Abstract
Non-shivering thermogenesis in brown adipose tissue (BAT) plays an important role in thermoregulation. In addition, activations of BAT have important implications for energy homeostasis due to the metabolic consumption of energy reserves entailed in the production of heat in this tissue. In this conceptual overview we describe the role of orexins/hypocretins within the central nervous system in the modulation of thermogenesis in BAT under several physiological conditions. Within this framework, we consider potential neural mechanisms underlying the pathological conditions associated with the absence of the central orexinergic modulation of BAT thermogenesis and energy expenditure. Overall, the experimental basis for our understanding of the role of central orexin in regulating body temperature and energy homeostasis provides an illustrative example that highlights several general principles and caveats that should help to guide future investigations of the neurochemical regulation of thermogenesis and metabolism.
Keywords: cannabinoid, narcolepsy, obesity, stress, ultradian rhythm
Introduction
The regulation of body temperature by the central nervous system is critical for mammalian survival in a wide variety of thermal environments. Small variations in core body temperature within the physiological range often occur during behavior and may be functionally relevant. However, large deviations in cellular temperature can sufficiently alter a variety of molecular properties, including enzymatic activities, diffusion capacity, and membrane fluidity, to impair cellular function. Severe disruptions in cellular function result in loss of motor coordination, mental confusion, loss of consciousness, significant respiratory and cardiovascular dysfunction, and eventual death.
Significant progress has been made in our understanding of the fundamental central nervous system circuitry that regulates body temperature (see [1-4]). Not surprisingly, the excitatory amino acid, glutamate, and the inhibitory amino acid, GABA, are the primary neurotransmitters within the fundamental central neural pathways for thermoregulation. In addition, body temperature can be influenced by an extensive array of peptides and other central neurotransmitters, including (but not limited to) hypocretin/orexin, galanin, melanin concentrating hormone, neuropeptide Y, oxytocin, glucagon-like peptide 1, cholecystokinin, cocaine and amphetamine related transcript, melanocortins, opioids, thyrotropin releasing hormone, corticotropin releasing factor, tuberoinfundibular peptide of 39 amino acid residues (TIP-39), dopamine, norepinephrine, epinephrine, serotonin, histamine, insulin and leptin. However, the precise neuroanatomical sites of action and the specific mechanisms by which many of these neurochemicals influence the fundamental neurocircuitry for thermoregulation remain unknown. In this paper, we will use an illustrative example, the influence of orexin on thermoregulation, to highlight some of the general principles and caveats that should guide experiments to understand the precise mechanisms by which neurochemicals may interact to influence thermoeffector activation.
Orexin neurons contribute to the regulation of BAT thermogenesis
Orexins/Hypocretins [5, 6] are peptides synthesized primarily by neurons located in the lateral, perifornical and dorsomedial areas of the hypothalamus. These orexinergic neurons have widespread projections in the brain that include many thermoregulatory areas such as the preoptic area, dorsomedial hypothalamus, parapyramidal area (PaPy) and the rostral raphe pallidus (rRPa) [7-9]. The rRPa is especially relevant to thermogenesis since it is the location of the sympathetic premotor neurons for BAT [10, 11]. We have recently demonstrated a role of orexin in the rRPa in the control of BAT thermogenesis [9]. Transynaptic retrograde virus tracers injected in BAT as well as injections of the retrograde tracer, cholera toxin subunit-b (CTb), in rRPa and PaPy highlighted a direct projection from orexinergic neurons to rRPa and PaPy sympathetic premotor neurons controlling BAT thermogenesis. Furthermore, nanoinjection of orexin in rRPa or PaPy, or nanoinjection of n-methyl-D-aspartate (NMDA) in the perifornical area of the lateral hypothalamus containing orexinergic neurons potentiated the ongoing BAT sympathetic nerve activity (SNA) elicited by mild cooling of core body temperature. However, during a slightly warm condition when the BAT sympathetic nerve was quiescent, neither nanoinjection of orexin in rRPa nor direct activation of orexinergic neurons by nanoinjections of NMDA in the perifornical area of the lateral hypothalamus increased BAT SNA [9]. Thus, orexin appears to serve as a modulator which changes the gain of the central thermoregulatory system to facilitate ongoing sympathetic activity to BAT and BAT thermogenesis.
