Abstract
Background
Helicobacter pylori is a spiral-shaped Gram-negative microaerophilic bacterium associated with a number of gastrointestinal disorders, including gastritis, peptic ulcers, and gastric cancer. Several studies have implicated a Th17 response as key to protective immunity against Helicobacter.
Materials and Methods
Wild type (WT) and MyD88 deficient (MyD88−/−) mice in the C57BL/6 background were infected with H. felis for 6 and 25 weeks and colonization density and host response evaluated. Real-time PCR was used to determine the expression of cytokines and antimicrobial peptides in the gastric tissue of mice.
Results
mRNA expression levels of the Th17 cytokines, interleukin-17A (IL-17A) and IL-22 were markedly up-regulated in WT compared to MyD88−/− mice both at 6 and 25 weeks in response to infection with H. felis, indicating that induction of Th17 responses depends on MyD88 signaling. Furthermore, reduction of expression of the Th17-dependent intestinal antimicrobial peptide lipocalin-2 was linked with increased bacterial burden in the absence of MyD88 signaling.
Conclusion
We provide evidence showing that MyD88-dependent signaling is required for the host to induce a Th17 response for the control of Helicobacter infection.
Keywords: Helicobacter, MyD88, IL-17A, IL-22, lipocalin-2
Introduction
Helicobacter pylori (H. pylori) is the most common human bacterial pathogen in the world, infecting approximately 50% of the world’s population (reviewed in [1]). H. pylori is responsible for most cases of inflammatory gastritis, peptic ulcer disease, and gastric cancer in the human population [1]. It has been widely reported that T lymphocytes play a central role in host immune response during Helicobacter infection [2]. We have previously described a predominantly Th1 response with increased IFN-γ production in infections with Helicobacter spp [3]. Recent studies have also shown a marked induction of a key Th17 cytokine, IL-17A during infection with H. pylori [4]. In mice infected with Helicobacter, IL-17A has been suggested to play a role in limiting the growth of Helicobacter following vaccination [5]. IL-17A is a potent inducer of CXCR2 cytokines [6, 7], which are implicated in neutrophil recruitment [8]. Antimicrobial peptide regulation at the mucosal surfaces is a potential additional yet important function of IL-17A. Indeed, IL-17A was reported to be essential for production of antimicrobial peptides in tissue during inflammation including induction of lipocalin-2 (Lcn2) [9]. Lcn2 is an antimicrobial peptide that inhibits bacterial growth through its interference with iron acquisition [10]. Up-regulation of Lcn2 was shown to improve host defense against bacterial pathogens such as Klebsiella pneumoniae [11], Escherichia coli [10], Mycobacterium tuberculosis [12], and Salmonella typhimurium [9]. Recently, Hornsby et al. [13] reported up-regulation of siderocalin in rhesus macaque monkeys infected with H. pylori, but the mechanism of this induction or the role of Lcn2 during infection with Helicobacter are not known. Additionally, the mechanism of Helicobacter-related induction of IL-17A remains unclear.
We recently demonstrated the importance of myeloid differentiation primary response gene 88 (MyD88) in host immune response to Helicobacter in vitro using bone marrow-derived macrophages [14]. MyD88 is a key intracellular adaptor molecule and mediates signals from most Toll-like receptors (TLRs) except for TLR3 [15]. We showed that MyD88 was required for H. pylori induction of many inflammatory cytokines including IL-6, IL-1β, IL-12, and IL-10 [14]. In the present study, we investigated the role of MyD88 in induction of Th17 responses (IL-17A and IL-22) during infection with H. felis. In addition, the effect of IL-17A expression during infection with H. felis was determined by examining the expression of IL-17A target genes, particularly antimicrobial peptides in the gastric tissue of mice.
Methods
Mice
Six- to ten- week-old wild type (WT) and MyD88 deficient (MyD88−/−) mice in the C57BL/6 background were used in this study. WT mice were purchased from The Jackson Laboratory (Bar Harbor, ME). MyD88−/− mice were from our breeding colony originally provided by Dr. Akira (Osaka University, Japan). All animal procedures were approved by the Animal Care Committee at the University of California, San Diego.
