Abstract
Objective
To determine the role of human papillomavirus (HPV) status on quality of life (QOL) in patients with oral cavity and oropharyngeal squamous cell carcinoma (OSCC). Since OSCC that are associated with high-risk HPV have an improved response to treatment and survival, we hypothesized that patients with these tumors would have better QOL trajectories.
Study Design
Prospective cohort study.
Setting
Tertiary care academic medical center and two affiliated hospitals.
Subjects and Methods
Head and neck-specific QOL was determined using the University of Washington Quality of Life (UW-QOL) scale version 4 in patients with newly diagnosed invasive OSSC (n=228).
Results
Pre-treatment QOL was higher in patients with high-risk HPV-associated tumors compared to patients with HPV-negative or low-risk HPV-associated tumors (p=0.015). Patients with high-risk HPV-associated tumors had larger decreases in QOL from pre-treatment to immediate post-treatment compared to patients with HPV-negative or low-risk HPV-associated tumors (p=0.041). There was no association between HPV status and one year post-treatment QOL.
Conclusion
Among OSCC patients, high-risk HPV-associated tumors were associated with higher pre-treatment QOL and a larger decrease in QOL from pre-treatment to immediate post-treatment, suggesting that treatment intensity in this unique population may adversely affect QOL.
Keywords: head and neck cancer, quality of life, epidemiology/outcomes research, head and neck surgery, human papillomavirus, oropharyngeal squamous cell carcinoma
Introduction
Head and neck squamous cell carcinomas (HNSCC) that are associated with high-risk human papillomavirus (HPV) have an improved response to treatment and survival.1 No prior studies have assessed the relationship between HPV status and quality of life (QOL) in patients with HNSCC. Given the increasing incidence of HPV-associated HNSCC,2 studying the factors that influence QOL will become more important, as these patients are younger and more likely to survive than patients with HNSCC unrelated to HPV. Understanding the relationship between HPV status and QOL could help clinicians tailor therapy and clinical management of HNSCC patients to optimize both survival and QOL. The goal of this study was to determine the role of HPV status on QOL in patients with oral cavity and oropharyngeal squamous cell carcinoma (OSCC). Since HPV is associated with an improved response to treatment and survival, we hypothesized that patients with high-risk HPV-associated OSCC would have better QOL trajectories.
Methods
Study Population
Patients were eligible if they were diagnosed with a first, previously untreated primary OSCC and underwent biopsy or surgical resection between December 2003 and June 2010, as previously described. Briefly, all patients were recruited from the University of Washington Medical Center, a tertiary care academic medical center, and from two affiliated hospitals in Seattle, WA (Harborview Medical Center and Puget Sound Veterans Affairs Health Care System). There were 313 patients who both met inclusion criteria and provided written informed consent. Of these, four had in situ tumors and were excluded from the study. Tumor specimens from 296 of the remaining patients were available and tested for the presence and type of HPV DNA (see below). In all but two patients, HPV status was determined, leaving 294 patients with invasive OSCC and known HPV status. Of these, there were 240 patients without missing demographic or cancer data and for whom the date treatment started could be determined. The study was approved by the institutional review boards of the respective institutions.
A structured questionnaire and a data abstraction form were used to determine demographic information, comorbidity, QOL, substance use history, tumor characteristics, and treatments for each patient. Race was one of the demographic variables assessed since it has been associated with differences in stage,3 HPV status,4 survival,3,4 and health-related QOL.5 Participants could choose their own race from a list of choices, with the option to choose more than one race category and to write in their own race.6 For this study, subjects’ race was classified as either white or non-white. Comorbidity was assessed using the Adult Comorbidity Scale-27 (ACE-27), a validated comorbidity index for patients with cancer.7 Patients were categorized at never, former, or current smokers. Patients’ alcohol consumption was determined and they were categorized as never drinkers, former drinkers, current drinkers drinking 0–14 drinks per week, or current drinks drinking greater than 14 drinks per week.
HPV Detection
Tumor tissue was obtained from patients at the time of surgical resection or diagnostic biopsy (prior to chemotherapy or radiation therapy). HPV DNA status was determined blind to patient characteristics and after patients had received treatment for their primary tumor, and thus was not used to guide clinical management. Tissue preservation, DNA extraction, HPV DNA detection, and HPV subtype determination were performed as previously described.8 Briefly, HPV positivity was determined by nested polymerase chain reaction (PCR). In all HPV-positive samples and a subset of negative samples, HPV subtype was determined using a LINEAR ARRAY HPV Genotyping Test (Roche, Indianapolis, Indiana) under a research-use-only agreement. Specimens were classified as HPV-negative, low-risk HPV (HPV-6, 11, 26, 33, 35, 39, 40, 42, 53, 54, 55, 61, 62, 64, 67, 69, 70, 71, 72, 81, 83, 84, IS39, and CP6108) or high-risk HPV (HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68).
