, and compared the combination treatment in paired TMZ-resistant cell lines.
MATERIALS & METHODS
Cell Culture & Antibodies
U251 and U87 malignant glioma cell lines were maintained in DMEM (Life Technologies, Inc.) supplemented with 10% fetal bovine serum, 1% penicillin and 1% streptomycin. U251 and U87 cells were cultured and passaged over 8 weeks in the presence of escalating concentrations of TMZ (30 to 300 microM) to generate TMZ resistant lines, which are denoted as U251TMZ and U87TMZ, respectively. Short-term explant cultures from the primary GBM12 xenograft line and a derivative resistant GBM12TMZ line were grown in Neurobasal media (Invitrogen catalog# A1050901)[5]. Antibodies specific for phospho-Chk1 (catalog #2341), phospho-Chk2 (catalog #2661), total Chk1 (catalog #2345), total Chk2 (catalog #2662), γ-H2AX (catalog #2577) were obtained from Cell Signaling, and phospho-ATM (catalog #ab81292) and ATM (catalog #10939) were obtained from Abcam. KU-55933 was synthesized by Ryss Laboratories Inc. and CP466722 (catalog #S2245) was purchased from Selleck Chemicals.
Cy-Quant Cell Proliferation Assay
U251 and U87 malignant glioma cell lines were plated at a density of 1000 and 500 cells per well, respectively, in 96-well plates, treated with 10, 30, 100 and 300 microM TMZ for 6 days and processed per manufacturer’s instructions (Invitrogen, CA).
Clonogenic assay
The effect of KU-55933 on TMZ sensitivity of U87 and U251 parental and TMZ resistant cells was assessed in a clonogenic survival assay as previously described [6, 7]. Cells were treated with 10, 30 or 300 microM TMZ alone or with a 1 hour pretreatment with 10 microM KU-55933 and cultured for 2 weeks. Resultant colonies were stained with Coomassie Blue and quantitated.
Western Blotting
Cells were treated with 30 microM TMZ with or without 10 microM KU-55933 and processed as previously described [8]. The effects of graded concentration of KU-55933 or CP466722 on ATM phosphorylation were evaluated similarly. Immunoblotting was carried out using nitrocellulose membranes and the indicated antibodies as previously described [6, 7].
Flow Cytometric Analysis
Cells treated with TMZ (10 microM) alone or in combination with KU-55933 (10 microM) were trypsinized, pelleted, fixed in 70% ethanol/PBS, stored at −20°C and analyzed by flow cytometry as described [6].
Immunocytochemistry
Cells cultured on coverslips were treated with 30 microM TMZ with or without 10 microM KU-55933 for 24 hours or 72 hours, fixed with 4% paraformaldehyde, permeabilized with 0.3 % Triton-X100 and stained overnight with a γ-H2AX specific primary antibody (Cell Signaling, #2557). A rhodamine conjugated secondary antibody (Jackson Immunolabs) and DAPI were used for the detection of foci and nuclei, respectively. Coverslips were mounted on slides with mounting media (Dako) and analyzed using a Zeiss LSM 510 Confocal Laser Scanning Microscope at 60X magnification and the number of nuclei positive for foci was quantitated.
Neurosphere Formation Assays
Short-term explant cultures from GBM12 and GBM12TMZ xenografts seeded in triplicate at (250 cells per well in a 96-well plate) and cultured in stem cell media (Invitrogen A1050901). Four hours post-seeding, TMZ, with or without either KU-55933 or CP-466722 were added to respective wells, and the number of neurospheres formed after 14 days of incubation were counted [5].