Culture of H. felis
H. felis, strain CSI (ATCC 49179) was purchased from American Type Culture Collection (Manassas, VA). H. felis was routinely maintained on solid medium, Columbia agar (Becton Dickinson, MD) supplemented with 5% laked blood under microaerophilic conditions (5% O2, 10% CO2, 85% N2) as previously described[14] in an incubator at 37°C. H. felis used to infect mice were subcultured into liquid medium, brain heart infusion broth (BHI, Becton Dickinson) supplemented with 5% fetal calf serum and cultured for 48 hours on a reciprocal shaker (100 rpm) at 37°C under microaerophilic conditions. Before infections, spiral bacteria were enumerated using a Petroff-Hausser chamber.
Infection of mice with H. felis
C57BL/6 WT and MyD88−/− mice were inoculated by oral gavage three times at 2-day intervals as described elsewhere [16–18] with 109 H. felis suspended in 0.3 ml BHI broth. At 6 and 25 weeks post-infection, mice were euthanized and the stomachs removed under aseptic conditions. The stomach was divided longitudinally into three parts and used for assessment of gene expression, histologic evaluation of inflammatory response, and recovery of H. felis to confirm colonization.
Histologic evaluation of colonization and inflammation
Mouse stomachs from C57BL/6 WT and MyD88−/− mice were processed for histology as in our previous studies [3, 19]. Briefly, longitudinal sections of mouse stomach tissue from each mouse were fixed in neutral-buffered 10% formalin, embedded in paraffin, and 5 µm sections stained with May-Grunwald Giemsa [20] for microscopic evaluation of colonization. Two longitudinal stomach sections per mouse with an average of ten 400X high-powered fields were evaluated. H. felis colonization density was determined by grading both the corpus and antrum by a blinded veterinary pathologist (Dr. Rickman) using a semiquantitative scoring system of 0 to 4 as described by Wang et al. [21]. Scores were assigned as follows: 0, none; 0.5, rare; 1, occasional pits and/or glands with individual bacteria; 2, frequent pits and/or glands with individual bacteria; 3, infrequent pits and/or gland with dense bacterial colonies; and 4, frequent pits and/or glands with dense bacterial colonies.
For inflammation, 5 µm stomach sections tissues were stained with hematoxylin and eosin. Gastric histopathology was graded using the paradigm developed by Rogers et al. [22]. Briefly, inflammation was scored with a grade of 0 – 4, with "0" representing no inflammation and 1 thru 4 representing a range from mild to severe inflammation.
RNA isolation and reverse transcription (RT)
A section of a stomach tissue from each WT and MyD88−/− mouse at sacrificing was immediately placed in RNA stabilizing solution (Ambion) and stored at −70°C. Total RNA from each mouse gastric tissue section was homogenized in 1 ml of Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The RNA preparations were further purified using the Turbo DNase treatment as directed by the manufacturer (Ambion). RNA was quantified by absorbance at 260 nm and integrity checked on a 1% agarose gel. 2 µg of RNA was reverse transcribed into complementary DNA (cDNA) using the High-capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA).
Gene expression in the mouse stomach by quantitative real-time PCR
Gene expression was determined by real-time PCR with SYBR Green dye (Eurogentec, San Diego, CA). Due to similar expression levels in in the initial three genes (glyceraldehyde-3-phosphate dehydrogenase, GAPDH; gamma interferon, IFN-γ; and IL-17A) performed in individual mice from the same treatment group, cDNA samples from mice in each treatment group were pooled in subsequent experiments and reactions set in 10 µl reaction mix in 96-well plates. For each gene of interest, reactions were performed in triplicates using an amplification program consisting of initial 1 cycle at 95°C for 5 minutes, followed by 40 cycles of amplification with denaturation at 95°C for 15 seconds, annealing at 60°C for 20 seconds, and extension at 72°C for 40 seconds. For each sample a melting curve was generated at the end of the reaction to ensure specificity. Gene expression levels were normalized to GAPDH and the data analyzed using a comparative cycle threshold calculations (ΔΔCT, Applied Biosystems). Data were expressed as fold change relative to uninfected mice. Each real-PCR experiment was run three times. Samples that were undetected after 40 cycles were considered negative and 40 cycles used to calculate the fold change. A list of genes analyzed and their respective primers are provided in Table 1.
Table 1.