QOL Assessment
QOL was assessed before and after treatment using the UW-QOL version 4, a validated head and neck cancer-specific health-related QOL questionnaire.9 It consists of 12 domain items, 3 global items, and a rating of the most important domains as perceived by the patient. Responses for each of the 12 domains were converted to a numerical score between 0 and 100, with 100 representing the best possible response. An overall QOL score was determined using the mean average of all the domain scores. A seven point difference in the overall UW-QOL score has been established as representing the minimal clinically important difference.10 The QOL scores were categorized into three time periods relative to the date treatment was started: pre-treatment (scores from questionnaires administered before treatment), immediate post-treatment (scores from questionnaires administered 3–8 months after treatment started), and one year post-treatment (scores from questionnaires administered 9–15 months after treatment started). One year post-treatment QOL has been shown to be a marker of longer term QOL.11 If a patient had multiple QOL questionnaires within one of these three periods, the QOL scores were averaged. The change in QOL scores from pre-treatment to immediate post-treatment and the change from pre-treatment to one year post-treatment were determined for each patient. The change in QOL was calculated by determining the difference in QOL scores between the two time points of interest; the possible range of values for change in QOL was −100 to 100.
Pre-treatment QOL data were available for 209 of the patients in the study; some patients completed QOL questionnaires soon after treatment started and these QOL scores were not used to determine pre-treatment QOL. There were 209 patients alive eight months after treatment started; attempts were made to contact 111 of them between three and eight months. Of these patients, 84 could be contacted and completed QOL questionnaires. Five additional patients completed QOL questionnaires between three and eight months but had died by the end of eight months. There were 182 patients alive 15 months after treatment started; attempts were made to contact 114 of them between nine and fifteen months. Of these patients, 83 could be contacted and completed QOL questionnaires. One additional patient completed a QOL questionnaire between nine and fifteen months but had died by the end of 15 months. Therefore, immediate post-treatment and one year post-treatment data were available for 89 and 84 patients, respectively.
For 12 and 2 patients we could not confirm that the baseline and one-year post treatment QOL data, respectively, had been collected according to the study protocol, and the corresponding data for these individuals were excluded, leaving 228 subjects in the study. The proportion of HPV positive patients with QOL data was similar during each of the three time periods used for this study (Appendix A).
Statistical Analysis
QOL was compared between groups using Mann-Whitney and Kruskal-Wallis tests. In cases in which the Kruskal-Wallis test indicated statistically significant differences, a test of trend was also performed. Linear regression models were generated for the multivariable analysis. This included models that controlled for age, sex, tumor site, stage, comorbidity, cigarette smoking, alcohol consumption, and treatment; prediction models that used stepwise modeling to pick covariates were also generated. Only the former models are presented but both types of models had similar results. All p-values were calculated using two-sided hypotheses and the threshold for statistical significance was set at p<0.05. All statistical analyses were conducted using STATA 11.1 Intercooled (Statacorp, College Station, TX).
Results
There were 158 patients with HPV-negative tumors, 4 with low-risk HPV-associated tumors, and 66 with high-risk HPV-associated tumors. Tumors were categorized for analyses as either: (1) HPV-negative or low-risk HPV, or (2) high-risk HPV. (The results below did not vary when patients with low-risk HPV-associated tumors were excluded). Patient characteristics, tumor data, and treatment were compared between these two groups (Table 1). High-risk-associated tumors were more likely to occur in males, to be located in the oropharynx, to have nodal metastases, and to be treated with multimodality therapy.
Table 1.