RESULTS
Characterization of parental and TMZ resistant cell lines
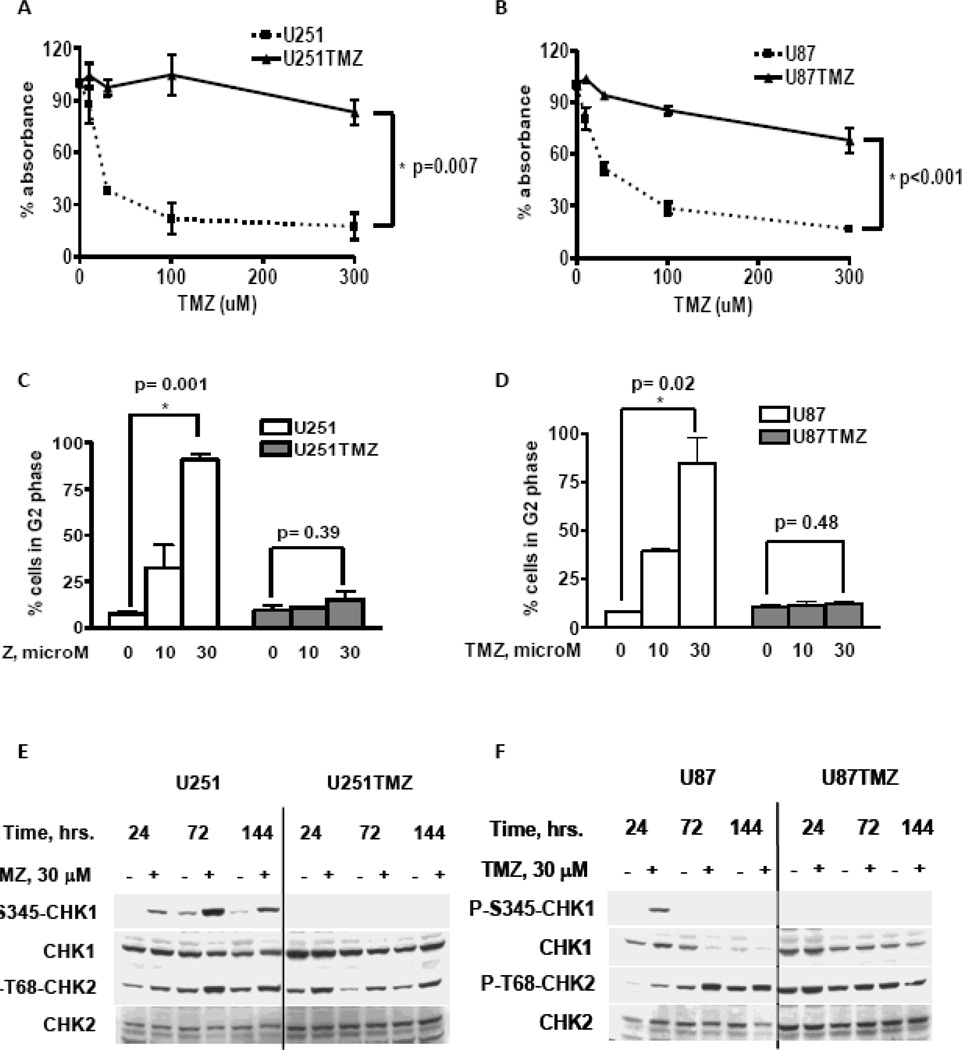
TMZ resistant sub-lines of U251 and U87 cells were generated by exposure to escalating doses of TMZ (30, 100 and 300 microM) over a period of two months. Surviving colonies were pooled to establish the TMZ-resistant U251TMZ and U87TMZ cell lines, respectively. In a CyQuant cell proliferation assay, these lines were markedly more resistant to the clinically relevant dose range of 30–100 microM TMZ compared to the parental lines (Figure 1A and 1B). Compared to control, treatment with 30 microM TMZ was associated with a relative absorbance of 38.2 ± 2.1% in U251 cells versus 97.4 ± 4.8% in U251TMZ cells (p<0.001) and 51.4 ±3.4% in U87 versus 94.1 ± 0.9% in U87TMZ (p<0.001). The TMZ-induced DNA damage response also was characterized in these lines by flow cytometry and western blotting. Treatment with 30 microM TMZ resulted in a marked increase in the fraction of cells arrested in G2/M, compared to untreated cells 72 hours after treatment for parental U251 (90.8 ± 3.3 % vs. 7.6 ± 1.4 %, respectively, p=0.001) and U87 cells (84.6 ± 13 % vs. 7.9 ± 1%, respectively, p=0.001). In contrast, the TMZ resistant cells did not accumulate in G2/M following treatment with TMZ (Figures 1C and D). Consistent with checkpoint activation, treatment of parental U251 and U87 cells with 30 microM TMZ resulted in prolonged induction of T68-Chk2 phosphorylation in the parental U251 and U87 cells at 24, 72 and 144 hour time points (Figure 1E and F). Similarly, increased phosphorylation of S345-Chk1 following TMZ treatment was observed at all 3 time points in U251 and only at 24 hours in U87 cells. In contrast, TMZ treatment in U251TMZ and U87TMZ lines was associated with a lack of Chk1 phosphorylation and marginal changes in Chk2 phosphorylation compared to untreated controls. Thus, in comparison to the parental lines, development of TMZ resistance in the U87TMZ and U251TMZ lines is associated with a loss of TMZ-induced G2/M arrest and associated checkpoint activation.
Figure 1.
Characterization of TMZ resistant GBM cell lines. (A–B) The indicated cell lines were plated in triplicates and exposed to graded concentrations of TMZ. After incubation for 144 hours, cell survival was quantified using the CyQuant colorimetric assay. The mean relative absorbance ± SE from two independent experiments is presented. (C–D) Cells treated with or without 30 microM TMZ were fixed at 72 hours and analyzed for cell cycle distribution by flow cytometric analysis of cellular DNA content. The percent of cells in G2/M (mean ± SEM from three independent experiments is presented. (E) Cells treated with or without TMZ were harvested at indicated time points, and phosphorylation and total levels of Chk1 and Chk2 were analyzed by western blot. Representative results from 3 independent experiments are shown.