Primer sequences for real-time PCR
| Gene | Direction | Sequence |
|---|---|---|
| IL-17A | Forward | AACATGAGTCCAGGGAGAGCTTCA |
| Reverse | AGTGTTTGGACACGCTGAGCTTTG | |
| IL-22 | Forward | GACAGGTTCCAGCCCTACAT |
| Reverse | ATCGCCTTGATCTCTCCACT | |
| IL-6 | Forward | ACCACTTCACAAGTCGGAGGCTTA |
| Reverse | TGGTACTCCAGAAGACCAGAGGAA | |
| IFN-γ | Forward | TCAAGTGGCATAGATGTGGAAGAA |
| Reverse | TGGCTCTGCAGGATTTTCATG | |
| Lcn2a | Forward | ACATTTGTTCCAAGCTCCAGGGC |
| Reverse | CATGGCGAACTGGTTGTAGTCCG | |
| Cnlpb | Forward | TCAACCAGCAGTCCCTAGAC |
| Reverse | AAGGCACATTGCTCAGGTAG | |
| mBD1 | Forward | GGCATTCTCACAAGTCTTGGACGAAG |
| Reverse | AGCTCTTACAACAGTTGGGCTTATCTGG | |
| GAPDH | Forward | TCAACAGCAACTCCCACTCTTCCA |
| Reverse | ACCCTGTTGCTGTAGCCGTATTCA | |
| FlaBc (H. felis) | Forward | TTCGATTGGTCCTACAGGCTCAGA |
| Reverse | TTCTTGTTGATGACATTGACCAACGCA |
Statistics
Colonization scores were compared between WT and MyD88−/− mice by a two-tailed nonparametric Mann-Whitney U-test. A P value of < 0.05 was considered statistically significant. Student t-test (Microsoft excel) was used for statistical analysis on ΔΔCT and SD real-time PCR results with significance at P < 0.01.
Results
H. felis colonization in WT and MyD88−/− mice
To test whether MyD88 signaling influences H. felis colonization, WT and MyD88−/− mice were infected with H. felis for 6 or 25 weeks. H. felis was detected in all infected WT and MyD88−/− mice. H. felis infection scores were higher in MyD88−/− than in WT mice (Fig. 1A). WT mice had significantly (p < 0.05) fewer bacteria at 6 weeks post-infection. At 25 weeks, WT mice still remained less heavily infected with H. felis when compared to MyD88−/− mice (Fig.1A) although the difference was not statistically significant. H. felis colonization of mice was also analyzed quantitatively by determining the relative mRNA levels for the H. felis flagellar filament B gene (FlaB) using real-time PCR [17, 18, 23]. As shown in Fig. 1B, H. felis colonization of MyD88−/− mice was significantly (p < 0.01) greater than that of WT mice at both 6 and 25 weeks. Quantitative analysis of H. felis colonization by real-time PCR was more sensitive in detecting significant differences in colonization levels between WT and MyD88−/− mice than the scoring system of bacterial density in histologic sections. Higher bacterial load has also been reported in MyD88−/− mice infected with H. pylori [24]. However, we chose to analyze H. felis infection of C57BL/6 mice because this system more accurately recapitulates disease progression similar to that occurring in humans during infection with H. pylori [25]. We did not observe differences in inflammation between WT and MyD88−/− mice (see supporting information). Rad et al. [24] reported marginal significant differences in inflammation between WT and MyD88−/− mice infected with H. pylori. Although WT mice had a higher inflammatory score than MyD88−/− mice the authors noted that some individual MyD88−/− mice had severe inflammation.
Figure 1.

Colonization of the mouse stomach tissue. WT and MyD88−/− mice were infected with H. felis for 6 and 25 weeks. (A) Two longitudinal stomach sections per mouse with an average of ten 400X high-powered fields were evaluated to determined H. felis colonization density. Both the corpus and antrum were graded using a semiquantitative scoring system and the difference in bacterial load between WT and MyD88−/− mice analyzed by a nonparametric Mann-Whitney U-test. (B) H. felis colonization of mice was also analyzed quantitatively by determining the relative gene expression of FlaB using real time PCR. Student t-test (Microsoft excel) was used to determine differences in expression of FlaB between WT and MyD88−/− mice as a measure of H. felis colonization. All infected mice were colonized. Data are presented as means ± SEM (WT: 6 weeks, n = 7; WT: 25 weeks, n = 7; MyD88−/−: 6 weeks, n = 6; MyD88−/−: 25 weeks, n = 9). *P < 0.05, **P < 0.01.