Selected characteristics of OSCC patients, by HPV DNA status, University of Washington Affiliated Hospitals, Seattle, WA, 2003–2010
| Characteristic | HPV-negative or low-risk HPV (n=162) |
High-risk HPV (n=66) |
p-value |
|---|---|---|---|
| Age, mean | 60 | 58 | 0.223 |
| Male | 100 (62%) | 56 (85%) | 0.001 |
| White | 148 (91%) | 59 (89%) | 0.642 |
| Tumor site | |||
| Oral | 144 (89%) | 22 (33%) | <0.001 |
| Oropharyngeal | 18 (11%) | 44 (67%) | |
| T stage | 0.079 | ||
| 1 | 62 (38%) | 19 (29%) | |
| 2 | 42 (26%) | 27 (41%) | |
| 3–4 | 58 (36%) | 20 (30%) | |
| N stage | <0.001 | ||
| 0 | 94 (58%) | 18 (27%) | |
| 1 | 21 (13%) | 6 (9%) | |
| 2–3 | 47 (29%) | 42 (64%) | |
| Cigarette smoking | 0.777 | ||
| Never | 43 (27%) | 15 (23%) | |
| Former | 48 (30%) | 19 (29%) | |
| Current | 71 (44%) | 32 (48%) | |
| Alcohol drinking | 0.407 | ||
| Never | 16 (10%) | 5 (8%) | |
| Former | 39 (24%) | 23 (35%) | |
| 0–14 drinks/week | 54 (33%) | 18 (27%) | |
| >14 drinks/week | 53 (33%) | 20 (30%) | |
| Comorbidity* | 0.545 | ||
| 0 | 35 (22%) | 16 (24%) | |
| 1 | 68 (42%) | 31 (47%) | |
| 2–3 | 59 (36%) | 19 (29%) | |
| Multimodality therapy | 80 (49%) | 47 (71%) | 0.003 |
| Surgery to primary site | 157 (97%) | 50 (76%) | <0.001 |
| Chemotherapy | 44 (27%) | 36 (55%) | <0.001 |
| Radiation therapy | 84 (52%) | 48 (73%) | 0.004 |
| Radiation dose in Greys, median** | 64 | 66 | 0.046 |
| Duration of treatment in days, median | 65 | 77 | 0.133 |
Comorbidity assessed with ACE-27, with higher scores indicating greater comorbidity.
Total radiation dose could be determined in 67 HPV-negative or low-risk HPV-associated OSCC patients and 45 high-risk HPV-associated OSCC patients.
Abbreviations: ACE-27: Adult Comorbidity Evaluation-27; HPV: Human Papillomavirus; OSCC: Oral cavity and oropharyngeal squamous cell carcinoma
Univariate associations between patient/tumor characteristics and QOL scores were determined (Table 2). The characteristics associated with pre-treatment QOL were T stage, N stage, and cigarette smoking status. Multimodality therapy, chemotherapy, and radiation therapy were associated with change in QOL scores from pre-treatment to one year post-treatment.
Table 2.
Univariate associations between QOL and selected patient and tumor characteristics in OSCC patients, University of Washington Affiliated Hospitals, Seattle, WA, 2003–2010
| Characteristic | Pre-treatment QOL | Change in QOL from pre-treatment to one year post-treatment* |
||||
|---|---|---|---|---|---|---|
| n | Median QOL score (IQR) |
p-value | n | Median QOL score (IQR) |
p-value | |
| Age | ||||||
| <60 | 102 | 79 (68–88) | 0.117 | 36 | −8 (−18 to 5) | 0.556 |
| ≥60 | 96 | 83 (70–93) | 36 | −2 (−15 to 3) | ||
| Sex | ||||||
| M | 137 | 82 (68–89) | 0.452 | 50 | −2 (−18 to 4) | 0.737 |
| F | 61 | 79 (68–88) | 22 | −3 (−13 to 6) | ||
| Race | ||||||
| White | 179 | 82 (69–90) | 0.115 | 68 | −3 (−18 to 4) | 0.439 |