ATM inhibitor KU-55933 sensitizes only parental GBM cell lines to TMZ
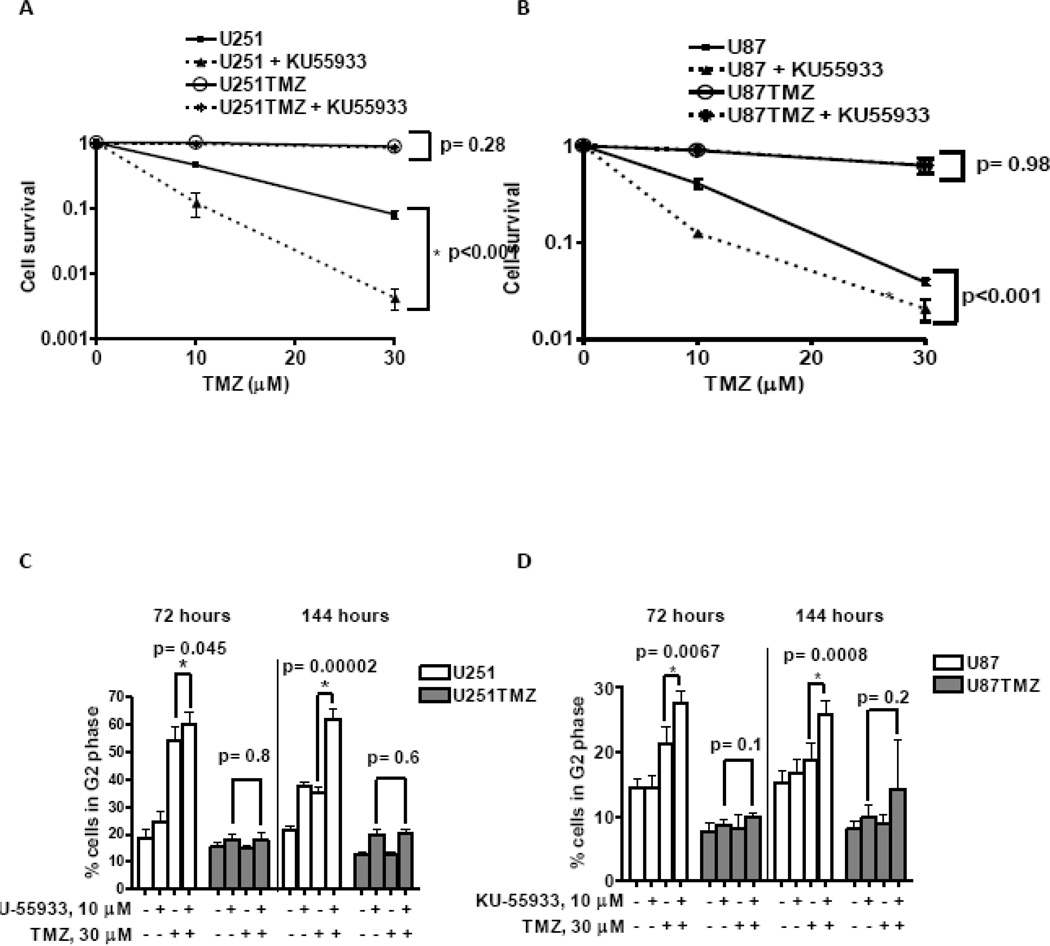
The effects of KU-55933 on cell survival were examined using a clonogenic assay. Treatment with 10 microM KU-55933 significantly sensitized U251 cells to TMZ (Figure 2A; survival after 30 microM TMZ 0.08 ± 0.01 without KU-55933 versus 0.004 ± 0.001 with KU-55933, p<0.001). U87 cells also were sensitized by KU-55933 treatment, although the extent of sensitization was less profound (Figure 2B; survival after 30 microM TMZ 0.04 ± 0.002 without KU-55933 versus 0.02 ± 0.005 with KU-55933. p<0.001). In contrast, the KU-55933 did not sensitize either TMZ resistant line to TMZ (U251TMZ survival: 0.84 ± 0.03 vs. 0.87 ± 0.01, respectively, p>0.1, and U87TMZ survival: 0.62 ± 0.03 vs 0.63 ± 0.09, respectively, p>0.1). These data suggest that KU-55933 selectively sensitizes parental but not TMZ-resistant GBM cells to TMZ.
Figure 2.
KU55933 sensitizes TMZ responsive GBM cells to TMZ treatment. The effect of KU55933 on TMZ sensitivity of U251 (A) and U87 (B) cells was assessed in a clonogenic assay. Cell survival (mean ± SEM from three independent experiments) is plotted relative to TMZ dose for treatment with or without 10 microM KU55933. (C–D) Cells were collected at indicated time points after treatment with 30 microM TMZ alone or in combination with 10 microM KU-55933, fixed, and analyzed for cell cycle distribution by flow cytometric analysis of cellular DNA content. The percent of cells in G2/M (mean ± SEM from three independent experiments) is presented.