Induction of gastric cytokines in response to infection with H. felis
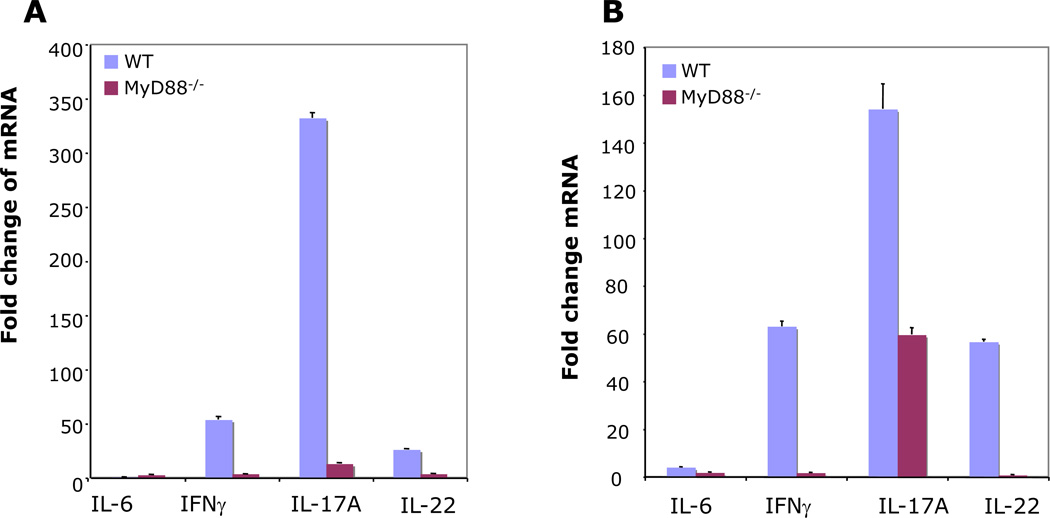
IL-17A expression during infection with Helicobacter has been reported [4]. However, the mechanism of this up-regulation is unknown. Additionally, the effect of MyD88 signaling on the expression of Th17 effector cytokines, IL-17A and IL-22 during Helicobacter infection has not been investigated. Real-time PCR analysis showed that the mRNA levels of IL-22, IL-17A, and IFN-γ exhibited substantial increases in response to infection with H. felis (Fig. 2). Infection of mice with H. felis increased the gastric mRNA levels of IL-17A, IL-22, and IFN-γ more in WT than in MyD88−/− mice, both at 6 and 25 weeks post-infection. There was a 53- and 26- fold change in expression of IFN-γ and IL-22, respectively in WT mice at 6 weeks (Fig. 2A). By 25 weeks post-infection the mRNA levels of IFN-γ and IL-22 had increased to 63- and 57- fold, respectively in WT mice (Fig. 2B). Among the cytokines we evaluated, IL-17A was the most prominently induced cytokine in response to infection with H. felis both at 6 and 25 weeks post-infection. Infection with H. felis resulted in a 332- and 12.9- fold change in IL-17A expression in WT and MyD88−/− mice, respectively at 6 weeks post-infection (Fig. 2A). At 25 weeks post-infection, H. felis infection resulted in 154-and 59- fold change in IL-17A expression in WT and MyD88−/− mice, respectively (Fig. 2B). Our study is the first to demonstrate that high level IL-17A/IL-22 expression during infection with Helicobacter requires activation of the MyD88 signaling pathway. Increased expression of IL-17A and IL-22, which was observed in WT mice, was associated with reduced H. felis colonization. Indeed, IL-17A is reported to be involved in protection against Helicobacter infection following vaccination [5]. IL-17A may contribute to limitation of Helicobacter growth in several ways, including induction of antimicrobial peptides.
Figure 2.
Cytokine expression in mouse gastric tissue. WT and MyD88−/− mice were infected with H. felis for 6 (A) and 25 (B) weeks. Expression of IL-6, IFN-γ, IL-17A, and IL-22 was determined by real-time RT-PCR and each normalized to GAPDH. Data are expressed as means fold change ± SEM compared to uninfected mice (WT: 6 weeks, n = 7; WT: 25 weeks, n = 7; MyD88−/−: 6 weeks, n = 6; MyD88−/−: 25 weeks, n = 9).
