Abstract
This research article reports halophilic and halotolerant bacteria isolated from mangrove forests located in Northern Vietnam. Several of these bacteria were able to synthesize polyhydroxyalkanoates (PHAs). PHAs are polyesters stored by microorganisms under the presence of considerable amounts of a carbon source and deficiency of other essential nutrient such as nitrogen or phosphorous. Mangrove forests in Northern Vietnam are saline coastal habitats that have not been microbiologically studied. Mangrove ecosystems are, in general, rich in organic matter, but deficient in nutrients such as nitrogen and phosphorus. We have found about 100 microorganisms that have adapted to mangrove forests by accumulating PHAs. The production of polyesters might therefore be an integral part of the carbon cycle in mangrove forests. Three of the strains (ND153, ND97, and QN194) isolated from the Vietnamese forests were identified as Bacillus species, while other five strains (QN187, ND199, ND218, ND240, and QN271) were phylogenetically close related to the α-proteobacterium Yangia pacifica. These strains were found to accumulate PHAs in noticeable amounts. Polymer inclusions and chemical structure were studied by transmission electron microscopy and proton nuclear magnetic resonance (NMR) spectroscopy analyses, respectively. Strains ND153, ND97, QN194, QN187, ND240, and QN271 synthesized poly(3-hydroxybutyrate) (PHB) from glucose, whereas strains ND199 and ND218 synthesized poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) from this carbohydrate. With the exception of strain QN194, the strains accumulated PHBV when a combination of glucose and propionate was included in the culture medium. The polymer yields and cell growth reached by one Bacillus isolate, strain ND153, and one Gram-negative bacterium, strain QN271, were high and worth to be researched further. For experiments performed in shake flasks, strain ND153 reached a maximum PHBV yield of 71 wt% and a cell dry weight (CDW) of 3.6 g/L while strain QN271 attained a maximum PHB yield of 48 wt% and a CDW of 5.1 g/L. Both strain ND153 and strain QN271 may only represent a case in point that exemplifies of the potential that mangrove forests possess for the discovery of novel halophilic and halotolerant microorganisms able to synthesize different types of biopolyesters.
Keywords: Biopolyesters, halophilic bacteria, halotolerant bacteria, mangrove forests, polyhydroxyalkanoates
Introduction
Microbial polyesters, also known as polyhydroxyalkanoates (PHAs), are synthesized by various microorganisms as a physiological strategy related to the utilization of nutritional resources in an ecosystem. When a carbon source is in excess in the ecosystem, while other essential nutrient (e.g., nitrogen, phosphorous, or oxygen) is insufficient to promote cell growth, the carbon source may be transformed to polyesters as intracellular carbon and energy storage compounds for the cells (Steinbüchel and Füchtenbush 1998) – such transformation was recognized as a very proficient survival strategy used by several microorganisms (Babel et al. 2001). The biopolyesters resemble to plastics or elastomers derived from petroleum depending on their chemical structure (Steinbüchel and Füchtenbush 1998), although PHAs can be completely metabolized to CO2 and water under aerobic conditions or to methane and CO2 in anaerobic environments by various organisms.
Microorganisms transform sugars and fatty acids to PHAs through metabolic pathways that involve as intermediate either acetyl-CoA or acyl-CoA and conclude with monomer polymerization by PHA synthases (Philip et al. 2007). The ability of microorganisms to synthesize a particular form of PHA is mainly due to the substrate specificity of PHA synthases; these enzymes may be divided into four classes (Rehm 2003). PHA synthases belonging to class I utilize CoA thioesters of 3-hydroxyalkanoates (3-HAs) comprising 3–5 carbon atoms, whereas class II polymerases direct their specificity toward CoA thioesters of 3-HAs with 6–14 carbon atoms. Synthases of both classes I and II are encoded by PhaC gene. Class III synthases are composed of two genes (PhaC and PhaE) that possess substrate specificities similar to class I, although the PhaCE subunit can also polymerize 3-HAs with 6–8 carbon atoms. Class IV synthases are also composed of two genes (PhaC and PhaR) that utilize 3-HA monomers with 3–5 carbon atoms (Rehm 2003). Poly(3-hydroxybutyrate) (PHB) is the most common type of the PHAs synthesized by microorganisms, and is rigid and brittle (Steinbüchel and Füchtenbush 1998; Philip et al. 2007). However, copolymers with varying monomer compositions can also be produced resulting in a high diversity of PHA molecules possessing a broad range of physico-chemical and mechanical properties, for example, poly(3-hydroxybutyrate-co-hydroxyvalerate) (PHBV) that is a more flexible material than PHB (Steinbüchel and Füchtenbush 1998; Philip et al. 2007). PHAs are also biocompatible and lack toxicity (Philip et al. 2007). Owning to this features, PHAs have been used to develop some devices for medical applications including biodegradable sutures, meniscus repair devices, bone plates, heart valves, nerve conduits, and drug delivery systems (Chen and Wu 2005; Wu et al. 2009).
Studies on production of PHAs by halophilic, salt (NaCl) requiring microorganisms, were recently initiated (Quillaguamán et al. 2010). The advantage of using of halophilic microorganisms in PHA production systems is related to their ability to grow optimally at high salt concentrations (Quillaguamán et al. 2010). At determined concentrations of salt, the growth of nonhalophilic microorganisms is prevented, hence allowing a process without strict sterile conditions and reducing the inherent costs, such as the costs of the energy required for sterilizing the equipment for fermentation and culture media (Quillaguamán et al. 2010). Nevertheless, salts in the medium are to be concentrated and recycled in order to reduce the overall process costs as well as to minimize ecological pollution implicit in the disposal of the fermentation residues (Quillaguamán et al. 2010). Several halophilic archaea and bacteria isolated from marine-related niches are able to accumulate PHA, albeit only a few reached yields and volumetric productivities high enough to be considered for industrial purposes (Quillaguamán et al. 2010). However, the biotechnological potential of halophiles remains to be studied further. Mangrove forests in Northern Vietnam are saline coastal habitats composed by shrubs and medium height trees. Mangrove ecosystems are rich in organic matter; however, they are usually nutrient-deficient, especially in nitrogen and phosphorus (Alongi et al. 1989). Many different microorganisms including bacteria, fungi, protozoa, and algae have been found in mangrove ecosystems (Holguin et al. 2001). Among these microbes, the bacterial population is many-fold greater than the others. Because of its diversity, bacterial activity is responsible for most of the mineral cycle and the carbon flux in the mangrove ecosystems, and act as a carbon sink (Holguin et al. 2001).