Consistent with the selective sensitizing effects of KU-55933 in the parental cells, KU-55933 increased TMZ-induced G2/M accumulation of cells compared to TMZ treatment alone. Both TMZ and TMZ + KU-55933 treatments resulted in a significant accumulation of U251 cells at G2/M 72 hours following treatment, but by 144 hours after treatment, combined treatment with KU-55933 and TMZ was associated with a persistent G2/M arrest (61.8 ± 1.1% cells in G2/M) as compared to treatment with TMZ alone (35 ± 0.8% cells in G2/M, p<0.001; Figure 2C). In U87 cells, the increased G2/M accumulation associated with combined TMZ/KU-55933 treatment compared to TMZ alone was observed both at 72 hours (27.5 vs. 21.4 respectively; p=0.007) and 144 hours (25.7 vs. 18.7 respectively; p<0.001) (Figure 2D). In contrast, co-treatment of the resistant lines with KU-55933 and TMZ did not result in an increase in the fraction of cells arrested in G2/M, as compared to monotherapy (U251TMZ G2/M fraction: 20 ± 0.6% vs. 19.7 ± 1.9% (p=0.58), respectively and U87TMZ G2/M fraction 14 ± 3.14% vs. 9.8 ± 1.9%, (p=0.2), respectively). Thus, the effects of KU-55933 on TMZ-induced G2/M arrest are significantly greater in the inherently sensitive U251 and U87 cells as compared to the TMZ-resistant lines.
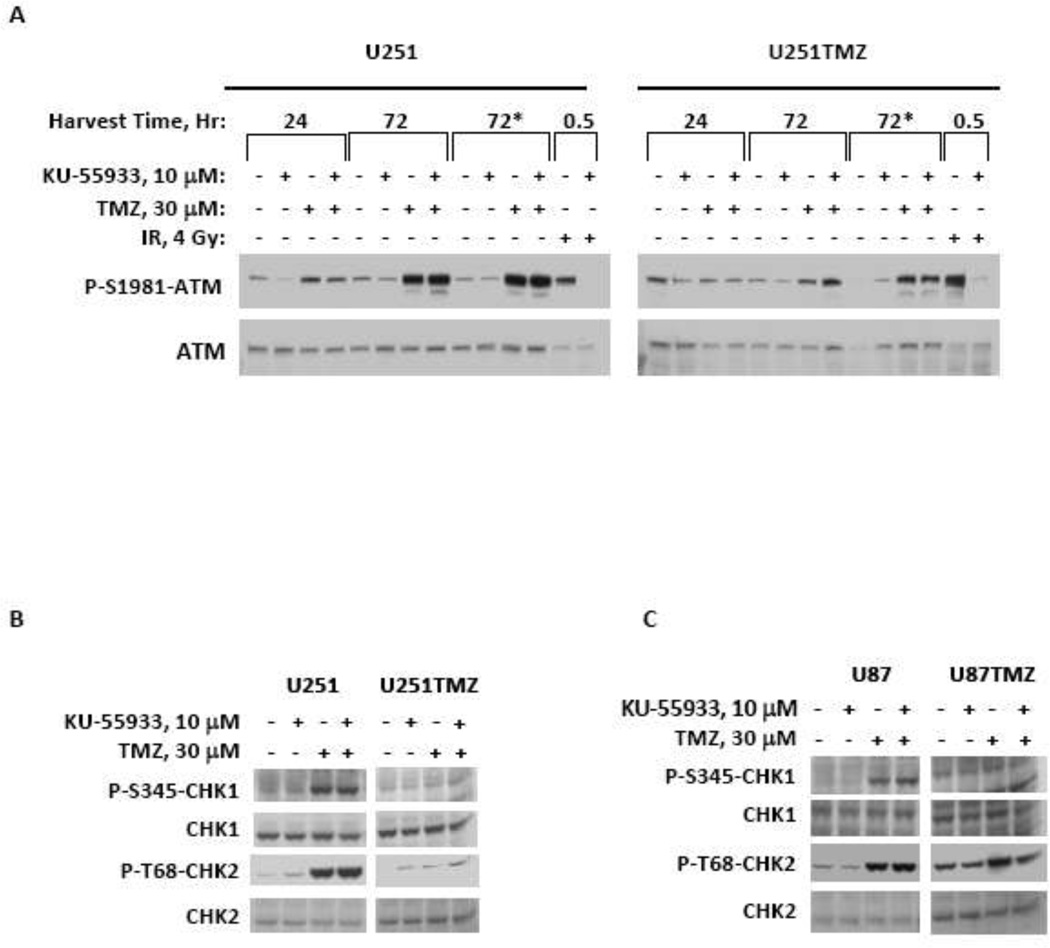
In conjunction with the cell cycle analysis, the effects of KU-55933 on TMZ-induced phosphorylation of ATM, Chk1 and Chk2 were characterized. Phosphorylation of Ser1981 on ATM has previously been reported as a marker of ATM activation, and in parental U251 cells, TMZ treatment induced ATM phosphorylation by 24 hours with robust activation by 72 hours (Figure 3A). Interestingly, co-treatment with KU55933 resulted in only minimal suppression of phosphorylation at either site 24 hours after treatment and had no effect at 72 hours after treatment despite robust suppression of radiation-induced ATM phosphorylation. In contrast, TMZ treatment in U251TMZ cells resulted in a delayed and blunted ATM phosphorylation that was not reproducibly affected by KU55933 co-treatment. Similar to the ATM activation pattern, treatment with TMZ with or without KU-55933 for 72 hours resulted in robust phosphorylation of S345-Chk1 and T68-Chk2 in both U251 (Figure 3B) and U87 cells (Figure 3C). In contrast, TMZ-induced phosphorylation of either Chk1 or Chk2 was markedly attenuated with either treatment in the corresponding U251TMZ and U87TMZ lines, and no reproducible differences were observed between therapy with TMZ alone vs. TMZ + KU-55933. These data suggest an attenuated damage signal induced by TMZ with or without KU-55933 in the TMZ-resistant cell lines.