Expression of antimicrobial peptides in mouse gastric tissue during infection with H. felis
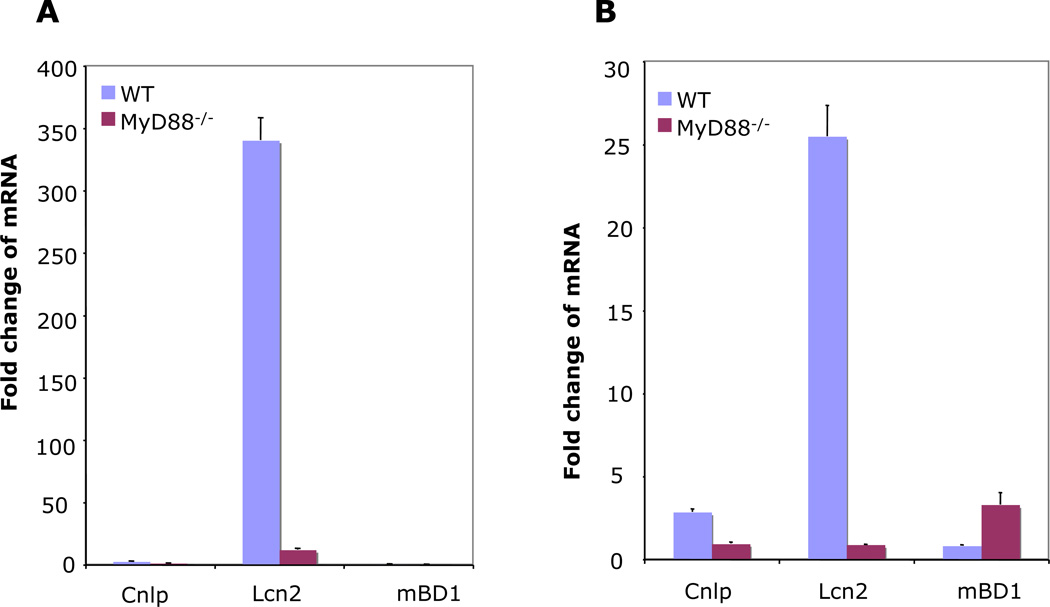
Because of the prominence of IL-17A expression we observed in gastric tissue of WT mice infected with H. felis, we reasoned that IL-17A target genes would be highly expressed in the gastric tissues. We have previously demonstrated in vitro that H. pylori up-regulates antimicrobial peptides [26]. We predicted that induction of Th17-regulated antimicrobial peptides, such as Lcn2, would be markedly reduced in the MyD88−/− mice compared to the WT. Real-time RT-PCR was performed on RNA isolated from gastric tissue of WT and MyD88 −/− mice at 6 and 25 weeks post-infection. As shown in Fig. 3, H. felis highly up-regulated expression of Lcn2 while the expression of other antimicrobial peptides including mouse β-defensin 1 (mBD1) and Cnlp, which encodes a cathelicidin-related antimicrobial peptide (CRAMP) was up-regulated only slightly ranging between 0.4- to 3- fold, both at 6 and 25 weeks post-infection. H. felis induced at least a 2-fold change in Cnlp in WT mice, both at 6 and 25 weeks post-infection. Expression of mBD1 was only detected at 25 weeks post-infection (Fig. 3B). The induction of Lcn2 was highest after six weeks of infection at 340-fold change in WT mice. The higher induction of Lcn2 expression in WT mice infected with H. felis was linked to higher IL-17A/IL-22 expression in these mice. In addition, H. felis induction of Lcn2 was dependent on MyD88 signaling, which was similar to what we observed for IL-17A and IL-22 expression. Our findings of lower Lcn2 expression in MyD88−/− mice are similar to those reported in the pulmonary K. pneumoniae infection mouse model [11] in which lung homogenates from MyD88−/− mice had reduced Lcn2. In the same study, it was shown that Lcn2 protein amounts were linked to bacterial burden. In our present study, higher expression of Lcn2 was related to reduced H. felis colonization.
Figure 3.
Antimicrobial peptide expression in mouse gastric tissue. WT and MyD88−/− mice were infected with H. felis for 6 (A) and 25 (B) weeks. Expression of Cnlp, mBD1, and Lcn2 was determined by real-time PCR and normalized to GAPDH. Data are expressed as means fold change ± SEM compared to uninfected mice (WT: 6 weeks, n = 7; WT: 25 weeks, n = 7; MyD88−/−: 6 weeks, n = 6; MyD88−/−: 25 weeks, n = 9).