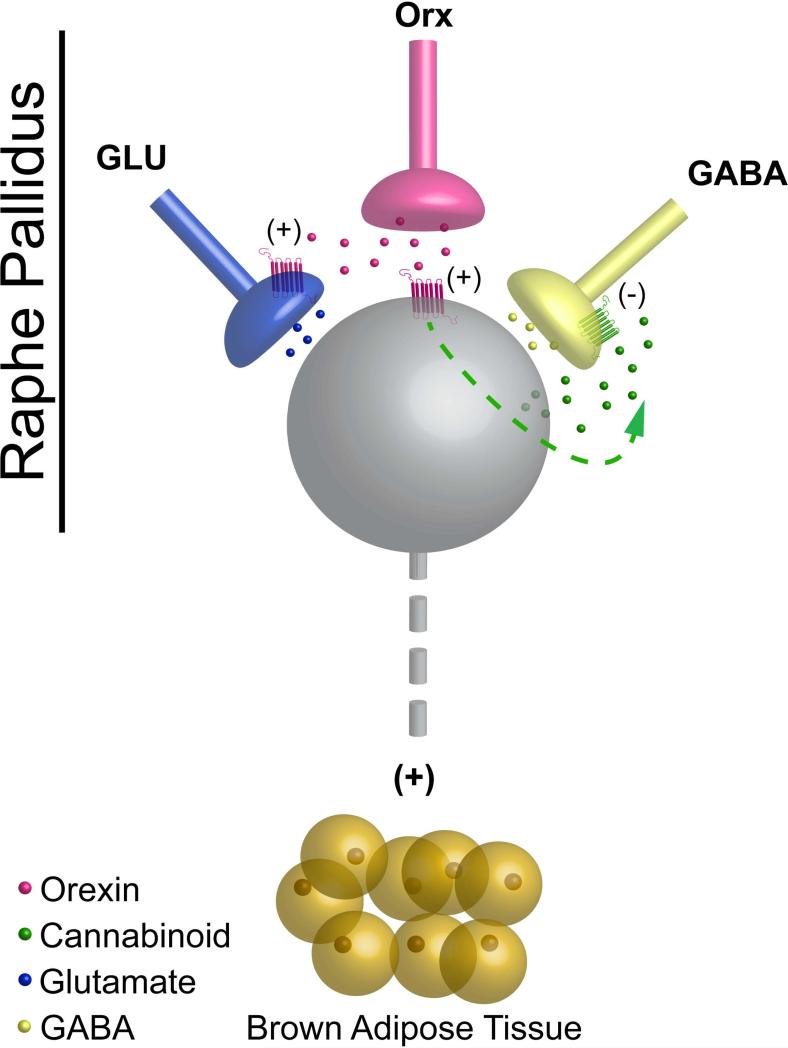
Figure 1 illustrates several potential mechanisms by which orexin-mediated facilitation of the activity of BAT sympathetic premotor neurons could occur within the rRPa. Orexin could act by inhibiting GABA release onto BAT sympathetic premotor neurons in rRPa, consistent with the increases in BAT SNA that can be driven by blockade of GABAA receptors in this region [12]. Interestingly, within the periaqueductal gray, orexin inhibits the release of GABA by cannabinoid-mediated retrograde neurotransmission, in which endocannabinoid, released from the postsynaptic cell in response to orexin receptor activation, diffuses retrogradely to inhibit GABA release from local presynaptic terminals [13]. Orexin could also presynaptically potentiate glutamate release onto BAT sympathetic premotor neurons of the rRPa, similar to mechanisms described for the potentiation of muscle tone with microinjection of orexin in somatic motor nuclei [14]. In addition, orexin could act directly on sympathetic premotor neurons in rRPa to alter their responsiveness to synaptic inputs. Although there are no data quantifying the phenotypes of the targets of orexin terminals within the rRPa, orexin fibers do form close appositions to serotonergic neurons (unpublished observation), consistent with the interesting possibility that the modulatory effects of orexin could be explained by effects on the serotonergic, BAT sympathetic premotor neurons in the rRPa. Indeed, similar to orexin's effect in rRPa, activation of serotonergic receptors in the spinal cord changes the gain of the spinal thermogenic network by potentiating glutamatergic excitation of BAT sympathetic preganglionic neurons [15-17].
Figure 1.