This article reports the production of PHAs by halophilic and halotolerant bacterial species isolated from mangrove soil samples in Northern Vietnam – these environments have not been microbiologically studied. The isolates were identified by molecular analysis of their 16S rDNA sequences and phenotypic characterization. The chemical structure of the polymer synthesized by these microorganisms was determined by nuclear magnetic resonance (NMR) spectroscopy analysis. The polymer inclusions, their size, number, and organization in the cells were studied under electron microscopy. Furthermore, the cell densities and polymer yields reached by the isolates were also evaluated using either glucose or a mixture of glucose and propionate in the culture medium.
Materials and Methods
Isolation of bacterial strains
Soil samples from mangroves in Northern Vietnam at Giao Thuy district, Nam Dinh province, and at Yen Hung district, Quang Ninh province, were collected and serially diluted with sterile sea water, and then 100 μL of the dilution was spread on solid HM medium (Quillaguamán et al. 2004), containing (g/L): NaCl, 30; MgSO4·7H2O, 0.25; CaCl2, 0.09; KCl, 0.5; NaBr, 0.06; peptone, 5.0; yeast extract, 10.0; glucose, 1.0; and granulated agar, 20; and pH adjusted to 7 using 2 N NaOH. The plates were incubated at 35°C for 30 h. Several hundreds of colonies were isolated by plating them again on fresh agar medium.
Detection of PHA in bacteria
Bacterial isolates were grown on a modified solid HM medium (HM-1) containing (g/L): NaCl, 30; MgSO4·7H2O, 0.25; CaCl2, 0.09; KCl, 0.5; NaBr, 0.06; KH2PO4, 0.25, yeast extract, 2.0; glucose, 20; granulated agar, 20; and Nile red (Sigma, Steinheim, Germany) (dissolved in dimethylsulfoxide) with a final concentration of 0.5 μg dye per mL of the medium (Spiekermann et al. 1999). Petri dishes were incubated at 35°C for 2 days. The agar plates were then exposed to untraviolet light (312 nm) to detect the presence of intracellular PHA granules in the bacteria (Spiekermann et al. 1999). The colonies with fluorescent bright orange were chosen for further studies.
Transmission electron microscopy observation
PHB-containing cells were fixed and observed under transmission electron microscopy (TEM) following a protocol reported previously (Quillaguamán et al. 2006). Cells were separated by centrifugation at 4000g for 7 min and fixed for 4 h at room temperature in a solution of 4% (v/v) glutaraldehyde in 0.1 mol/L sodium cacodylate, pH 7.1, and 0.1% (w/v) Brij 35, followed by an overnight treatment in the same solution without Brij 35. The cells were then rinsed with 0.1 mol/L sodium cacodylate, pH 7.1, transferred to 2% osmium tetroxide for 8 h at room temperature and subsequently to 2% uranyl acetate in 10% ethanol for 40 min. The cells were dehydrated through a graded series of ethanol–water solutions with a final treatment in propylene oxide, and embedded in epon/araldite resin that was then cut with a diamond knife. The fine sections of 50 nm were placed on Formvar-coated copper grids, contrasted with a 2% aqueous solution of uranyl acetate and examined under a JEM-1010 transmission electron microscope (Jeol Korea Ltd., Korea).
Phylogenetic and phenotypic characterization of the selected PHA-accumulating bacteria
The morphological and physiological properties of the selected PHA-accumulating bacteria were investigated according to Bergey's Manual of Determinative Bacteriology. For phylogenetic studies, the 16S rDNA of the bacteria was amplified by PCR using universal primers: 314F (5′-CCTACGGGAGGCAGCAG-3′) and 907R (5′-CCGTCAATTCCTTTGAGTTT-3′), and 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). Sequencing of the amplified DNA fragment was performed at Bioneer, Korea. GenBank and Ribosomal Database Project databases were used to seek for 16S rDNA gene similarities. Phylogenetic analysis based on the 16S rDNA gene was performed with the aid of the Mega 5 software package (Tamura et al. 2011), using the neighbor-joining distance correction methods (Saitou and Nei 1987). For constructing a phylogenetic tree, only sequences from the type strains of species whose names have been validly published were taken into account. Almost complete sequences (c.a. 1400 bp) of the 16S rDNA genes of the strains isolated in Vietnam were deposited at GenBank/EMBL/DDBJ databases and were used in the analysis.
Production of PHA by the isolated strains
The selected bacterial strains were grown in 20 mL of HM medium in 100 mL flasks at optimum NaCl concentration and temperature for each strain with rotary shaking at 180 rpm for 13 h. Subsequently, 2.5 mL of each culture was inoculated in 250 mL Erlenmeyer flasks containing 50 mL of HM-1 medium with optimum NaCl concentration for each strain. The pH of this medium was initially adjusted to 7.0 using 5 mol/L NaOH. The cultures were incubated at 32°C for Gram-negative bacteria and at 37°C for Gram-positive bacteria with rotary shaking at 180 rpm. In all cases, samples were withdrawn at 30 h of cultivation for cell dry weight (CDW) determination and PHA content analysis.
Quantitative analysis
CDW was determined by centrifuging 3 mL of the culture samples at 4000 g for 10 min in a preweighed centrifuge tubes, the pellet was washed once with 3 mL distilled water, centrifuged and dried at 105°C until constant weight was obtained. The centrifuge tube was weighed again to calculate the CDW.
PHA content analysis was performed using a gas-chromatographic method (Huijberts et al. 1994). For this, about 10 mg of freeze-dried cells was mixed with 1 mL of chloroform and 1 mL of methanol solution containing 15% (v/v) sulfuric acid and 0.4% (w/v) benzoic acid. The mixture was incubated at 100°C for 3 h to convert the constituents to their methyl esters. After cooling to room temperature, 0.5 mL of distilled water was added and the mixture was shaken for 30 sec. The lower chloroform layer was transferred into a fresh tube and used for GC analysis to determine the PHA content. Sample volume of 2 μL was injected into the gas chromatography column (VARIAN, Factor Four Capillary Column, CP8907). The injection temperature was 250°C, the detector temperature was 240°C, and the column temperature was 60°C for the first 5 min and then increased at 3°C/min until 120°C was reached. PHB and PHBV containing 12% valerate (Sigma) were used as a standard for calibration.