Figure 3.
KU-55933 inhibits ATM-mediated phosphorylation events. (A) Cells treated with indicated concentrations of TMZ or radiation in combination with or without KU-55933, were harvested and processed for western blot analysis of phospho- and total levels of ATM at 24 and 72 hours (* indicates KU-55933 was added a second time to the media 48 hours after initial treatment). (B–C) Cells treated with 30 microM TMZ alone or in combination with 10 microM KU-55933 were harvested at 72 hours and phospho- and total levels of Chk1 and Chk2 were analyzed by western blot. Representative results from three independent experiments are presented where paired parental and TMZ resistant lines were run on the same gel but intermediate non-relevant lanes were cropped out of the image.
Influence of KU-55933 on DNA damage processing
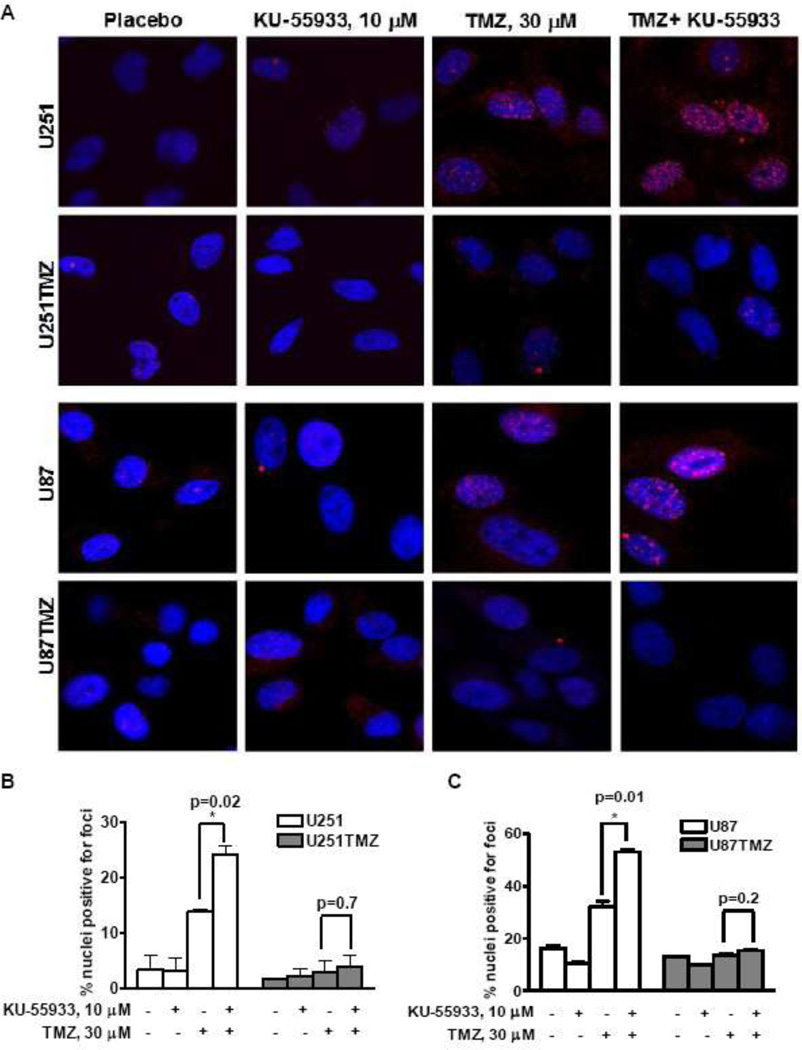
Unrepaired DSBs appear as residual punctuate nuclear γ-H2AX foci indicative of incomplete or defective DSB repair [9]. Hence, γ-H2AX was used to assess the repair of TMZ-induced DNA damage in the sensitive and resistant lines. KU-55933 increased TMZ-induced percent nuclei positive for γ-H2AX foci 72 hours after treatment compared to TMZ alone in the parental lines (Figure 3 - U251 % nuclei positive for foci: 24 ± 1.5% vs. 13.7 ± 0.5%, respectively, p=0.02; U87 % nuclei positive for foci: 52.9 ± 1% vs. 32.5 ± 2.5%, respectively, p=0.01). In contrast, neither treatment with TMZ alone nor TMZ in combination with KU-55933 induced a significant increase in γ-H2AX foci in U251TMZ and U87TMZ. Collectively, these results demonstrate that KU-55933 significantly enhances the number of residual DNA DSBs induced by TMZ only in the inherently sensitive U251 and U87 cell lines.