Schematic diagram summarizing several proposed models of the detailed mechanisms responsible for the modulation of brown adipose tissue (BAT) sympathetic nerve activity and thermogenesis evoked by the actions of orexin in the raphe pallidus. Orexin peptide released from an orexinergic terminal (pink terminal) in the raphe pallidus may: 1) act at postsynaptic orexin receptors on a sympathetic premotor neuron (gray neuron) for BAT to increase its responsiveness to other excitatory synaptic inputs; 2) act at orexin receptors on glutamatergic terminals (blue terminal) to presynaptically potentiate glutamate release onto sympathetic premotor neurons for BAT; 3) act on sympathetic premotor neurons for BAT to stimulate the release of endocannabinoids that subsequently serve as retrograde neurotransmitters to inhibit ongoing GABAergic input (yellow terminal) to sympathetic premotor neurons for BAT.
Role of orexin in the ultradian cycle of BAT thermogenesis
Ultradian rhythms are those with a periodicity of less than 24-hrs. Ultradian rhythms in rats have been described for sleep/wake cycles, motor activity, arterial pressure, and heart rate. An ultradian rhythm in BAT thermogenesis has also been demonstrated, with increases in BAT temperature occurring every ~1-2 hours [18, 19]. A role for orexin in the regulation of the ultradian rhythm of BAT thermogenesis has been suggested [18, 20]. Consistent with this hypothesis, injection of orexin into the cerebral ventricles increases BAT SNA and BAT temperature [21] and the activity of orexinergic neurons as well as the levels of orexin in the extracellular fluid of the CNS correlate well with the sleep/wake state of the animal, with higher indices of orexin activity during the waking state [22-24]. Unfortunately, the presence (or absence) of an ultradian rhythm in BAT temperature in orexin-null mice has not yet been described, although these animals do have disrupted ultradian sleep/wake cycles characterized by shorter bouts of wakefulness compared to control mice [19, 25]. We postulate that the ultradian increases in BAT temperature are mediated by the periodic release of orexin in the rRPa and PaPy areas, resulting in increases in the gain of thermogenic neurotransmission that, in turn, increase body and brain temperatures. What are the functional implications of orexin's ability to increase the gain of the thermogenic system? An interesting, though untested hypothesis is that the ultradian rhythm of BAT thermogenesis facilitates neural activity and thereby improves brain function [26]. Similarly, given the close correlation of BAT thermogenesis and the resulting increase in body temperature with periods of wakefulness, thermogenesis in this tissue may act to optimize metabolic function during periods requiring enhanced performance in general, and, conversely, low levels of thermogenesis during sleep states may act to conserve limited metabolic resources. Likewise, changing the gain of the excitatory neurotransmitter system driving a thermogenic effector such as BAT, which consumes energy stores to produce heat, would have clear functional consequences for energy homeostasis (see below).
Role of orexin neurons in the stress-induced increase in body temperature
Zhang and colleagues have demonstrated that the increase in core body temperature evoked by repeated handling stress is attenuated in mice that lack orexin neurons compared to wild type mice [27]. Surprisingly, prepro-orexin knock-out mice that do not produce orexin peptides have relatively normal stress-evoked increases in core body temperature [27]. Based on these data it has been suggested that orexin-containing neurons may be involved in stress-evoked increases in body temperature via the release of neurochemicals other than orexin (e.g. dynorphin or glutamate, which are normally co-expressed in orexin neurons) [27].
Particular caution is warranted when interpreting genetically-driven ablation and knockout models as there is always the possibility for developmental changes or compensatory mechanisms, as well as unintended consequences on additional cell populations beyond those specifically targeted (e.g. due to developmental expression patterns that differ from those in adult animals, see [28]). This may also be true in the case of orexin. Orexin is required for the development and differentiation of BAT and orexin administration during gestation in orexin-null dams rescues this developmental defect in the newborn pups [29]. The placenta may be the source of the orexin required for BAT development [29]. But how could this defect explain the differences observed between prepro-orexin knock-out mice and orexin neuron-ablated mice? Since the source of the functionally-important orexin may be the placenta, the genotype of the dam could determine the development of BAT in the offspring. The prepro-orexin knock-out mice were generated by breeding heterozygotes [27], therefore the dams will produce orexin as adults [30]. Unfortunately, it is not clear from the methodological description of the generation of orexin neuron-ablated mice whether the dams are devoid of orexin (the specific genotype of the dams is not stated) [27]. However, if orexin neuron-ablated dams were used, then the differential expression of orexin in the placenta of the dams would be expected to result in differences in the development of BAT that could contribute to a difference in the stress-evoked increase in body temperature that is independent of alterations in the central nervous system of the pups. There is also an apparent controversy concerning the UCP-1 expression levels in orexin knock-out mice (compare, for instance, Figure S1E in [29] to Figure 3 in [27]). Obviously, additional insights are necessary to resolve these issues.