PHA isolation for NMR spectroscopic analysis
NMR analysis was performed as described previously (Quillaguamán et al. 2006). The selected bacterial cells containing the polymer were harvested from 300 mL of culture broth by centrifugation at 6000 g for 10 min, washed twice with distilled water and lyophilized. PHA was recovered from lyophilized cells by extraction for 30 h with chloroform in a Soxhlet apparatus, and concentrated by evaporating the solvent under vacuum. The polymer was precipitated from the concentrated solution with 10 volumes of ethanol and the resulting PHA granulates were filtered twice. The 1H NMR spectrum was recorded at 500 MHz with a Bruker ARX500 Spectrometer (Bruker, Sikerstrifen, Germany) at room temperature using deuterated chloroform as internal reference solvent. The spectrum was evaluated using standard Bruker UXNMR software.
Results
Isolation and screening of PHA-accumulating bacteria
Soil samples collected from mangrove forests at Giao Thuy district, Nam Dinh province, and at Yen Hung district, Quang Ninh province in Northern Vietnam (Fig. 1), were used for this study. Mangrove forests are specialized ecosystems situated at the interphase between land and sea of tropical and subtropical areas (Spalding et al. 2010). We hypothesized that the presence of excess carbon and limitation of nitrogen and phosphorus in mangrove forests (Spalding et al. 2010) could be a favorable condition for the existence of microbes that have the ability to accumulate PHA.
Figure 1.
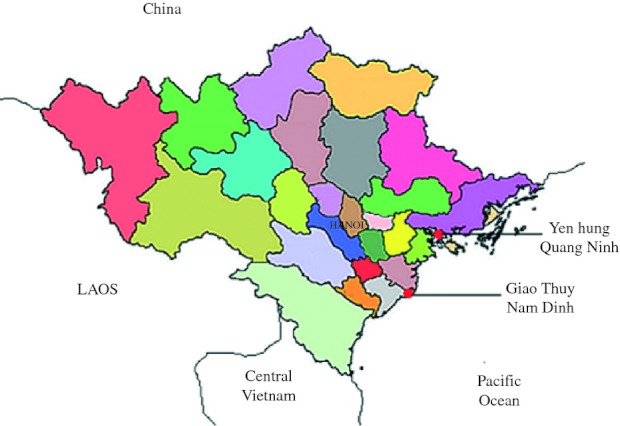
Regions in Vietnam where mangrove forests are located. Mangrove forests located at Giao Thuy district in Nam Dinh province and Yen Hung district in Quang Ninh province were selected to isolate halophilic and halotolerant microorganisms.
The soil samples of the forests were inoculated on solid HM medium, which was previously used to isolate halophilic microorganisms (Quillaguamán et al. 2004). After 30 h of incubation at 35°C, about 500 bacterial colonies were obtained on agar plates. Bacterial colonies were stained with Nile Red dye and PHA-producing bacteria were identified by examining them in a fluorimeter. About 100 fluorescent bacteria were observed to accumulate PHAs; among them, eight bacterial strains that exhibited a very strong fluorescence were selected to be studied further. The strains were named as ND97, ND153, ND199, ND218, ND240 (ND standing for the strains isolated from Nam Dinh), and QN187, QN194, QN271 (QN standing for the strains isolated from Quang Ninh).
Phylogenetic studies based on 16S rDNA sequences
The phylogenetic affiliation of the eight bacterial strains selected was analyzed using their 16S rDNA sequences. The sequences of strains ND153, ND97, and QN194 share a close relationship with sequences of Bacillus species (Fig. 2A). Strains ND153 and ND97 clustered together and had a 16S rDNA similarity of 98.3%. The closest similarity of strains ND153 and ND97 was shared with Bacillus cereus, 99.3% and 98%, respectively, whereas the 16S rDNA sequence of strain QN194 was 98.2% similar to the sequences of B. aryabhattai and B. megaterium (Fig. 2A).
Figure 2.
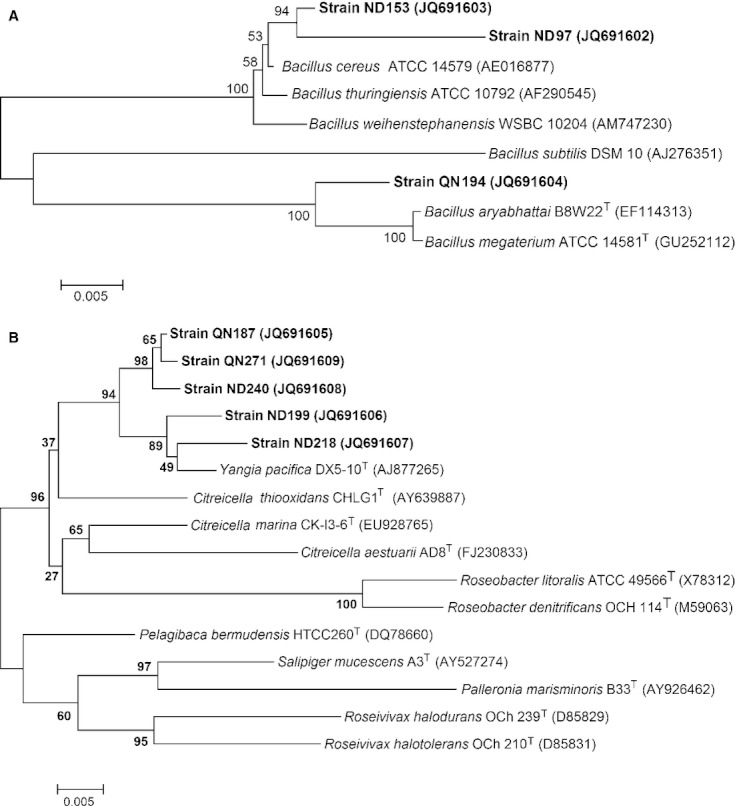
Phylogenetic trees constructed using 16S rDNA gene sequences of (A) Gram-positive bacteria belonging to the genus Bacillus and (B) Gram-negative bacteria within the α-Proteobacteria. Bar, five substitutions per 1000 nucleotides. Numbers at branching points refer to bootstrap values (500 resamplings).