Effect of ATM inhibitors on primary GBM xenograft lines
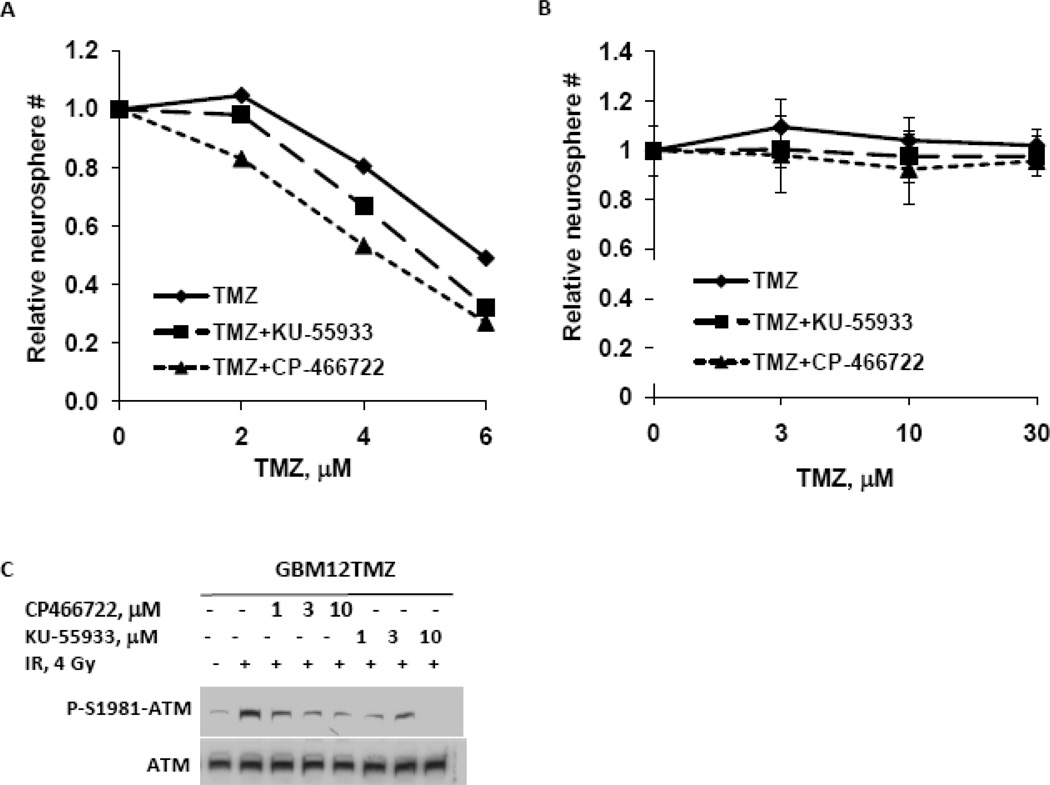
The effects of ATM inhibition on TMZ sensitivity were extended by testing either KU-55977 or a second ATM inhibitor, CP-466722 [10], in short-term explant cultures derived from the primary GBM12 and derivative GBM12TMZ xenograft line. Treatment of GBM12 cells with either KU-55933 or CP-466722 sensitized GBM12 cells to TMZ (Figure 4B; survival after 6 microM TMZ alone 0.49 ± 0.09 versus 0.32 ± 0.05 with TMZ and KU-55933, p=0.06 or 0.20 ± 0.06 with TMZ and CP-466722, p=0.02). In contrast, combination of TMZ with either KU-55933 or CP-466722 had no effect on neurosphere formation in the TMZ-resistant GBM12TMZ line (Figure 4C; survival after 30 microM TMZ alone 1.02 ± versus 0.98 ± 0.08 with KU-55933, p=0.5 or 0.96 ± 0.04 with KU-55933, p=0.4). To test whether the lack of sensitization in the GBM12TMZ resistant line was due to lack of ATM inhibition, the effects of either ATM inhibitor on radiation-induced ATM phosphorylation was assessed in GBM12TMZ neurospheres. As seen in Figure 4C, both inhibitors effectively suppressed radiation induced Ser-1981-ATM phosphorylation, suggesting that the lack of TMZ sensitization by ATM inhibitors in the TMZ-resistant neurospheres is specific to TMZ induced damage. Collectively, these data further support the idea that ATM inhibitors may enhance the efficacy of TMZ in tumors that are inherently sensitive to TMZ.
Figure 4.