| Non-white | 19 | 74 (58–88) | 4 | 1 (−8 to 7) | ||
| Tumor site | ||||||
| Oral | 141 | 80 (65–89) | 0.098 | 48 | −2 (−12 to 4) | 0.191 |
| Oropharynx | 57 | 84 (75–90) | 24 | −9 (−23 to 5) | ||
| T stage | 0.899 | |||||
| 1 | 70 | 87 (78–94) | <0.001 | 35 | −2 (−13 to 5) | |
| 2 | 59 | 81 (70–88) | 25 | −5 (−19 to 4) | ||
| 3–4 | 69 | 72 (60–85) | 12 | −3 (−13 to 4) | ||
| N stage | 0.058 | |||||
| 0 | 95 | 85 (73–92) | 0.047 | 38 | −1 (−9 to 6) | |
| 1 | 25 | 79 (68–85) | 9 | −9 (−16 to 10) | ||
| 2–3 | 78 | 76 (65–89) | 25 | −10 (−24 to 0) | ||
| Cigarette smoking | ||||||
| Never | 51 | 87 (72–93) | 0.010 | 20 | −3 (−9 to 6) | 0.808 |
| Former | 59 | 83 (73–93) | 22 | 0 (−19 to 3) | ||
| Current | 88 | 77 (64–85) | 30 | −3 (−19 to 6) | ||
| Alcohol drinking | ||||||
| Never | 18 | 88 (72–95) | 0.137 | 5 | −3 (−16 to −2) | 0.264 |
| Former | 55 | 83 (68–90) | 21 | 0 (−5 to 10) | ||
| 0–14 drinks/week | 62 | 83 (73–89) | 22 | −9 (−19 to 3) | ||
| >14 drinks/week | 63 | 76 (65–87) | 24 | −3 (−16 to 4) | ||
| Comorbidity** | 0.320 | |||||
| 0 | 42 | 85 (68–93) | 0.323 | 14 | −9 (−20 to −2) | |
| 1 | 85 | 82 (70–89) | 34 | 0 (−17 to 5) | ||
| 2–3 | 71 | 79 (64–87) | 24 | −2 (−15 to 7) | ||
| Multimodality therapy | ||||||
| No | 32 | 0 (−9 to 8) | 0.007 | |||
| Yes | 38 | −8 (−21 to 1) | ||||
| Surgery to primary site | ||||||
| No | 7 | −1 (−18 to 8) | 0.732 | |||
| Yes | 65 | −3 (−16 to 3) | ||||
| Chemotherapy | ||||||
| No | 48 | −1 (−12 to 6) | 0.043 | |||
| Yes | 22 | −13 (−24 to 2) | ||||
| Radiation therapy | ||||||
| No | 31 | 0 (−9 to 8) | 0.004 | |||
| Yes | 39 | −10 (−21 to 1) | ||||
| Radiation dose | ||||||
| <65 Gy | 16 | −4 (−19 to 1) | 0.272 | |||
| ≥65 Gy | 17 | −14 (−25 to −3) | ||||
Statistically significant differences in QOL are in bold font.
One year post-treatment: data collected 9–15 months after treatment started
Comorbidity assessed with ACE-27, with higher scores indicating greater comorbidity.
Abbreviations: ACE-27: Adult Comorbidity Evaluation-27; HPV: Human Papillomavirus; IQR: Interquartile range; OSCC: Oral cavity and oropharyngeal squamous cell carcinoma; QOL: Quality of life
Overall (composite) QOL scores were calculated and compared by HPV status using univariate methods (Figure 1). Pre-treatment QOL scores were significantly higher in patients with high-risk HPV-associated OSCC compared to patients with HPV negative or low risk-associated OSCC (86 vs 79, p=0.015). Immediate post-treatment QOL scores were lower in patients with high-risk HPV-associated OSCC (63 vs 73, p=0.062). QOL scores between the high-risk HPV and HPV-negative or low-risk HPV-associated OSCC groups were similar one year post-treatment (75 vs 77, p=0.733).
Figure 1.
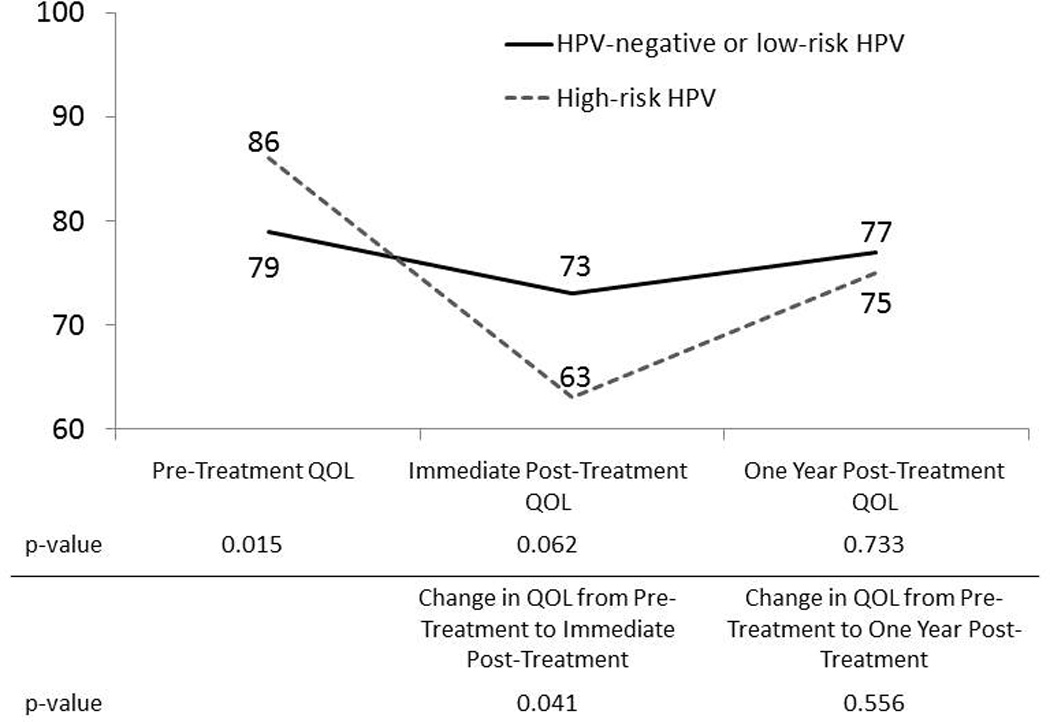
Relationship between HPV DNA status and QOL in OSCC patients, University of Washington Affiliated Hospitals, Seattle, WA, 2003–2010