On the other hand, strains QN187, ND199, ND218, ND240, and QN271 gathered with the α-proteobacterium Yangia pacifica (Fig. 2B). The 16S rDNA sequences of these strains were 98.4–98.9% similar to Y. pacifica. Other α-proteobacteria that belong to the genera Citreicella and Roseivivax were found in different clades, and sheared a similarity of 97.4% or lower with the strains isolated from Vietnam.
Phenotypic characterization of the selected bacterial strains isolated from mangroves
The morphological and physiological characteristics of the eight selected strains are summarized in Table 1. All strains were aerobic, rod-shaped, and motile. They were also catalase and oxidase positive and showed a negative test for indol formation (Table 1). All strains were mesophilic with optimum temperatures for growth varying from 30 to 40°C and were able to grow with an optimum pH between 6 and 7 (Table 1). Three strains (ND97, ND153, and QN194) were Gram positive, spore forming, and halotolerant (optimum growth between 0% and 1% NaCl), the remaining five strains (ND199, ND218, ND240, QN187, and QN271) were Gram negative, nonspore forming, and halophilic bacteria (optimum growth between 3% and 7% NaCl) (Table 1). Moreover, the Gram-positive strains were gelatinase, caseinase, and amylase positive and were urease negative, which differ from the tests for Gram-negative strains (Table 1). Regarding the assimilation of carbon sources, l-arabitol, carboxy methyl cellulose, α-methyl-d-mannose, α-methyl-d-glucose were not suitable substrates for the growth of the eight strains, whereas d-raffinose, maltodextrin, maltose, fructose, inulin, glucose, sucrose, cellobiose, and cane molasses promoted the growth of all strains (Table 1).
Table 1.
Phenotypic characteristics of the bacteria isolated from soil at mangrove forests in Vietnam and the reference strains Bacillus cereus and Yangia pacifica
| ND97 | ND153 | QN194 | B. cereus | QN187 | ND199 | ND218 | ND240 | QN271 | Y. pacifica | |
|---|---|---|---|---|---|---|---|---|---|---|
| Morphological characteristics | ||||||||||
| Shape | Rod | Rod | Rod | Rod | Rod | Rod | Rod | Rod | Rod | Rod |
| Size (μm) | 0.7–1.2 × 1.6–3.0 | 0.8–1.2 × 2.0–3.5 | 0.5–0.7 × 1.0–2.5 | >0.9 × >3.0 | 0.4–0.7 × 1.5–3.0 | 0.8–1.1 × 1.2–2.2 | 0.4–0.6 × 1.0–3.0 | 0.3–0.5 × 1.5–3.5 | 0.4–0.7 × 1.4–2.5 | 0.8 × 1.0–1.5 |
| Motility | + | + | + | + | + | + | + | + | + | + |
| Gram staining | + | + | + | + | − | − | − | − | − | − |
| Spore formation | + | + | + | + | − | − | − | − | − | − |
| Growth conditions | ||||||||||
| Optimum temperature (°C) | 35–37 | 35–37 | 37–40 | 37 | 30–33 | 30–33 | 33–35 | 30–33 | 33–35 | 37 |
| Optimum pH | 6–7 | 6–7 | 6–7 | 6–8 | 6.5–7.5 | 6.5–7.5 | 7–8 | 7–8 | 6.5–7.5 | 7.5 |
| Optimum NaCl (%, w/v) | 0–1.0 | 0–1.0 | 0–1.0 | <2.0 | 2–3 | 4–5 | 6–7 | 4–5 | 4–5 | 5 |
| Aerobic conditions | + | + | + | ± | + | + | + | + | + | + |
| Physiological characteristics | ||||||||||
| Catalase | + | + | + | + | + | + | + | + | + | + |
| Oxidase | + | + | + | + | + | + | + | + | + | ± |
| Urease | − | − | − | − | + | + | + | + | + | + |
| Gelatinase | + | + | + | + | − | − | − | − | − | + |
| Caseinase | + | + | + | + | − | − | − | − | − | + |
| Amylase | + | + | + | + | − | − | − | − | − | + |
| Indol formation | − | − | − | − | − | − | − | − | − | − |
| Methyl red test | + | + | − | + | − | − | − | − | − | − |
| Voges–Proskauer test | + | + | − | + | − | − | − | − | − | − |
| Growth on | ||||||||||
| Citrate | − | − | + | + | + | + | + | + | + | NR |
| d-Galactose | − | − | + | − | + | + | + | + | + | NR |
| d-Gluconate | − | − | + | − | + | + | + | + | + | NR |
| d-Fucose | − | − | − | NR | + | + | + | + | + | NR |
| l-Fucose | − | − | − | NR | + | + | + | + | + | NR |
| l-Arabitol | − | − | − | NR | − | − | − | − | − | NR |
| Starch | + | + | + | + | − | − | − | − | − | + |
| d-Raffinose | + | + | + | − | + | + | + | + | + | NR |
| Maltodextrin | + | + | + | NR | + | + | + | + | + | NR |
| Maltose | + | + | + | + | + | + | + | + | + | + |
| Mannitol | − | − | + | − | + | + | + | + | + | − |
| Lactose | − | − | + | − | + | + | + | + | + | − |
| Fructose | + | + | + | + | + | + | + | + | + | − |
| l-Rhamnose | − | − | − | − | + | + | + | + | + | NR |
| d-Xylose | − | − | + | − | + | + | + | + | + | NR |
| Inositol | + | + | + | − | + | − | − | + | + | − |
| Sorbitol | − | − | + | − | + | + | + | + | + | − |
| Inulin | + | + | + | NR | + | + | + | + | + | NR |
| Salicin | − | − | + | + | − | − | − | − | − | NR |
| Glucose | + | + | + | + | + | + | + | + | + | − |
| Sucrose | + | + | + | + | + | + | + | + | + | − |
| Carboxy methyl cellulose | − | − | − | NR | − | − | − | − | − | NR |
| Cellobiose | + | + | + | + | + | + | + | + | + | NR |
| α-Methyl-d-mannose | − | − | − | NR | − | − | − | − | − | NR |
| α-Methyl-d-glucose | − | − | − | NR | − | − | − | − | − | NR |
| Dextrin | + | + | + | NR | + | + | + | + | + | NR |
| Glycerol | − | − | + | + | + | + | + | + | + | NR |
| Cane molasses | + | + | + | NR | + | + | + | + | + | NR |
Data for B. cereus were reported by Priest et al. (1988) and data for Y. pacifica were determined by Dai et al. (2006). +, positive; −, negative; NR, not reported.
