Abstract
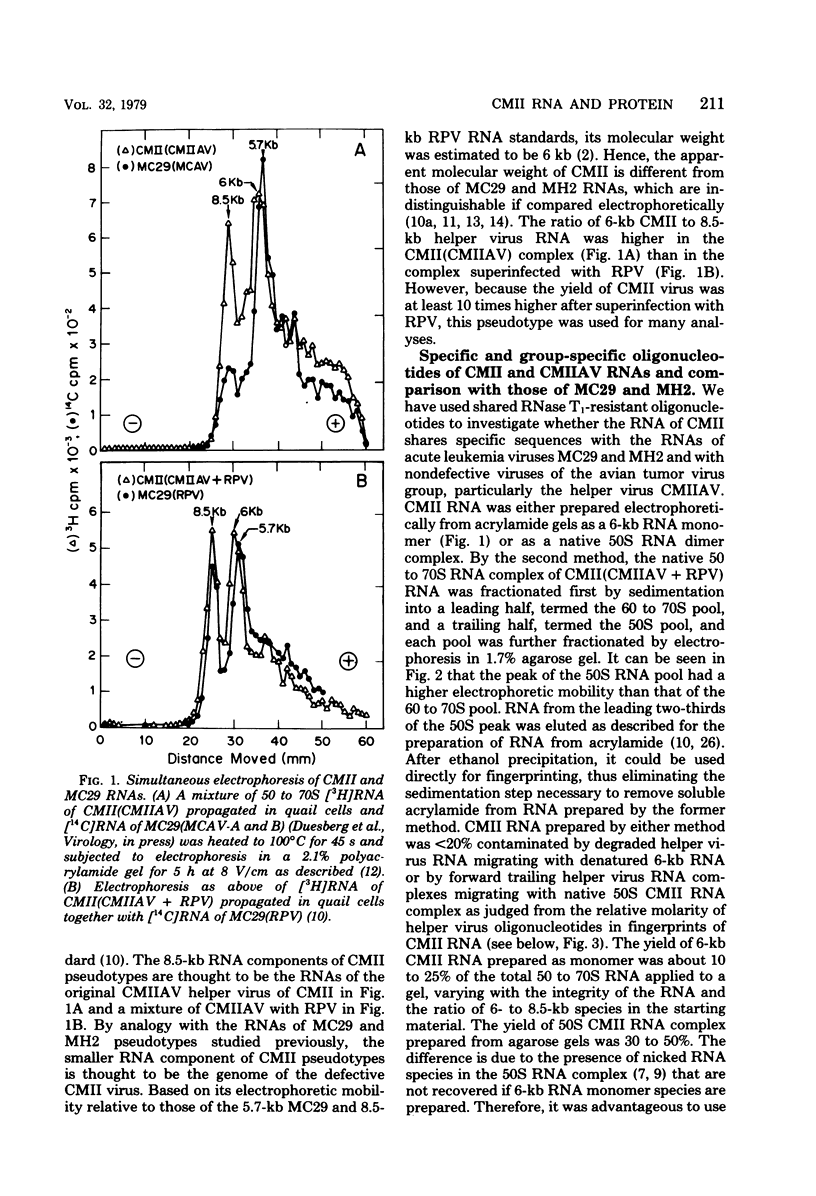
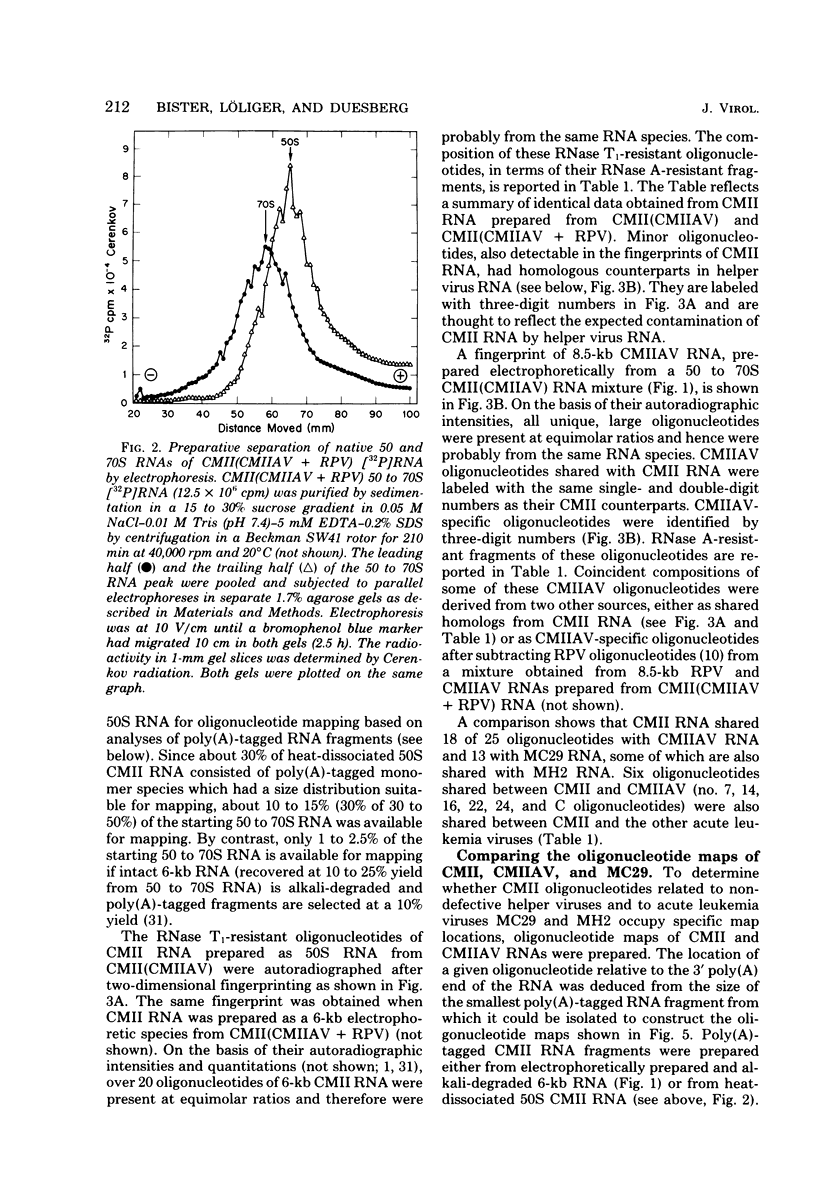
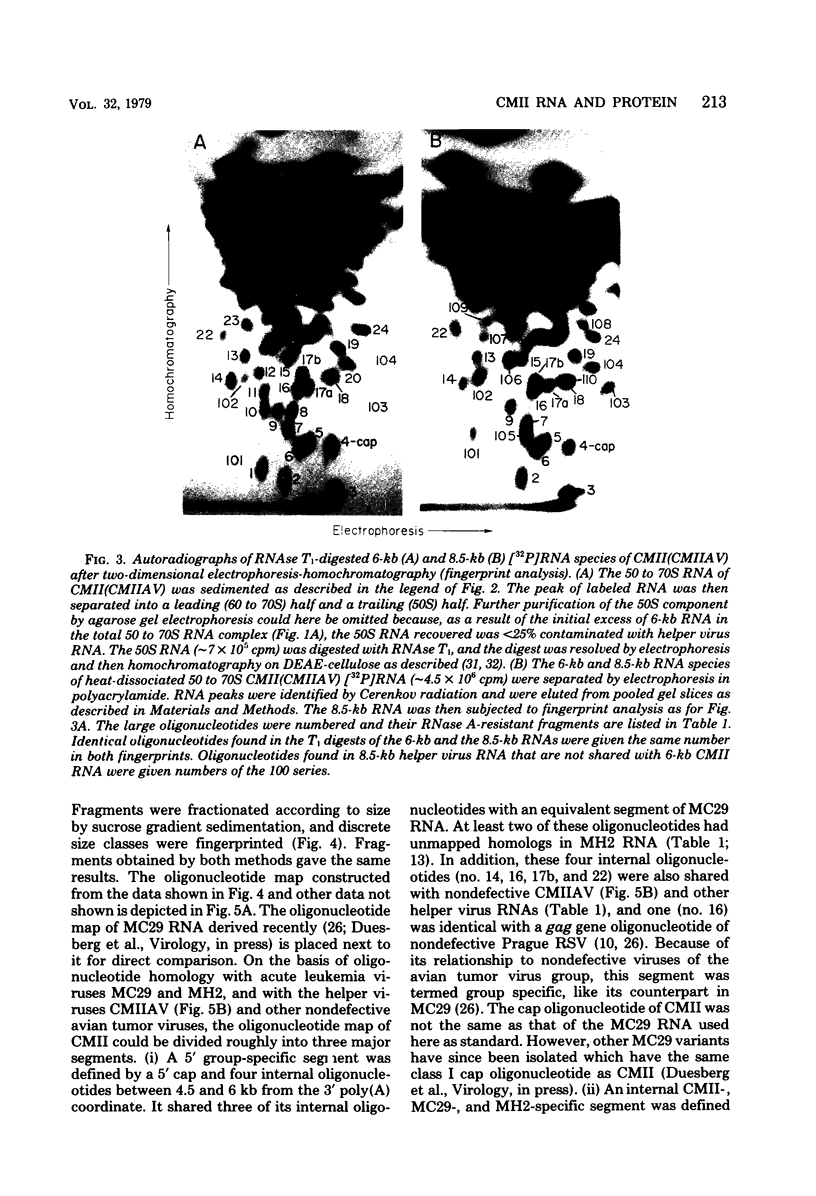
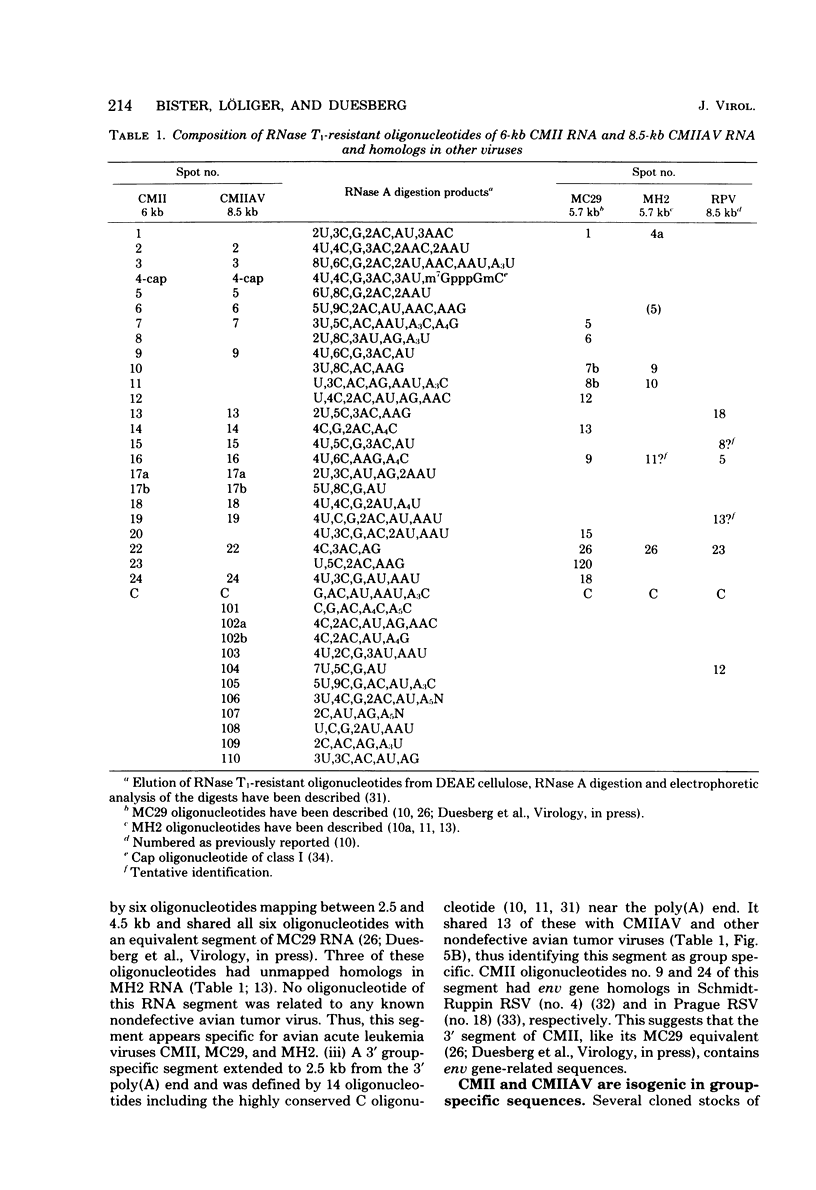
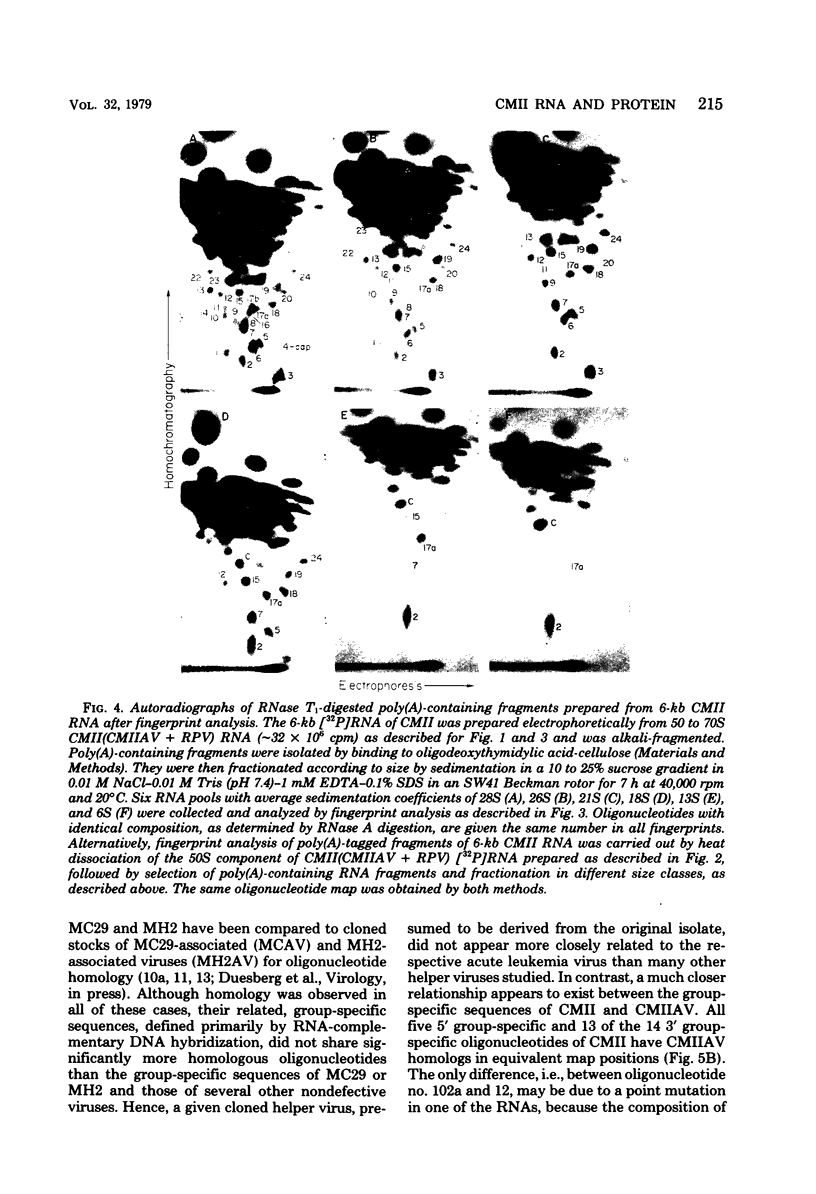
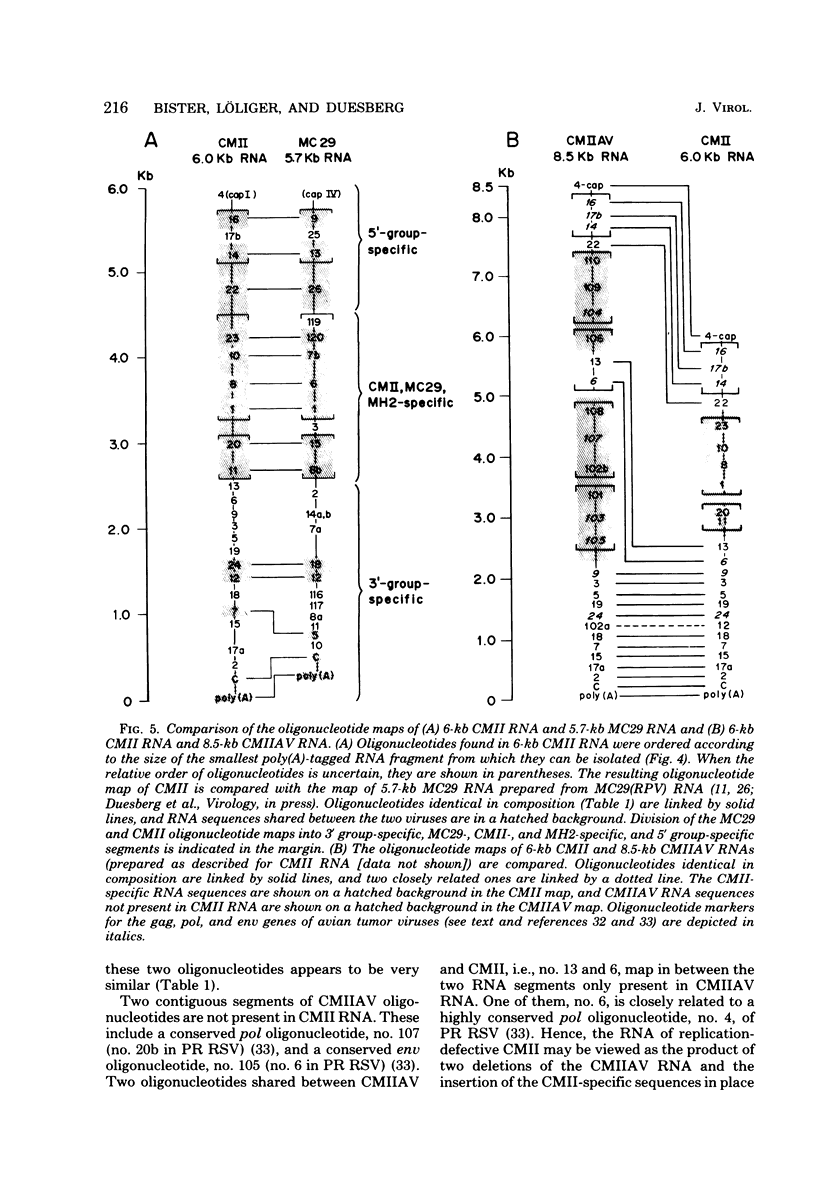
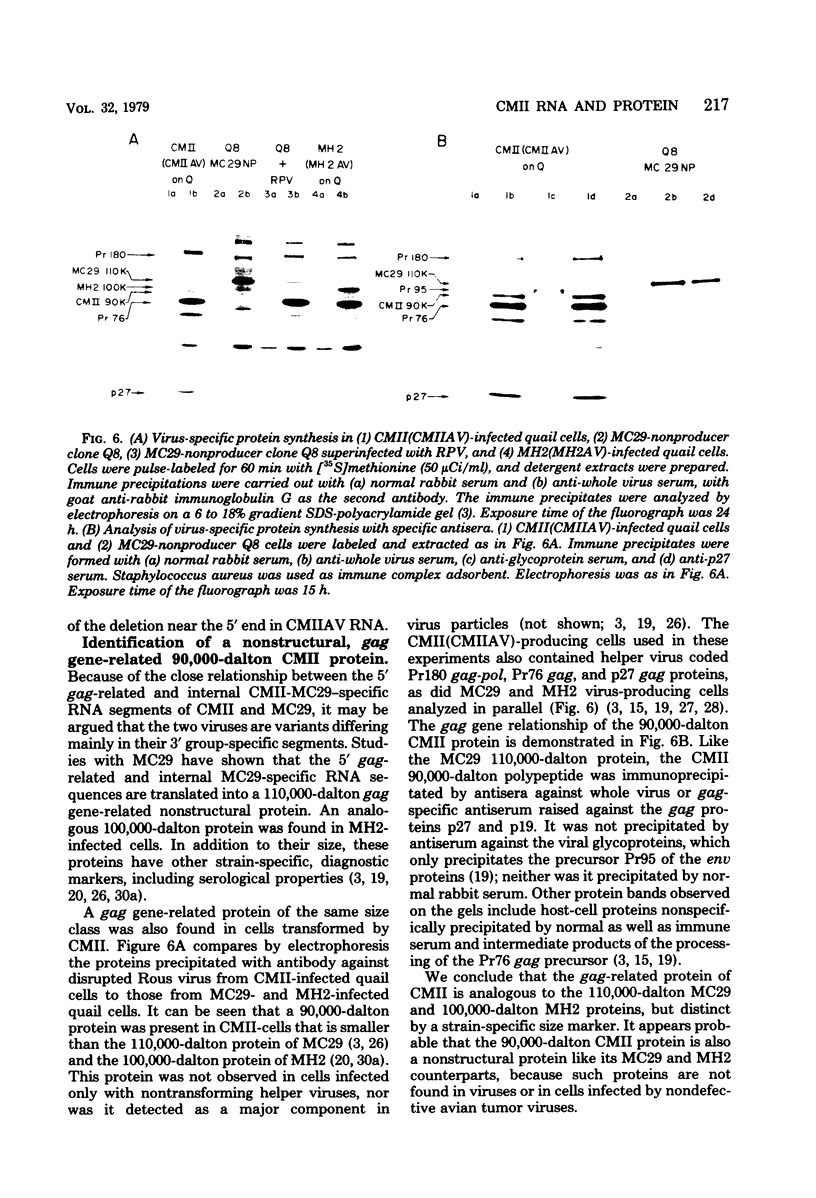
RNA and protein of the defective avian acute leukemia virus CMII, which causes myelocytomas in chickens, and of CMII-associated helper virus (CMIIAV) were investigated. The RNA of CMII measured 6 kilobases (kb) and that of CMIIAV measured 8.5 kb. By comparing more than 20 mapped oligonucleotides of CMII RNA with mapped and nonmapped oligonucleotides of acute leukemia viruses MC29 and MH2 and with mapped oligonucleotides of CMIIAV and other nondefective avian tumor viruses, three segments were distinguished in the oligonucleotide map of CMII RNA: (i) a 5' group-specific segment of 1.5 kb which was conserved