Immediate post-treatment: data collected 3–8 months after treatment started
One year post-treatment: data collected 9–15 months after treatment started
Abbreviations: HPV: Human Papillomavirus; OSCC: Oral cavity and oropharyngeal squamous cell carcinoma; QOL: Quality of life
Patients with high-risk HPV tumors experienced a significantly larger decrease in their QOL scores from pre-treatment to immediate post-treatment compared to the HPV-negative or low-risk HPV-associated group (−18 vs −6, p=0.041). The change in QOL scores from pre-treatment to one year post-treatment was similar between the high-risk HPV group and the HPV-negative or low-risk HPV group (−1 vs −3, p=0.556).
The covariates used in the multivariable analysis (Table 3) were age, sex, race, tumor site, HPV status, T stage, N stage, comorbidity, cigarette smoking, and alcohol consumption. The three treatment modalities were included as covariates in the models in which change in QOL was the outcome. HPV status was associated with pre-treatment QOL (regression coefficient 4.61, p=0.117) and change in QOL from pre-treatment to immediate post-treatment (regression coefficient −4.54, p=0.275).
Table 3.
Multivariable associations between QOL and HPV DNA status and other patients and tumor characteristics in OSCC patients, University of Washington Affiliated Hospitals, Seattle, WA, 2003–2010
| Outcome | ||||||
|---|---|---|---|---|---|---|
| Pre-treatment QOL (n=198) |
Change in QOL from pre-treatment to immediate post- treatment* (n=83) |
Change in QOL from pre-treatment to one year post-treatment** (n=70) |
||||
| Covariate† | Coefficient†† | p-value | Coefficient | p-value | Coefficient | p-value |
| High-risk HPV DNA | 4.61 | 0.117 | −4.54 | 0.275 | −0.26 | 0.965 |
| Age | 0.19 | 0.042 | −0.14 | 0.385 | 0.00 | 0.998 |
| Sex | −4.34 | 0.078 | 0.68 | 0.879 | 1.89 | 0.697 |
| Race | −4.51 | 0.209 | −0.89 | 0.89 | 4.49 | 0.609 |
| Tumor site | 3.65 | 0.214 | 2.89 | 0.561 | −1.96 | 0.76 |
| T stage: | ||||||
| 2 | −5.27 | 0.056 | −0.54 | 0.902 | 3.03 | 0.551 |
| 3–4 | −9.96 | <0.001 | 2.81 | 0.572 | 7.25 | 0.248 |
| N stage: | ||||||
| 1 | −4.21 | 0.212 | −6.04 | 0.378 | 1.80 | 0.779 |
| 2–3 | −3.93 | 0.130 | −11.26 | 0.051 | −3.68 | 0.557 |
| Comorbidity:‡ | ||||||
| 1 | −2.31 | 0.463 | 2.02 | 0.686 | 1.83 | 0.762 |
| 2–3 | −2.83 | 0.394 | 2.76 | 0.592 | 4.37 | 0.557 |
| Cigarette smoking: | ||||||
| Former | −0.71 | 0.807 | −7.81 | 0.199 | −4.53 | 0.421 |
| Current | −4.17 | 0.148 | −5.74 | 0.329 | −5.18 | 0.361 |
| Alcohol drinking | ||||||
| Former | −2.70 | 0.534 | 3.36 | 0.645 | 13.31 | 0.176 |
| 0–14 drinks/week | −2.12 | 0.602 | 1.57 | 0.811 | 6.67 | 0.488 |
| >14 drinks/week | −6.05 | 0.158 | 1.04 | 0.882 | 8.51 | 0.390 |
| Surgery to primary site | 4.88 | 0.478 | −6.30 | 0.421 | ||
| Chemotherapy | 4.97 | 0.389 | 0.62 | 0.927 | ||
| Radiation therapy | −9.11 | 0.054 | −9.65 | 0.087 | ||
Statistically significant differences in QOL are in bold font.