Furthermore, the phenotypic characteristics were compared with those of B. cereus. Most characteristics of the Gram-positive strains and B. cereus were similar (Table 1); only some biochemical tests and growth on a few carbohydrates were different between the strains isolated from mangroves and B. cereus (Table 1). The phenotypic features of the Gram-negative strains QN187, ND199, ND218, ND240, and QN271 and those reported for Y. pacifica were also compared (Table 1). The Gram-negative strains and Y. pacifica differed with respect to the hydrolysis of casein, gelatine, and starch (Table 1). They also differ regarding the growth on starch, mannitol, lactose, fructose, inositol, sorbitol, glucose, and sucrose (Table 1).
Electron microscopy observation of microbial isolates containing PHA inclusions
The presence of PHA in the eight selected bacterial strains was analyzed using TEM. The cells with the highest contents of PHA were chosen for the micrographs (Fig. 3A–D). The Gram-positive strains ND253, ND97, and QN194 were rod cells with size ranging from 4.4 × 1.4 to 4.4 × 1.7 μm and coccid-shaped cells with an average size of 1.5 × 1.2 μm (Fig. 3A and B). Furthermore, these cells contained PHA inclusions with three different average diameters, c.a. 0.17, 0.27, and 1.2 μm. Coccoid cells contained one, two, or three PHA inclusions, while rods stored from four to nine PHA granules shifting in size (Fig. 3A and B). The Gram-negative strains QN187, ND199, ND218, ND240, and QN271 were short rods with sizes ranging from 2 × 0.3 to 2.3 × 1 μm and coccoid cells with an average diameter of 0.3 μm (Fig. 3C and D). The inclusions in Gram-negative bacteria had three average diameters, c.a. 0.18, 0.30, and 0.91 μm. The coccoid cells were completely filled with either one PHA inclusion or may store up to four inclusions (Fig. 3C and D). Moreover, short rods contained from 1 to 9 inclusions (Fig. 3C and D).
Figure 3.
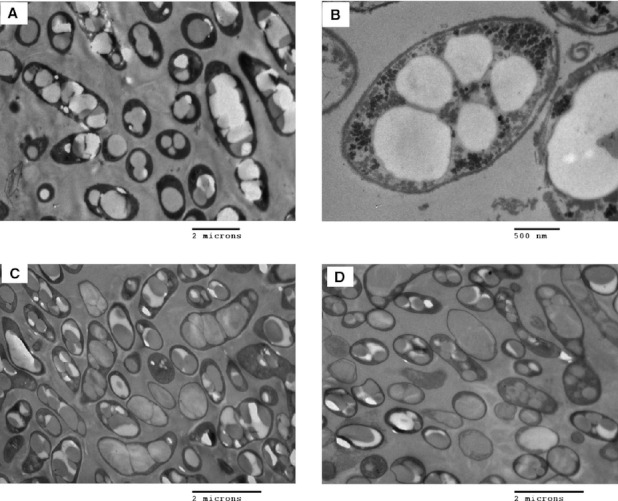
Transmission electron microscope pictures of (A) Strain ND153, (B) Strain ND97, (C) Strain QN187, and (D) Strain QN271 grown on HM-1 medium. The samples were taken after 30 h of cultivation.
PHA production by halophilic and halotolerant strains isolated from mangroves
The PHA produced by the eight selected strains was studied using either one or two sources of carbon. For a first set of experiments, an initial concentration of 20 g/L glucose was used as sole carbon source. For a second set of experiments, propionate was also added to the culture medium to reach an initial concentration of 0.2 g/L after 10 h of cultivation. CDW, PHA content, and the polymer composition were evaluated in all assays (Table 2). For the first group of experiments, we determined that strains ND199 and ND218 were able to synthesize the copolymer PHBV from glucose while the remaining strains accumulated PHB. Table 2 shows also that strain ND153 accumulated the largest amount of PHA (65 wt%). Under the same culture conditions, strains ND97, QN187, and QN271 stored the polymer in yields between 44 and 48 wt%. The remaining strains attained yields lower than 35 w% (Table 2). The addition of propionate in the culture medium enhanced the final yield of PHA reached by strains ND97 and ND153, and induced the synthesis of PHBV in seven of the eight strains (Table 2). The cell growth, as determined by the CDW, reached by seven of the strains was between 2 and 3.8 g/L while strain QN271 achieved a slightly higher CDW, c.a. 4.7–5.1 g/L (Table 2). The chemical structures of the PHAs were confirmed further by 1H-NMR analysis (Fig. 4). The proton signals and chemical shifts clearly showed that two different types of PHAs were synthesized by the strains, that is, PHB (Fig. 4A) and PHBV (Fig. 4B).
Table 2.
Cell growth, PHA content, and composition attained by halophilic and halotolerant strains isolated from soil at mangrove forests in Vietnam
| Carbon substrate | ||||||||
|---|---|---|---|---|---|---|---|---|
| Glucose | Glucose + Propionate1 | |||||||
| PHA composition | PHA composition | |||||||
| Strain | CDW (g/L) | PHA content (wt%) | 3HB (mol%) | 3HV (mol%) | CDW (g/L) | PHA content (wt%) | 3HB (mol%) | 3HV (mol%) |
| ND97 | 3.1 | 48 | 100 | 0 | 3.3 | 53 | 86 | 14 |
| ND153 | 3.1 | 65 | 100 | 0 | 3.6 | 71 | 91 | 15 |
| QN194 | 2.2 | 26 | 100 | 0 | 1.8 | 11 | 100 | 0 |
| ND199 | 2.6 | 34 | 98 | 2 | 2.1 | 12 | 56 | 44 |
| ND218 | 2.3 | 24 | 97 | 3 | 2 | 11 | 79 | 21 |
| QN271 | 5.1 | 48 | 100 | 0 | 4.7 | 31 | 95 | 5 |
| QN187 | 3.8 | 44 | 100 | 0 | 3.1 | 27 | 90 | 10 |
| ND240 | 3.2 | 28 | 100 | 0 | 2.6 | 12 | 87 | 13 |
All experiments were performed in shake flasks.
A propionate concentration of 0.2 g/L was fed into the culture after 10 h of growth.
Figure 4.