among CMII, MC29, and MH2 and also homologous with gag-related oligonucleotides of CMIIAV and other helper viruses (hence, group specific); (ii) an internal segment of 2 kb which was conserved specifically among CMII, MC29, and MH2 and whose presence in CMII lends new support to the view that this class of genetic elements is essential for oncogenicity, because it was absent from an otherwise isogenic, nontransforming helper, CMIIAV; and (iii) a 3' group-specific segment of 2.5 kb which shared 13 of 14 oligonucleotides with CMIIAV and included env oligonucleotides of other nondefective viruses of the avian tumor virus group (hence, group specific). This segment and analogous map segments of MC29 and MH2 were not conserved at the level of shared oligonucleotides. CMII-transformed cells contained a nonstructural, gag gene-related protein of 90,000 daltons, distinguished by its size from 110,000-daltom MC29 and 100,000-dalton MH2 counterparts. The gag relatedness and similarity to the 110,000-dalton MC29 counterpart indicated that the 90,000-dalton CMII protein is translated from the 5' and internal segments of CMII RNA. The existence of conserved 5' and internal RNA segments and conserved nonstructural protein products in CMII, MC29, and MH2 indicates that these viruses belong to a related group, termed here the MC29 group. Viruses of the MC29 group differ from one another mainly in their 3' RNA segments and in minor variations of their conserved RNA segments as well as by strain-specific size markers of their gag-related proteins. Because (i) the conserved 5' gag-related and internal RNA segments and their gag-related, nonvirion protein products correlate with the conserved oncogenic spectra of the MC29 group of viruses and because (ii) the internal RNA sequences and nonvirion proteins are not