Influence of KU55933 on the processing of TMZ induced DNA damage. (A) Seventy-two hours after TMZ treatment, unrepaired DSBs were visualized by staining γ-H2AX foci (red) formed in the nuclei (blue). (B–C) Percent nuclei positive for foci (mean ±S.E from two independent experiments) are presented.
DISCUSSION
TMZ is an integral component of chemotherapy for glioblastoma (GBM). Unrepaired TMZ induced O6 methyl guanine (O6MG) lesions result in replication-associated DSBs which trigger cell death if left unrepaired [11]. Hence, targeting DSB repair pathways in combination with TMZ therapy may be a useful therapeutic strategy in GBM. The related kinases ATM, ATR, and DNA-PKcs play key roles in DNA repair and development of inhibitors for each of these kinases has been actively pursued. The ATM inhibitor, KU-55933, inhibits ATM at 100-fold lower concentrations compared to ATR or DNA-PKcs, and inhibits ATM-specific DNA repair events [4]. Similarly, CP-466722, an ATM-specific inhibitor identified by screening a targeted compound library, suppresses ATM-dependent phosphorylation and disrupts ATM function but does not affect ATR and DNA-PKcs activity [10]. In this study, both CP-466722 and KU-55933 enhanced the efficacy of TMZ only in inherently sensitive tumor lines and this effect was associated with increased DSB accumulation in inherently TMZ sensitive GBM tumor lines consistent with disruption of ATM-mediated DNA repair processes. These data support the concept of combining next generation ATM inhibitors with TMZ in a selected subset of tumors based on predicted TMZ sensitivity.
The selective sensitization of inherently TMZ-sensitive tumor cells suggests that induction of DNA damage by TMZ may be important for the sensitizing effects of KU-55933. TMZ treatment in parental tumor lines induced robust checkpoint activity up to 144 hours after treatment and was associated with an increase in the formation of γ-H2AX foci, which is indicative of DNA DSB induction. These data are consistent with a model in which O6MG lesions mis-pair with thymidine during replication and are recognized by the mismatch repair (MMR) pathway [3]. Resulting futile cycles of MMR result in stalled replication forks that subsequently can degenerate into lethal DSBs [3]. In contrast to the parental lines, treatment of the TMZ resistant lines was associated with a distinct lack of checkpoint activation or γ-H2AX foci induction. Given the importance of ATM in regulating DNA damage repair, we hypothesize that inhibition of ATM suppresses DNA repair of the replication-induced DSBs, which can account for the selective sensitizing effect in the tumor lines inherently sensitive to TMZ. The extent of KU55933 mediated TMZ sensitization was much lower in the parental U87 cells as compared to U251 cells. U87 is wild-type for p53 while U251 has mutant p53, and previous studies with caffeine at concentrations that inhibit ATM and ATR suggest that ATM inhibitors may selectively radiosensitize tumors specifically with deficient p53 status. Our preliminary results also suggest that p53 status may be important for TMZ-sensitizing effects, although this needs to be addressed in future studies using isogenic cell lines wild-type or deficient for p53.
The mechanism of TMZ resistance does not appear to have a significant impact on the lack of sensitizing effects of ATM inhibitors in our models. High level MGMT expression is a well-established mechanism of TMZ resistance, and resistance in GBM12TMZ is mechanistically linked to MGMT over-expression [5]. In contrast, MGMT is not overexpressed in either U87TMZ or U251TMZ lines (supplemental data Figure 1). While previous studies have established an association between TMZ resistance and mutations in the MSH6 and MSH2 mismatch repair genes, sequencing of these 2 genes did not reveal any mutations unique to the TMZ resistant lines and associated with the emergence of TMZ resistance (data not shown). While these lines could harbor mutations in in the other MMR genes such as PMS2 and MLH1, at this point the mechanism of TMZ resistance in U87TMZ or U251TMZ is clearly different from GBM12TMZ. Regardless of the mechanism of TMZ resistance, the results in this study demonstrate, in 3 different glioma models that the sensitizing effects of ATM-inhibition are limited to the parental lines, and at least in U87 and U251, the sensitizing effects of combined treatment were associated with significantly increased H2AX foci. These data suggest that the lack of sensitizing effects of KU55933 or CP-466722 in the resistant lines may be related to the absence of TMZ induced DSBs in these resistant lines.
Although the classic description of the ATR and ATM signaling pathways place ATR upstream from Chk1 and ATM upstream from Chk2, there is significant cross-talk between the 2 pathways. Specifically relevant to the current studies, ATR can mediate Chk2 phosphorylation in response to replicative stress [3]. Recent studies demonstrate that ATR can phosphorylate ATM at Ser1981 in addition to autophosphorylation by ATM [12].This may explain why KU-55933 did not block either ATM P-Ser1981 or Chk2 phosphorylation at 72 hours. Similarly, both ATR and ATM, can phosphorylate H2AX in response to damage; in the present study γ-H2AX foci were increased following treatment with KU-55933, which is keeping with a role for ATR in H2AX phosphorylation following replicative DSBs [13]. Although there is functional overlap between ATM and ATR in sensing DSBs, ATM plays a separate role in the processing of DSBs through direct interaction with key DNA repair proteins [14]. Hence, while ATM inhibition by KU-55933 did not inhibit checkpoint activation or H2AX phosphorylation, the KU-55933-associated increase in TMZ-induced residual γ-H2AX foci is consistent with a model where ATM inhibition suppresses repair of TMZ induced replicative-induced DNA DSBs.