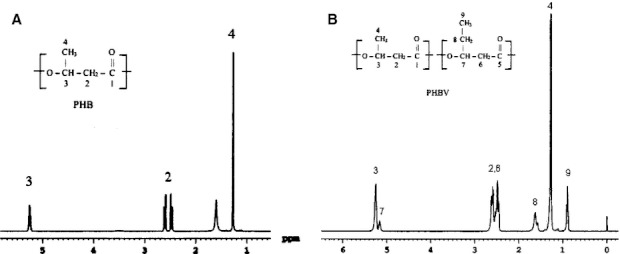
500 MHz 1H-NMR spectra of (A) purified PHB isolated from strain QN194 grown on glucose (2%, v/w) as carbon source, (B) purified PHBV isolated from strain ND153 grown on glucose (2%, v/w) and propionate (0.2%, v/w) as carbon sources.
Discussion
Mangrove forests are characterized for presenting a much higher content of organic matter than nitrogen or phosphorous sources (Alongi et al. 1989). Moreover mangrove forests in Vietnam are coastal areas, whereby they are constantly in contact with salts coming from sea; bacterial diversity in mangroves is known to be responsible of most of the carbon cycle (Holguin et al. 2001). Bacteria that succeeded in such ecosystems should have adapted their metabolisms to variations in salt concentrations, excess in carbon sources, and limited amounts of other essential nutrients such as nitrogen or phosphorous. Several halophilic and halotolerant bacteria are able to tolerate a wide range of NaCl concentrations (Oren 2008), whereas various species accumulate polyesters (Quillaguamán et al. 2010). In this regard, we sought for halophilic or halotolerant microorganisms in Vietnamese mangrove forests that were able to synthesize PHAs in large amounts.
We obtained about 100 halophilic and halotolerant isolates able to produce PHAs from mangrove forests located at the provinces of Nam Dinh and Quang Ninh in Vietnam. Therefore, synthesis of PHA shows to be a strategy of adaptation that microorganisms follow in such ecosystems, and might have a role to play in the carbon cycle in the mangrove forests. Three of the eight selected strains belonged to the genus Bacillus (Fig. 2A). Different Bacillus species share 16S rDNA similarities above 99% and exhibit only a few phenotypic differences, but DNA relatedness among them revealed that they are genetically distinct species (Priest et al. 1988; Nakamura and Jackson 1995). Consequently, DNA–DNA hybridization studies may help to discern the species association of strains ND97, ND153, and QN194. Members of the genus Bacillus are highly ubiquitous in nature. (Priest et al. 1988; Nakamura and Jackson 1995; Lechner et al. 1998). Strains of B. cereus, B. thuringiensis, B. megaterium, B. aryabhattai, and other bacilli that form part of their phylogenetic cluster were also found in marine-related ecosystems (Ettoumi et al. 2009; Jung et al. 2011; Antony et al. 2012). Moreover, strains of B. amyloliquefaciens and B. megaterium were isolated in mangrove forests; the former possessed larvicidal activity and the latter was able to reduce selenite (Geetha et al. 2011; Mishra et al. 2011). In this sense, strains ND97, ND153, and QN194 could also interact with other organisms at the coastal areas that they habit, besides their capacity to synthesize polyesters.
The first studies on the production of PHB by microorganisms were performed on B. megaterium (Lemoigne 1926). Several other Bacillus species including B. thuringiensis and B. cereus were also found to be able to store PHB (Kominek and Halvorson 1965; Chen et al. 1991). Bacillus megaterium, B. thuringiensis, and B. cereus include genes that encode class IV PHA synthases in their genomes (Tseng et al. 2006; Hyakutake et al. 2011). Class IV synthases polymerize PHAs containing short-chain-length monomers such as PHB and PHBV (Rehm 2003). Strains ND97, ND153, and QN194 cells had one or three PHA inclusions, while rods stored from four to nine PHA granules varying in size (Fig. 3A and B). A similar number of PHA inclusions were found in B. megaterium (McCool et al. 1996). Strain ND153 accumulated large amounts of PHA, 65–71 wt% (Table 2), which are higher than the largest obtained by most Bacillus species, with yields ranging between 40 and 47 wt% (Valappil et al. 2007). It is also noteworthy that strain ND153 assimilates sucrose and sugarcane molasses (Table 1). These substrates are cheaper alternatives than glucose for the production of polyesters by Bacillus species (Valappil et al. 2007; Kumar et al. 2009; Akaraonye et al. 2012). The use of cheap substrates leads to feasible bioprocesses, which also become environmentally friendly when agricultural surplus such as molasses are used as the source for polyester production.
Gram-negative strains isolated from Vietnamese mangrove forests, that is, strains QN187, ND199, ND218, ND240, and QN271, were phylogenetically gathered with Y. pacifica (Fig. 2B). However, these strains showed various biochemical and nutritional differences with Y. pacifica (Table 1), implying that they are different strains from the type strain of Y. pacifica. Additional DNA relatedness studies are required to establish whether these strains belong to the Y. pacifica species. On the other hand, there are no reports on the production of PHA by Y. pacifica, albeit the strains isolated from Vietnam were able to produce PHA (Fig. 3C and D Table 2). The number and size of PHA granules accumulated by Gram-negative bacteria fluctuate depending on the phylogenetic group. The β-proteobacterium Cupriavidus necator (formerly called Ralstonia eutropha) cells store between 8 and 12 PHB granules with varying diameters in the range of 0.24–0.50 μm (Anderson and Dawes 1990), whereas the γ-proteobacterium Azotobacter vinelandii can accumulate more than 40 granules per cell, with sizes of 0.5–1.4 μm (Page et al. 1995). Moreover, the halophilic γ-proteobacterium Halomonas boliviensis commonly synthesizes one or two granules (0.20–0.64 μm) per cell, although occasionally the formation of up to five granules in elongated cells was observed (Quillaguamán et al. 2006). The organization, size, and number of inclusions found in the strains isolated from Vietnam (Fig. 3C and D) are similar to those found in H. boliviensis. The formation of such large and uniform PHB granules is suggested to be advantageous for the purification and quality of the polymer (Steinbüchel et al. 1995).