Discussion
Despite a robust immune response during infection with Helicobacter, the bacteria persists causing disease conditions including gastritis, peptic ulcer, mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer [1]. Understanding the host immune response during infection with this organism may lead to the definition of mechanisms involved in the clearance of these bacteria. We have previously demonstrated in vitro that MyD88 is critical in the H. pylori-induced inflammatory response [14]. However, the effects of MyD88 on Th17 effector cytokines had not been investigated. In addition, the role of IL-17A and/or IL-22 in induction of antimicrobial peptides during Helicobacter infection had not been previously described. We show here for the first time that H. felis induction of IL-17A and IL-22 is dependent on MyD88 signaling. Furthermore, we demonstrated an inverse relationship between IL-17A/IL-22 expression and H. felis colonization level in the mouse gastric mucosa. IL-17A has been shown to play a major role in host protection against bacterial and fungal infections especially at the mucosal surface [27]. In particular, IL-17A was reported to be involved in protection against Helicobacter infection following vaccination [5]. We observed a strong association between increased expression of IL-17A/IL-22 with reduced H. felis colonization. IL-17A may contribute to limitation of Helicobacter growth in several ways, including through induction of antimicrobial peptides. We identified antimicrobial peptides released during gastric inflammation in response to H. felis infection and report for the first time Helicobacter induction of the mouse Cnlp gene, which encodes the mouse cathelicidin, CRAMP. In addition, Lcn2, an antimicrobial peptide involved in binding microbial iron-siderophore complexes, was also markedly up-regulated in response to infection with H. felis. In particular, high expression of Lcn2 was linked to reduced H. felis colonization at both 6 and 25 weeks post-infection.
Elevated expression of Lcn2 has recently been reported in humans [28] and rhesus macaques [13] in response to H. pylori infection although the mechanism of this induction is unknown. Here we show that H. felis induction of Lcn2 expression was associated with up-regulation of IL-17A and IL-22 and the expression of all three was dependent on MyD88 signaling. Additionally, it was reported in H. pylori-infected rhesus macaque monkeys that induction of Lcn2 was dependent on the presence of a cag pathogenicity island [13]. Our observation of high Lcn2 induction by H. felis, which lacks the cag pathogenicity island suggests involvement of a different system in this induction in mice. Mice that had high expression levels of IL-17A/IL-22, which coincided with high induction of Lcn2 had significantly reduced H. felis colonization both at 6 and 25 weeks post-infection. Our data suggesting that IL-17A may be involved in induction of Lcn2 during infection with Helicobacter is in agreement with a study showing that IL-17A induces Lcn2 in response to infection with Klebsiella [29]. In addition, it has been reported that the growth of S. typhimurium was inhibited by production of Lcn2 upon stimulation of intestinal epithelial cells with IL-17A and IL-22 in vitro [9]. Studies using Lcn2−/− mice suggest that Lcn2 is pivotal in innate immune response to bacterial infections and its absence can lead to sepsis and death of these mice [10, 11]. These studies together with our data suggest that Lcn2 could have a role in the reduced H. felis colonization we observed in WT compared to MyD88−/− mice. To the best of our knowledge, we are the first to show evidence connecting expression of Lcn2 and limitation of H. felis growth in mice.
In summary, we show for the first time that Helicobacter induction of IL-17A and IL-22 expression is dependent on MyD88 signaling. Increased IL-17A/IL-22 expression was accompanied with enhanced expression of Lcn2. We propose that higher expression of IL-17A/IL-22 in conjunction with up-regulation of Lcn2 may contribute to the reduced H. felis colonization levels we observed in WT mice.
Supplementary Material
Acknowledgments
We thank Professor S. Akira (Osaka University, Osaka, Japan) for the generous gift of MyD88−/− breeder mice. We also thank Elaine Hanson for designing primer sequences. This study was supported in part by a Public Health Service grant NIH # DK080506.