Role of orexin in obesity
Narcolepsy is a neurological disorder principally characterized by altered sleep/wake cycles, in most cases attributable to reduced orexin neurotransmission [30-32]. In addition to the disturbances in sleep/wake cycles there is a well-recognized association between narcolepsy and obesity in human patients and animal models of the disease [33-38]. The increase in body weight in human patients with orexin deficiencies occurs despite a reduced caloric intake [39] and a normal total physical activity compared either to healthy control subjects [40] or to patients with idiopathic hypersomnia [34]. Similarly in mice that lack orexin, weight gain occurs despite reduced food intake; however, in mouse models, a decrease in spontaneous motor activity likely contributes to the weight gain [41]. Impaired thermogenesis in BAT has also been suggested to play a role in the propensity for weight gain in orexin-null mice [29]. There are currently no studies demonstrating a role for diminished activation of BAT in the increased incidence of obesity of narcoleptic patients, however this hypothesis would be consistent with the recent demonstrations of an inverse relationship between the activity of BAT and body mass index in adult humans [42-45]. Conversely, augmented orexin activity prevents diet-induced obesity and could contribute to a lean phenotype [46].
Highlights.
Orexin has pleiotropic effects on thermogenesis in brown adipose tissue. These include synergistic contributions to the development and differentiation of brown adipocytes and to the neurally-regulated activation of this tissue under various physiological conditions. In addition, orexin neurons may contribute to thermogenesis via the release of neurotransmitters other than the orexin peptide.
Neurochemicals may act as modulators which by themselves may be insufficient to drive thermoeffector systems, but instead act to change the gain of neural activity driven primarily by other neurotransmitters.
Because neurons express multiple neurotransmitters, neurochemical phenotyping may have limited utility until the unique and the ‘interactive’ roles of each neurochemical in target cell regulation have been addressed. This is especially important when using genetic methods to specifically alter a ‘neurochemically-specific’ cell population as the important neurotransmitter under a given condition may indeed differ from the genotype used to target the cell.
‘Knockout’-models must be interpreted with caution due to developmental compensation and/or potential for changes in development.
Abbreviations
- BAT
brown adipose tissue
- PaPy
parapyramidal area
- rRPa
rostral raphe pallidus
- Glu
glutamate
- Orx
orexin
Biography
 Dr Christopher J. Madden is an Assistant Professor in the Department of Neurological Surgery at Oregon Health and Science University. Dr. Madden received his Ph.D. in Neuroscience from the University of Pittsburgh in 2002. He was a Post doctoral Fellow in the laboratory of Dr. Shaun F. Morrison in the Neurological Sciences Institute at Oregon Health and Science University from 2002-2008 and then a Staff Scientist at the Oregon National Primate Research Center until obtaining his current position in 2010. Dr. Madden's research is focused on the functional organization of the central neural circuits regulating metabolism of adipose tissue, glucose homeostasis, cardiovascular function, and thermogenesis, and how alterations in this regulation contribute to disease states and pathological conditions such as obesity, diabetes, and hypertension. Current techniques in use in Dr. Madden's lab include direct simultaneous recordings of peripheral nerve activities and central nervous system single cell discharge, as well as, pharmacological, functional neuroanatomical, molecular biological, and optogenetic techniques to investigate the central neural circuits that regulate cardiovascular function, blood glucose homeostasis, body temperature, and energy metabolism.
Dr Christopher J. Madden is an Assistant Professor in the Department of Neurological Surgery at Oregon Health and Science University. Dr. Madden received his Ph.D. in Neuroscience from the University of Pittsburgh in 2002. He was a Post doctoral Fellow in the laboratory of Dr. Shaun F. Morrison in the Neurological Sciences Institute at Oregon Health and Science University from 2002-2008 and then a Staff Scientist at the Oregon National Primate Research Center until obtaining his current position in 2010. Dr. Madden's research is focused on the functional organization of the central neural circuits regulating metabolism of adipose tissue, glucose homeostasis, cardiovascular function, and thermogenesis, and how alterations in this regulation contribute to disease states and pathological conditions such as obesity, diabetes, and hypertension. Current techniques in use in Dr. Madden's lab include direct simultaneous recordings of peripheral nerve activities and central nervous system single cell discharge, as well as, pharmacological, functional neuroanatomical, molecular biological, and optogenetic techniques to investigate the central neural circuits that regulate cardiovascular function, blood glucose homeostasis, body temperature, and energy metabolism.