Interestingly, two of the Gram-negative strains, that is, ND199 and ND218 (Table 2), could synthesize the copolymer PHBV using glucose as carbon source. Only a few bacteria such as Rhodococcus species (Valentin and Dennis 1996) and halophilic archaea (Quillaguamán et al. 2010) are able to synthesize PHBV without the inclusion of propionic or valeric acid in the microbial culture medium. The halophilic archaea able to accumulate PHBV from carbohydrates possess PHA synthases that belong to the class III (Quillaguamán et al. 2010). Molecular analysis supports that the genes encoding PHA synthases of class III were transferred between bacteria and archaea able to thrive in marine-related ecosystems (Quillaguamán et al. 2010). Nevertheless, halophilic Halomonas species harbor genes that encode synthases phylogenetically close related to class I (Quillaguamán et al. 2010; Guzmán et al. 2012). The closest identities of the enzymes found in halophilic bacteria are shared with Proteobacteria of different subgroups, that is, α, β, and γ (Quillaguamán et al. 2010; Guzmán et al. 2012). Both class I and class III PHA synthases direct the production of PHBV when a carbohydrate and propionate form part of the production medium of the microorganisms (Rehm 2003); therefore, only additional molecular studies will reveal the type of PHA synthases that are expressed by the strains isolated from Vietnam. The maximum PHB yield and CDW reached in batch systems by strain QN271 were 48 wt% and 5.1 g/L, respectively (Table 2), which are rather lower than those reached by H. boliviensis (54 wt% and 14 g/L) (Quillaguamán et al. 2007), Cupriavidus necator (54 wt% and 9.4 g/L) (Doi et al. 1988), and a recombinant Escherichia coli strain (80.8 wt% and 8.9 g/L) (Lee et al. 1994). These bacteria attained among the highest productions of PHB and are recognized for their potential and current utilization at industrial scales. Studies on the optimization of the culture medium of strain QN271 that lead to higher polymer yields and cell growth using combinations of carbon sources are in progress. The studies should discern the potential that strain QN271 has for large-scale production systems.
Acknowledgments
The authors are grateful to the National Foundation for Science and Technology Development (NAFOSTED: code 106.03-2010.64) and International Foundation for Science (IFS: code F/5021-1) for supporting this work.
Conflict of Interest
None declared.
References
- Akaraonye E, Moreno C, Knowles JC, Keshavarz T, Roy I. Poly(3-hydroxybutyrate) production by Bacillus cereus SPV using sugarcane molasses as the main carbon source. Biotechnol. J. 2012;7:293–303. doi: 10.1002/biot.201100122. [DOI] [PubMed] [Google Scholar]
- Alongi D, Boto K, Tirendi F. Effect of exported mangrove litter on bacterial productivity and dissolved organic carbon fluxes in adjacent tropical nearshore sediments. Mar. Ecol. Prog. Ser. 1989;56:133–144. [Google Scholar]
- Anderson AJ, Dawes EA. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony R, Krishnan KP, Laluraj CM, Thamban M, Dhakephalkar PK, Engineer A, et al. Diversity and physiology of culturable bacteria associated with a coastal Antarctic ice core. Microbiol. Res. 2012;167:372–380. doi: 10.1016/j.micres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Babel W, Ackermann J, Breuer U. Physiology, regulation, and limits of the synthesis of poly (3HB) In: Scheper T, Babel W, Steinbüchel A, editors. Advances in biochemical engineering/biotechnology: biopolyesters. Berlin: Springer; 2001. pp. 125–157. [DOI] [PubMed] [Google Scholar]
- Chen G, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26:6565–6578. doi: 10.1016/j.biomaterials.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Konig KH, Lafferty RM. Occurrence of poly-D(−)-3-hydroxyalkanoates in the genus Bacillus. FEMS Microbiol. Lett. 1991;68:173–176. doi: 10.1016/0378-1097(91)90123-r. [DOI] [PubMed] [Google Scholar]
- Dai X, Wang BJ, Yang QX, Jiao NZ, Liu SJ. Yangia pacifica gen. nov., sp. nov., a novel member of the Roseobacter clade from coastal sediment of the East China Sea. Int. J. Syst. Evol. Microbiol. 2006;56:529–533. doi: 10.1099/ijs.0.64013-0. [DOI] [PubMed] [Google Scholar]
- Doi Y, Tamaki A, Kunioka M, Soga K. Production of copolyesters of 3-hydroxybutyrate and 3-hydroxyvalerate by Alcaligenes eutrophus from butyric and pentanoic acids. Appl. Microbiol. Biotechnol. 1988;28:330–334. [Google Scholar]
- Ettoumi B, Raddadi N, Borin S, Daffonchio D, Boudabous A, Cherif A. Diversity and phylogeny of culturable spore-forming Bacilli isolated from marine sediments. J. Basic Microbiol. 2009;49:S13–S23. doi: 10.1002/jobm.200800306. [DOI] [PubMed] [Google Scholar]
- Geetha I, Manonmani AM, Prabakaran G. Bacillus amyloliquefaciens: a mosquitocidal bacterium from mangrove forests of Andaman and Nicobar islands, India. Acta Trop. 2011;120:155–159. doi: 10.1016/j.actatropica.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Guzmán D, Balderrama-Subieta A, Cardona-Ortuño O, Guevara-Martínez M, Callisaya-Quispe N, Quillaguamán J. Evolutionary patterns of carbohydrate transport and metabolism in Halomonas boliviensis as derived from its genome sequence: influences on polyester production. Aquat. Biosyst. 2012;8:9. doi: 10.1186/2046-9063-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin G, Vazquez P, Bashan Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol. Fertil. Soils. 2001;33:265–278. [Google Scholar]
- Huijberts GNM, Wilkinson H, van der Wal C, Eggink G. Gas-chromatographic analysis of poly(3-hydroxyalkanoates) in bacteria. Biotechnol. Tech. 1994;8:187–192. [Google Scholar]
- Hyakutake M, Saito Y, Tomizawa S, Mizuno K, Tsuge T. Polyhydroxyalkanoate (PHA) synthesis by class IV PHA synthases employing Ralstonia eutropha PHB(-)4 as host strain. Biosci. Biotechnol. Biochem. 2011;75:1615–1617. doi: 10.1271/bbb.110229. [DOI] [PubMed] [Google Scholar]