Immediate post-treatment: data collected 3–8 months after treatment started
One year post-treatment: data collected 9–15 months after treatment started
Reference/baseline categories are as follow: HPV: HPV-negative or low-risk HPV; Sex: male; Race: white; Tumor site: oral; T stage: 1; N stage: 0; Comorbidity: ACE-27 score 0; Cigarette smoking: never; Alcohol drinking: never.
From the regression model, representing the difference in the QOL score (pre-treatment) or change in QOL score (pre-treatment to immediate post-treatment, or pre-treatment to one-year post-treatment) associated with the respective covariate.
Comorbidity assessed with ACE-27, with higher scores indicating greater comorbidity.
Abbreviations: ACE-27: Adult Comorbidity Evaluation-27; HPV: Human Papillomavirus; OSCC: Oral cavity and oropharyngeal squamous cell carcinoma; QOL: Quality of life
Discussion
Changes in the patterns of HNSCC over the last three decades suggest an increasing incidence of HPV-associated cancers as opposed to those related to alcohol and tobacco.2,12 Patients with high-risk HPV-associated oropharyngeal cancer have higher rates of survival, which has been hypothesized to be at least partially due to an increased responsiveness to radiation therapy.1,13 Given the improved survival in patients with HPV-associated OSCC, our a priori hypothesis was that patients with high-risk HPV tumors would have better QOL trajectories. Surprisingly, we did not find a difference in one year post-treatment QOL or change in QOL from pre-treatment to one year post-treatment by HPV status. However, we found that patient with high-risk HPV tumors had higher pre-treatment QOL and a greater decrease in QOL from pre-treatment to immediate post-treatment.
Because the current study included patients with both oral cavity and oropharyngeal cancer, we controlled for tumor site in the multivariable analysis. The association between high-risk HPV and higher pre-treatment QOL as well as the association between high-risk HPV and greater change in QOL did not remain significant after controlling for tumor site and other covariates, although a strong trend remained apparent. One possible reason that the former association showed a trend but did not remain significant in the multivariable analysis is the limited sample size. Also, patients with high-risk HPV-associated OSCC were more likely than patients with HPV-negative or low-risk HPV-associated OSCC to have oropharyngeal tumors and advanced nodal disease. They were also more likely to be treated with chemotherapy, radiotherapy, and multimodality therapy. Each of these factors was also associated with a larger decrease in QOL from pre-treatment to one year post-treatment, which could explain why there was a statistically significant association between high-risk HPV status and worse QOL trajectories in the unadjusted analysis, but not in the adjusted analysis.
The finding that patients with high-risk HPV tumors experience a greater decrease in their QOL from pre-treatment to the immediate post-treatment could have implications for clinicians treating these patients. Studies have shown associations between QOL and mood disorders, such as anxiety and depression, in patients with head and neck cancer.14,15 Because of their significant decrease in QOL from pre-treatment to immediate post-treatment, patients with high-risk HPV-associated OSCC may be at greater risk for mood disorders and may require closer monitoring as they recover from treatment.
To our knowledge, this is the only study to examine the association between HPV status and QOL in patients with OSCC. In addition, unlike many studies on QOL in patients with HNSCC, we collected QOL data both before and after treatment, allowing us to compare QOL scores at various time periods as well as the change in QOL scores. There were limitations to this study, however. First, as with any observational study, causal relations between HPV status and QOL cannot be made. In addition, patients who completed QOL questionnaires were well enough to respond. Some patients were missing QOL data at certain time points, which could be biasing the results. The majority of the patients in this study were white. Therefore, our results may not be representative of more diverse populations of OSCC patients.
In conclusion, the most important finding in our study is that patients with high-risk HPV-associated OSCC did not have better one year QOL compared to other OSCC patients. Although patients with high-risk HPV-associated oropharygeal tumors have been shown to have greater response to treatment, it is interesting that the clinical features of these tumors (i.e. heavier nodal burden) mandates intensification of therapy, which may account for some of our findings. For this reason, further work exploring the interplay of HPV status, QOL, and survival is warranted. Treatment de-intensification has been suggested for patients with high-risk HPV-associated oropharyngeal squamous cell carcinoma since they are younger and have better survival than other patients with OSCC.16 This is currently being explored in ongoing clinical trials. If patients with high-risk HPV-associated oropharyngeal cancers can be treated with less morbid therapies without affecting survival, improved QOL may be realized. The current study illustrates the importance of determining HPV status of specimens from patients with OSCC and exploring treatment de-intensification for HPV-associated oropharyngeal cancers. Doing so may optimize both survival and QOL trajectories in this unique patient population.