 Dr. Domenico Tupone obtained his Ph. D. in Neurophysiology in 2010 from Alma Mater Studiorum, University of Bologna, Bologna, Italy. He was honored by the Italian Sleep Research Society (SIRS) with the annual “Igino Fagioli” award for the best doctoral thesis in basic sleep research, for his neurophysiological studies on the relationship between REM sleep and central regulation of metabolism. Dr. Tupone is currently a postdoctoral fellow in the laboratory of Prof. Shaun F. Morrison, in the Department of Neurological Surgery at the Oregon Health and Science University, where he studies the central neural regulation of body temperature. Dr. Tupone's principal research interest is the pharmacological manipulation of the central autonomic circuits controlling body temperature for the development of clinical approaches to controlled hypothermia. His studies implement in vivo, electrophysiological and anatomical approaches in anesthetized, awake head-restrained and free-behaving rats.
Dr. Domenico Tupone obtained his Ph. D. in Neurophysiology in 2010 from Alma Mater Studiorum, University of Bologna, Bologna, Italy. He was honored by the Italian Sleep Research Society (SIRS) with the annual “Igino Fagioli” award for the best doctoral thesis in basic sleep research, for his neurophysiological studies on the relationship between REM sleep and central regulation of metabolism. Dr. Tupone is currently a postdoctoral fellow in the laboratory of Prof. Shaun F. Morrison, in the Department of Neurological Surgery at the Oregon Health and Science University, where he studies the central neural regulation of body temperature. Dr. Tupone's principal research interest is the pharmacological manipulation of the central autonomic circuits controlling body temperature for the development of clinical approaches to controlled hypothermia. His studies implement in vivo, electrophysiological and anatomical approaches in anesthetized, awake head-restrained and free-behaving rats.
 Dr. Shaun F. Morrison is a Professor in the Department of Neurological Surgery at Oregon Health and Science University. Dr. Morrison received his Ph.D. in Physiology and Biophysics from the University of Vermont. He was an Assistant Professor in the Division of Neurobiology at Cornell University Medical College and subsequently became a Professor in the Department of Physiology at Northwestern University Medical School. Dr. Morrison became a Senior Scientist in the Neurological Sciences Institute at Oregon Health and Science University in 2001, and to his current position in 2010. His research integrates neurophysiological examination of the central thermoregulatory pathways, from cutaneous thermal afferents to motor outflows controlling thermoregulatory effectors, with physiological analyses of the interrelationship among thermoregulatory, cardiovascular, respiratory and metabolic control systems required to maintain the balance of homeostasis.
Dr. Shaun F. Morrison is a Professor in the Department of Neurological Surgery at Oregon Health and Science University. Dr. Morrison received his Ph.D. in Physiology and Biophysics from the University of Vermont. He was an Assistant Professor in the Division of Neurobiology at Cornell University Medical College and subsequently became a Professor in the Department of Physiology at Northwestern University Medical School. Dr. Morrison became a Senior Scientist in the Neurological Sciences Institute at Oregon Health and Science University in 2001, and to his current position in 2010. His research integrates neurophysiological examination of the central thermoregulatory pathways, from cutaneous thermal afferents to motor outflows controlling thermoregulatory effectors, with physiological analyses of the interrelationship among thermoregulatory, cardiovascular, respiratory and metabolic control systems required to maintain the balance of homeostasis.