- Jung MY, Kim JS, Paek WK, Lim J, Lee H, Kim PI, et al. Bacillus manliponensis sp. nov., a new member of the Bacillus cereus group isolated from foreshore tidal flat sediment. J. Microbiol. 2011;49:1027–1032. doi: 10.1007/s12275-011-1049-6. [DOI] [PubMed] [Google Scholar]
- Kominek LA, Halvorson HO. Metabolism of poly-b-hydroxybutyrate and acetoin in Bacillus cereus. J. Bacteriol. 1965;90:1251–1259. doi: 10.1128/jb.90.5.1251-1259.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar T, Singh M, Purohit HJ, Kalia VC. Potential of Bacillus sp. to produce polyhydroxybutyrate from biowaste. J. Appl. Microbiol. 2009;106:2017–2023. doi: 10.1111/j.1365-2672.2009.04160.x. [DOI] [PubMed] [Google Scholar]
- Lechner S, Mayr R, Francis KP, Prub BM, Kaplan T, Wiebner-Gunkel E, et al. Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Int. J. Syst. Evol. Microbiol. 1998;48:1373–1382. doi: 10.1099/00207713-48-4-1373. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee KM, Chang HN, Steinbüchel A. Comparison of recombinant Escherichia coli strains for synthesis and accumulation of poly-(3-hydroxybutyric acid) and morphological changes. Biotechnol. Bioeng. 1994;44:1337–1347. doi: 10.1002/bit.260441110. [DOI] [PubMed] [Google Scholar]
- Lemoigne M. Produits de deshydration et de polymerisation de l' acide b-oxybutyrique. Bull. Soc. Chim. 1926;8:770–782. [Google Scholar]
- McCool GJ, Fernandez T, Li N, Cannon MC. Polyhydroxyalkanoate inclusion-body growth and proliferation in Bacillus megaterium. FEMS Microbiol. Lett. 1996;138:41–48. [Google Scholar]
- Mishra RR, Prajapati S, Das J, Dangar TK, Das N, Thatoi H. Reduction of selenite to red elemental selenium by moderately halotolerant Bacillus megaterium strains isolated from Bhitarkanika mangrove soil and characterization of reduced product. Chemosphere. 2011;84:1231–1237. doi: 10.1016/j.chemosphere.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Nakamura LK, Jackson MA. Clarification of the taxonomy of Bacillus mycoides. Int. J. Syst. Bacteriol. 1995;45:46–49. [Google Scholar]
- Oren A. Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Systems. 2008;4:2. doi: 10.1186/1746-1448-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page WJ, Sherburne R, D'Elia L, Graham LL. Poly(b-hydroxybutyrate) extrusion from pleomorphic cells of Azotobacter vinelandii UWD. Can. J. Microbiol. 1995;41(Suppl. 1):22–31. [Google Scholar]
- Philip S, Keshavarz T, Roy I. Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007;82:233–247. [Google Scholar]
- Priest FG, Goodfellow M, Todd C. A numerical classification of the genus Bacillus. J. Gen. Microbiol. 1988;134:1847–1882. doi: 10.1099/00221287-134-7-1847. [DOI] [PubMed] [Google Scholar]
- Quillaguamán J, Hatti-Kaul R, Mattiasson B, Alvarez MT, Delgado O. Halomonas boliviensis sp. nov., an alkalitolerant, moderate halophile bacterium isolated from soil around a Bolivian hypersaline lake. Int. J. Syst. Evol. Microbiol. 2004;54:721–725. doi: 10.1099/ijs.0.02800-0. [DOI] [PubMed] [Google Scholar]
- Quillaguamán J, Delgado O, Mattiasson B, Hatti-Kaul R. Poly(β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1. Enzyme Microb. Technol. 2006;38:148–154. doi: 10.1111/j.1365-2672.2005.02589.x. [DOI] [PubMed] [Google Scholar]
- Quillaguamán J, Muñoz M, Mattiasson B, Hatti-Kaul R. Optimizing conditions for poly(β-hydroxybutyrate) production by Halomonas boliviensis LC1 in batch culture with sucrose as carbon source. Appl. Microbiol. Biotechnol. 2007;74:981–986. doi: 10.1007/s00253-006-0754-2. [DOI] [PubMed] [Google Scholar]
- Quillaguamán J, Guzmán H, Van-Thuoc D, Hatti-Kaul R. Synthesis and production of polyhydroxyalkanoates by halophiles: current potential and future prospects. Appl. Microbiol. Biotechnol. 2010;85:1687–1696. doi: 10.1007/s00253-009-2397-6. [DOI] [PubMed] [Google Scholar]
- Rehm BHA. Polyester synthases: natural catalysts for plastics. Biochem. J. 2003;376:15–33. doi: 10.1042/BJ20031254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Spalding M, Kainuma M, Collins L. World atlas of mangroves. Washington, DC: Earthscan LLC; 2010. [Google Scholar]
- Spiekermann P, Rehm BAH, Kalscheuer R, Baumeister D, Steinbüchel A. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 1999;171:73–80. doi: 10.1007/s002030050681. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A, Füchtenbush B. Bacterial and other biological systems for polyester production. Trends. Biotechnol. 1998;16:419–427. doi: 10.1016/s0167-7799(98)01194-9. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A, Aerts K, Babel W, Folner C, Leibergesell M, Wieczorek R. Considerations on the structure and biochemistry of bacterial polyhydroxyalkanoic acid inclusions. Can. J. Microbiol. 1995;41(Suppl. 1):94–105. doi: 10.1139/m95-175. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C-L, Chen H-J, Shaw G-C. Identification and characterization of the Bacillus thuringiensis phaZ gene, encoding new intracellular poly-3-hydroxybutyrate depolymerase. J. Bacteriol. 2006;188:7592–7599. doi: 10.1128/JB.00729-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valappil SP, Boccaccini AR, Bucke C, Roy I. Polyhydroxyalkanoates in Gram-positive bacteria: insight from the genera Bacillus and Streptomyces. Antonie Van Leeuwenhoek. 2007;91:1–17. doi: 10.1007/s10482-006-9095-5. [DOI] [PubMed] [Google Scholar]
- Valentin HE, Dennis D. Metabolic pathway for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) formation in Nocardia corallina: inactivation of mutB by chromosomal integration of a kanamycin resistance gene. Appl. Environ. Microbiol. 1996;62:372–379. doi: 10.1128/aem.62.2.372-379.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wang Y, Chen GQ. Medical application of microbial biopolyesters polyhydroxyalkanoates. Artif. Cells Blood Substit. Immobil. Biotechnol. 2009;37:1–12. doi: 10.1080/10731190802664429. [DOI] [PubMed] [Google Scholar]