found in nondefective viruses, we propose that the conserved RNA and protein elements are necessary for oncogenicity and probably are the onc gene products of the MC29 group of viruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bister K., Vogt P. K. Genetic analysis of the defectiveness in strain MC29 avian leukosis virus. Virology. 1978 Jul 15;88(2):213–221. doi: 10.1016/0042-6822(78)90278-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Champion M., Chabot F. Nucleotide sequence relationships between the genomes of an endogenous and an exogenous avian tumor virus. J Virol. 1978 Dec;28(3):972–991. doi: 10.1128/jvi.28.3.972-991.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Duesberg P. H., Mangel W. F. Electron microscope measurements of rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):835–843. doi: 10.1101/sqb.1974.039.01.097. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Vogt P. K. The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4320–4324. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Avian acute leukemia viruses MC29 and MH2 share specific RNA sequences: evidence for a second class of transforming genes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1633–1637. doi: 10.1073/pnas.76.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Duesberg P., Mellon P., Pawson A., Bister K., Vogt P. K. Anatomy of the RNA and gene products of MC29 and MH2, two defective avian tumor viruses causing acute leukemia and carcinoma: evidence for a new class of transforming genes. Haematol Blood Transfus. 1979;23:241–260. doi: 10.1007/978-3-642-67057-2_31. [DOI] [PubMed] [Google Scholar]
- Eisenman R., Vogt V. M., Diggelmann H. Synthesis of avian RNA tumor virus structural proteins. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1067–1075. doi: 10.1101/sqb.1974.039.01.122. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Hu S. S., Moscovici C., Vogt P. K. The defectiveness of Mill Hill 2, a carcinoma-inducing avian oncovirus. Virology. 1978 Aug;89(1):162–178. doi: 10.1016/0042-6822(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Ishizaki R., Langlois A. J., Chabot J., Beard J. W. Component of strain MC29 avian leukosis virus with the property of defectiveness. J Virol. 1971 Dec;8(6):821–827. doi: 10.1128/jvi.8.6.821-827.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mellon P., Pawson A., Bister K., Martin G. S., Duesberg P. H. Specific RNA sequences and gene products of MC29 avian acute leukemia virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5874–5878. doi: 10.1073/pnas.75.12.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Marciani D. J., Papas T. S. Cell-free synthesis of the precursor polypeptide for avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4951–4954. doi: 10.1073/pnas.74.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Fanshier L., Bishop J. M. Identification of nucleotide sequences which may encode the oncogenic capacity of avian retrovirus MC29. J Virol. 1978 Nov;28(2):600–610. doi: 10.1128/jvi.28.2.600-610.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Robins T., Yokota H., Vogt P. K. The terminal oligonucleotides of avian tumor virus RNAs are genetically linked. Virology. 1977 Oct 15;82(2):472–492. doi: 10.1016/0042-6822(77)90020-4. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Mellon P., Vogt P. K. Distribution of envelope-specific and sarcoma-specific nucleotide sequences from different parents in the RNAs of avian tumor virus recombinants. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1073–1077. doi: 10.1073/pnas.73.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]