Acknowledgements
We wish to acknowledge the resources from and use of facilities at the VA Puget Sound Health Care System, University of Washington Medical Center and Harborview Medical Center, Seattle, Washington. This study was supported by grant R01CA095419 from National Cancer Institute, National Institutes of Health, Bethesda, MD; grant 1K1L2RR025015-01 from the National Center for Research Resources; and by funds from the Fred Hutchinson Cancer Research Center.
Appendix A
Selected characteristics of OSCC patients by whether QOL was available during each of the three time periods used for this study, University of Washington Affiliated Hospitals, Seattle, WA, 2003–2010
| Characteristic | Pre-treatment QOL data available? |
Immediate post- treatment QOL data available? |
One year post- treatment QOL data available? |
|||
|---|---|---|---|---|---|---|
| No (n=30) |
Yes (n=198) |
No* (n=115) |
Yes (n=84) |
No** (n=101) |
Yes (n=81) |
|
| Age, mean | 59 | 60 | 58 | 60 | 58 | 59 |
| Male | 19 (63%) | 137 (69%) | 77 (67%) | 58 (69%) | 67 (66%) | 55 (68%) |
| White | 28 (93%) | 179 (90%) | 12 (10%) | 8 (10%) | 87 (86%) | 76 (94%) |
| High-risk HPV DNA | 11 (37%) | 55 (28%) | 31 (27%) | 30 (36%) | 32 (32%) | 24 (29%) |
| Tumor site | ||||||
| Oral | 25 (83%) | 141 (71%) | 89 (75%) | 57 (68%) | 73 (73%) | 56 (69%) |
| Oropharyngeal | 5 (17%) | 57 (29%) | 29 (25%) | 27 (32%) | 28 (28%) | 25 (31%) |
| T stage | ||||||
| 1 | 11 (37%) | 70 (35%) | 41 (36%) | 39 (46%) | 37 (37%) | 38 (47%) |
| 2 | 10 (33%) | 59 (30%) | 36 (31%) | 26 (31%) | 30 (30%) | 28 (35%) |
| 3–4 | 9 (30%) | 69 (35%) | 38 (33%) | 19 (23%) | 34 (34%) | 15 (19%) |
| N stage | ||||||
| 0 | 17 (57%) | 95 (48%) | 61 (53%) | 46 (55%) | 57 (56%) | 44 (54%) |
| 1 | 2 (7%) | 25 (13%) | 17 (15%) | 7 (8%) | 12 (12%) | 9 (11%) |
| 2–3 | 11 (37%) | 78 (39%) | 37 (32%) | 31 (37%) | 32 (32%) | 28 (35%) |
| Cigarette smoking | ||||||
| Never | 7 (23%) | 51 (26%) | 31 (27%) | 19 (23%) | 24 (24%) | 24 (30%) |
| Former | 8 (27%) | 59 (30%) | 33 (29%) | 28 (33%) | 31 (31%) | 25 (31%) |
| Current | 15 (50%) | 88 (44%) | 51 (44%) | 37 (44%) | 46 (46%) | 32 (40%) |
| Alcohol drinking | ||||||
| Never | 3 (10%) | 18 (9%) | 8 (7%) | 11 (13%) | 10 (10%) | 7 (9%) |
| Former | 7 (23%) | 55 (28%) | 31 (27%) | 24 (29%) | 26 (26%) | 23 (28%) |
| 0–14 drinks/week | 10 (33%) | 62 (31%) | 33 (29%) | 26 (31%) | 30 (30%) | 25 (31%) |
| >14 drinks/week | 10 (33%) | 63 (32%) | 43 (37%) | 23 (27%) | 35 (35%) | 26 (32%) |
| Comorbidity† | ||||||
| 0 | 9 (30%) | 42 (21%) | 26 (23%) | 23 (27%) | 28 (28%) | 18 (22%) |
| 1 | 14 (47%) | 85 (43%) | 50 (43%) | 35 (42%) | 39 (39%) | 38 (47%) |
| 2–3 | 7 (23%) | 71 (36%) | 39 (34%) | 26 (31%) | 34 (34%) | 25 (31%) |
| Multimodality therapy | 13 (43%) | 114 (58%) | 65 (57%) | 44 (52%) | 56 (55%) | 42 (52%) |
| Surgery to primary site | 28 (93%) | 179 (90%) | 105 (91%) | 75 (89%) | 93 (92%) | 74 (91%) |
| Chemotherapy | 9 (30%) | 71 (36%) | 41 (36%) | 28 (33%) | 35 (35%) | 24 (30%) |
| Radiation therapy | 13 (43%) | 119 (60%) | 68 (59%) | 46 (55%) | 60 (60%) | 43 (53%) |
Only includes patients who were also alive 8 months after treatment started.