The current prognosis of GBM patients is extremely poor with a 15 month median survival with combined radiation and TMZ therapy, and novel therapeutic strategies are desperately needed. The studies presented here in both established and primary GBM models provide a framework for evaluation of ATM inhibitors currently in development. While the currently available ATM inhibitors, including KU-55933 and CP-466722, lack appropriate pharmacokinetic properties to allow in vivo efficacy studies, our data suggests inherently TMZ-sensitive tumors are more likely to respond to combination of an ATM inhibitor with TMZ compared to TMZ resistant or recurrent tumors. Multiple studies have demonstrated that tumor MGMT promoter hypermethylation is associated with increased responsiveness to TMZ, although these patients still have an ultimately very poor prognosis [11]. This suggests that MGMT hypermethylated tumors may benefit most from an ATM-inhibitor based TMZ sensitizing strategy. Normal tissues generally express high-level MGMT and are resistant to TMZ compared to TMZ-sensitive tumor cells that have suppressed MGMT expression[15]. Hence, an ATM inhibitor may effectively sensitize this subset of MGMT-methylated tumors to TMZ without marked sensitization of the bone marrow cells. This is in contrast to previous attempts to employ an MGMT inhibitor O6-benzyl guanine (O6BG) as a TMZ-sensitizing agent in which combinations with TMZ was associated with enhanced myelotoxicity [16]. In conclusion, selective TMZ sensitization by KU55933 or CP-466722 of inherently sensitive cells provides a strong rationale for testing next generation ATM inhibitors in GBM models with differing sensitivities to TMZ.
Supplementary Material
Figure 5.
ATM-inhibition sensitizes TMZ responsive patient xenograft derived stem-like neurospheres to TMZ. (A–B) The effect of two ATM inhibitors, KU-55933 and CP466722 on the TMZ sensitivity of parental GBM12 and TMZ resistant GBM12TMZ neurospheres was assessed by a neurosphere formation assay. Neurosphere survival (Mean ± SEM from three independent experiments) is plotted relative to TMZ concentration with or without either KU-55933 (10 micromolar) or CP-466722 (3 micromolar).
Acknowledgments
Funding: Grant support provided by NIH SPORE grant CA108961, NIH RO1 CA127716, and the Brain Tumor Funder’s Consortium (all to JNS).
The authors thank James Tarara and the Mayo Flow Cytometry and Optical Morphology Core personnel.
Footnotes
Conflict of Interest: JNS is a recipient of a research grant from Merck Pharmaceuticals for unrelated research.
References
- 1.Helleday T, et al. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8(3):193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 2.Kaina B, et al. BER, MGMT, and MMR in defense against alkylation-induced genotoxicity and apoptosis. Prog Nucleic Acid Res Mol Biol. 2001;68:41–54. doi: 10.1016/s0079-6603(01)68088-7. [DOI] [PubMed] [Google Scholar]
- 3.Caporali S, et al. DNA damage induced by temozolomide signals to both ATM and ATR: role of the mismatch repair system. Mol Pharmacol. 2004;66(3):478–491. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 4.Hickson I, et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64(24):9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 5.Kitange GJ, et al. Inhibition of Histone Deacetylation Potentiates the Evolution of Acquired Temozolomide Resistance Linked to MGMT Upregulation in Glioblastoma Xenografts. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkaria JN, et al. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58(19):4375–4382. [PubMed] [Google Scholar]
- 7.Eshleman JS, et al. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res. 2002;62(24):7291–7297. [PubMed] [Google Scholar]
- 8.Alderton GK, et al. Regulation of mitotic entry by microcephalin and its overlap with ATR signalling. Nat Cell Biol. 2006;8(7):725–733. doi: 10.1038/ncb1431. [DOI] [PubMed] [Google Scholar]
- 9.Banath JP, et al. Residual gammaH2AX foci as an indication of lethal DNA lesions. BMC Cancer. 10:4. doi: 10.1186/1471-2407-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rainey MD, et al. Transient inhibition of ATM kinase is sufficient to enhance cellular sensitivity to ionizing radiation. Cancer Res. 2008;68(18):7466–7474. doi: 10.1158/0008-5472.CAN-08-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkaria JN, et al. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(10):2900–2908. doi: 10.1158/1078-0432.CCR-07-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stiff T, et al. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 2006;25(24):5775–5782. doi: 10.1038/sj.emboj.7601446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276(51):47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 14.Cortez D, et al. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286(5442):1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 15.Hegi ME, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 16.Quinn JA, et al. Phase II trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J Clin Oncol. 2009;27(8):1262–1267. doi: 10.1200/JCO.2008.18.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.