References
- 1.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 2.Robinson K, Argent RH, Atherton JC. The inflammatory and immune response to Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:237–259. doi: 10.1016/j.bpg.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Obonyo M, Guiney DG, Fierer J, Cole SP. Interactions between inducible nitric oxide and other inflammatory mediators during Helicobacter pylori infection. Helicobacter. 2003;8:495–502. doi: 10.1046/j.1523-5378.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 4.Algood HM, Gallo-Romero J, Wilson KT, Peek RM, Jr, Cover TL. Host response to Helicobacter pylori infection before initiation of the adaptive immune response. FEMS Immunol Med Microbiol. 2007;51:577–586. doi: 10.1111/j.1574-695X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- 5.Velin D, Favre L, Bernasconi E, Bachmann D, Pythoud C, Saiji E, Bouzourene H, Michetti P. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology. 2009;136:2237 el–2246 e1. doi: 10.1053/j.gastro.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 6.O'Connell AE, Redding KM, Hess JA, Lok JB, Nolan TJ, Abraham D. Soluble extract from the nematode Strongyloides stercoralis induces CXCR2 dependent/IL-17 independent neutrophil recruitment. Microbes Infect. 2011 doi: 10.1016/j.micinf.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 9.Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, Tsolis RM, Bevins CL, Solnick JV, Dandekar S, Baumler AJ. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 11.Chan YR, Liu JS, Pociask DA, Zheng M, Mietzner TA, Berger T, Mak TW, Clifton MC, Strong RK, Ray P, Kolls JK. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol. 2009;182:4947–4956. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117:1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornsby MJ, Huff JL, Kays RJ, Canfield DR, Bevins CL, Solnick JV. Helicobacter pylori induces an antimicrobial response in rhesus macaques in a cag pathogenicity island-dependent manner. Gastroenterology. 2008;134:1049–1057. doi: 10.1053/j.gastro.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obonyo M, Sabet M, Cole SP, Ebmeyer J, Uematsu S, Akira S, Guiney DG. Deficiencies of myeloid differentiation factor 88, Toll-like receptor 2 (TLR2), or TLR4 produce specific defects in macrophage cytokine secretion induced by Helicobacter pylori. Infect Immun. 2007;75:2408–2414. doi: 10.1128/IAI.01794-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Lee H, Schaffer L, Gilmartin TJ, Head SR, Takaishi S, Wang TC, Nakayama J, Fukuda M. A distinctive set of genes is upregulated during the inflammation-carcinoma sequence in mouse stomach infected by Helicobacter felis. J Histochem Cytochem. 2007;55:263–274. doi: 10.1369/jhc.6A7097.2006. [DOI] [PubMed] [Google Scholar]
- 17.Stoicov C, Fan X, Liu JH, Bowen G, Whary M, Kurt-Jones E, Houghton J. T-bet knockout prevents Helicobacter felis-induced gastric cancer. J Immunol. 2009;183:642–649. doi: 10.4049/jimmunol.0900511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takaishi S, Tu S, Dubeykovskaya ZA, Whary MT, Muthupalani S, Rickman BH, Rogers AB, Lertkowit N, Varro A, Fox JG, Wang TC. Gastrin is an essential cofactor for Helicobacter-associated gastric corpus carcinogenesis in C57BL/6 mice. Am J Pathol. 2009;175:365–375. doi: 10.2353/ajpath.2009.081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obonyo M, Guiney DG, Harwood J, Fierer J, Cole SP. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect Immun. 2002;70:3295–3299. doi: 10.1128/IAI.70.6.3295-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutton P, O'Rourke J, Wilson J, Dixon MF, Lee A. Immunisation against Helicobacter felis infection protects against the development of gastric MALT Lymphoma. Vaccine. 2004;22:2541–2546. doi: 10.1016/j.vaccine.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, Koh TJ, Fox JG. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 22.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–10715. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 23.Sayi A, Kohler E, Hitzler I, Arnold I, Schwendener R, Rehrauer H, Muller A. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J Immunol. 2009;182:7085–7101. doi: 10.4049/jimmunol.0803293. [DOI] [PubMed] [Google Scholar]
- 24.Rad R, Brenner L, Krug A, Voland P, Mages J, Lang R, Schwendy S, Reindl W, Dossumbekova A, Ballhorn W, Wagner H, Schmid RM, Bauer S, Prinz C. Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori. Gastroenterology. 2007;133:150 e3–163 e3. doi: 10.1053/j.gastro.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 25.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, Obonyo M, Gallo RL, Eckmann L, Kagnoff MF. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology. 2003;125:1613–1625. doi: 10.1053/j.gastro.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 27.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alpizar-Alpizar W, Laerum OD, Illemann M, Ramirez JA, Arias A, Malespin-Bendana W, Ramirez V, Lund LR, Borregaard N, Nielsen BS. Neutrophil gelatinase-associated lipocalin (NGAL/Lcn2) is upregulated in gastric mucosa infected with Helicobacter pylori. Virchows Arch. 2009;455:225–233. doi: 10.1007/s00428-009-0825-8. [DOI] [PubMed] [Google Scholar]
- 29.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.