Only includes patients who were also alive 15 months after treatment started
Comorbidity assessed with ACE-27, with higher scores indicating greater comorbidity.
There were no differences in mean age or the number of patients in each of the above categories when comparing whether QOL was available during the three time periods.
Abbreviations: ACE-27: Adult Comorbidity Evaluation-27; HPV: Human Papillomavirus; OSCC: Oral cavity and oropharyngeal squamous cell carcinoma; QOL: Quality of life
References
- 1.Ragin CCR, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int J Cancer. 2007;121(8):1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence Trends for Human Papillomavirus-Related and -Unrelated Oral Squamous Cell Carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 3.Ragin CC, Langevin SM, Marzouk M, Grandis J, Taioli E. Determinants of head and neck cancer survival by race. Head Neck. 2010 doi: 10.1002/hed.21584. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Settle K, Posner MR, Schumaker LM, et al. Racial Survival Disparity in Head and Neck Cancer Results from Low Prevalence of Human Papillomavirus Infection in Black Oropharyngeal Cancer Patients. Cancer Prev Res. 2009;2(9):776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao D, Debb S, Blitz D, Choi S, Cella D. Racial/Ethnic Differences in the Health-Related Quality of Life of Cancer Patients. J Pain Symptom Manage. 2008;36(5):488–496. doi: 10.1016/j.jpainsymman.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winker MA. Measuring Race and Ethnicity: Why and How? JAMA. 2004 Oct 6;292(13):1612–1614. doi: 10.1001/jama.292.13.1612. 2004. [DOI] [PubMed] [Google Scholar]
- 7.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic Importance of Comorbidity in a Hospital-Based Cancer Registry. JAMA. 2004 May 26;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. 2004. [DOI] [PubMed] [Google Scholar]
- 8.Lohavanichbutr P, Houck J, Fan W, et al. Genomewide gene expression profiles of HPV-positive and HPV-negative oropharyngeal cancer: potential implications for treatment choices. Arch Otolaryngol Head Neck Surg. 2009 Feb;135(2):180–188. doi: 10.1001/archoto.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preedy VR, Watson RR. Handbook of Disease Burdens and Quality of Life Measures. Springer Science+Business Media; 2010. [Google Scholar]
- 10.El-Deiry MW, Futran ND, McDowell JA, Weymuller EA, Jr, Yueh B. Influences and predictors of long-term quality of life in head and neck cancer survivors. Arch Otolaryngol Head Neck Surg. 2009 Apr;135(4):380–384. doi: 10.1001/archoto.2009.18. [DOI] [PubMed] [Google Scholar]
- 11.Rogers SN, Hannah L, Lowe D, Magennis P. Quality of life 5–10 years after primary surgery for oral and oro-pharyngeal cancer. J Craniomaxillofac Surg. 1999;27(3):187–191. doi: 10.1016/s1010-5182(99)80049-3. [DOI] [PubMed] [Google Scholar]
- 12.Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978–2007: Focus on human papillomavirus associated sites. Int J Cancer. 2010 doi: 10.1002/ijc.25699. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Allen CT, Lewis JS, Jr, El-Mofty SK, Haughey BH, Nussenbaum B. Human papillomavirus and oropharynx cancer: biology, detection and clinical implications. Laryngoscope. 2010 Sep;120(9):1756–1772. doi: 10.1002/lary.20936. [DOI] [PubMed] [Google Scholar]
- 14.Horney DJ, Smith HE, McGurk M, et al. Associations between quality of life, coping styles, optimism, and anxiety and depression in pretreatment patients with head and neck cancer. Head Neck. 2011;33(1):65–71. doi: 10.1002/hed.21407. [DOI] [PubMed] [Google Scholar]
- 15.D'Antonio LL, Long SA, Zimmerman GJ, Peterman AH, Petti GH, Chonkich GD. Relationship between quality of life and depression in patients with head and neck cancer. Laryngoscope. 1998 Jun;108(6):806–811. doi: 10.1097/00005537-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Ihloff AS, Petersen C, Hoffmann M, Knecht R, Tribius S. Human papilloma virus in locally advanced stage III/IV squamous cell cancer of the oropharynx and impact on choice of therapy. Oral Oncology. 2010;46(10):705–711. doi: 10.1016/j.oraloncology.2010.07.006. [DOI] [PubMed] [Google Scholar]