References
- 1.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Experimental Physiology. 2008;93:773–97. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Frontiers in bioscience : a journal and virtual library. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison SF. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol. 2011;110:1137–49. doi: 10.1152/japplphysiol.01227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Frontiers in Endocrinology. 2012;3:1–19. doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 7.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochemistry and cell biology. 2005;123:147–56. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- 9.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;31:15944–55. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. American Journal of Physiology. 1999;276:R962–73. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. Journal of Neuroscience. 2004;24:5370–80. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol. 1999;276:R290–7. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- 13.Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, Liao HT, Mackie K, Chiou LC. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci. 2011;31:14600–10. doi: 10.1523/JNEUROSCI.2671-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peever JH, Lai YY, Siegel JM. Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. J Neurophysiol. 2003;89:2591–600. doi: 10.1152/jn.00968.2002. [DOI] [PubMed] [Google Scholar]
- 15.Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. Journal of Physiology. 2006;577:525–37. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madden CJ, Morrison SF. Brown adipose tissue sympathetic nerve activity is potentiated by activation of 5-hydroxytryptamine (5-HT)1A/5-HT7 receptors in the rat spinal cord. Neuropharmacology. 2008;54:487–96. doi: 10.1016/j.neuropharm.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madden CJ, Morrison SF. Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2010;298:R776–83. doi: 10.1152/ajpregu.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ootsuka Y, de Menezes RC, Zaretsky DV, Alimoradian A, Hunt J, Stefanidis A, Oldfield BJ, Blessing WW. Brown adipose tissue thermogenesis heats brain and body as part of the brain-coordinated ultradian basic rest-activity cycle. Neuroscience. 2009;164:849–61. doi: 10.1016/j.neuroscience.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mochizuki T, Klerman EB, Sakurai T, Scammell TE. Elevated body temperature during sleep in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R533–40. doi: 10.1152/ajpregu.00887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Zeitzer JM, Sakurai T, Nishino S, Mignot E. Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol. 2007;581:649–63. doi: 10.1113/jphysiol.2007.129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monda M, Viggiano A, De Luca V. Paradoxical [correction of parodoxical] effect of orexin A: hypophagia induced by hyperthermia. Brain Res. 2003;961:220–8. doi: 10.1016/s0006-8993(02)03953-7. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–70. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, Mignot E, Nishino S. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–81. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 24.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–62. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blessing W, Mohammed M, Ootsuka Y. Heating and eating: Brown adipose tissue thermogenesis precedes food ingestion as part of the ultradian basic rest-activity cycle in rats. Physiol Behav. 2011;105:966–74. doi: 10.1016/j.physbeh.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J Physiol. 2010;588:4117–29. doi: 10.1113/jphysiol.2010.195099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nature medicine. 2010;16:403–5. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–90. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 31.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 32.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 33.Dahmen N, Bierbrauer J, Kasten M. Increased prevalence of obesity in narcoleptic patients and relatives. European archives of psychiatry and clinical neuroscience. 2001;251:85–9. doi: 10.1007/s004060170057. [DOI] [PubMed] [Google Scholar]
- 34.Kok SW, Overeem S, Visscher TL, Lammers GJ, Seidell JC, Pijl H, Meinders AE. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes Res. 2003;11:1147–54. doi: 10.1038/oby.2003.156. [DOI] [PubMed] [Google Scholar]
- 35.Nishino S, Ripley B, Overeem S, Nevsimalova S, Lammers GJ, Vankova J, Okun M, Rogers W, Brooks S, Mignot E. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Annals of neurology. 2001;50:381–8. doi: 10.1002/ana.1130. [DOI] [PubMed] [Google Scholar]
- 36.Schuld A, Hebebrand J, Geller F, Pollmacher T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355:1274–5. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- 37.Schuld A, Beitinger PA, Dalal M, Geller F, Wetter TC, Albert ED, Hebebrand J, Pollmacher T. Increased body mass index (BMI) in male narcoleptic patients, but not in HLA-DR2-positive healthy male volunteers. Sleep medicine. 2002;3:335–9. doi: 10.1016/s1389-9457(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 38.Poli F, Plazzi G, Di Dalmazi G, Ribichini D, Vicennati V, Pizza F, Mignot E, Montagna P, Pasquali R, Pagotto U. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep. 2009;32:1491–7. doi: 10.1093/sleep/32.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lammers GJ, Pijl H, Iestra J, Langius JA, Buunk G, Meinders AE. Spontaneous food choice in narcolepsy. Sleep. 1996;19:75–6. doi: 10.1093/sleep/19.1.75. [DOI] [PubMed] [Google Scholar]
- 40.Middelkoop HA, Lammers GJ, Van Hilten BJ, Ruwhof C, Pijl H, Kamphuisen HA. Circadian distribution of motor activity and immobility in narcolepsy: assessment with continuous motor activity monitoring. Psychophysiology. 1995;32:286–91. doi: 10.1111/j.1469-8986.1995.tb02957.x. [DOI] [PubMed] [Google Scholar]
- 41.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 42.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. New England Journal of Medicine. 2009;360:1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 43.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One. 2011;6:e17247. doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–31. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, Yanagisawa M. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]