Abstract
Objectives
We sought to determine the prevalence, characteristics and outcomes of asymptomatic left ventricular (LV) systolic dysfunction in patients with severe aortic stenosis (AS).
Background
Management of asymptomatic patients with severe AS remains controversial. In these patients, LV systolic dysfunction, defined in the guidelines as ejection fraction < 50%, is a class I(C) indication for aortic valve replacement (AVR), but its prevalence is unknown.
Methods
A retrospective study of adults ≥ 40 years with severe valvular AS (peak velocity ≥ 4 m/sec, mean gradient > 40 mmHg, aortic valve area (AVA) < 1 cm2, or AVA index < 0.6 cm2/m2) from 1984 through 2010 was undertaken. Patients with prior cardiac surgery, severe coronary artery disease, or greater than moderate aortic regurgitation were excluded.
Results
Of 9940 patients with severe AS, 43 (0.4 %) patients had asymptomatic LV dysfunction. Age was 73 ± 14 years and 70% were male. Hypertension (78%) and LV hypertrophy (LV mass index 143 ± 36 g/m2) were characteristic. Fifty-three percent of these patients developed symptoms at 21 ± 19 months after diagnosis. During 7.5 ± 6.7 years follow-up, 5-year mortality was 48%. After multivariable adjustment, there was no survival advantage with AVR in asymptomatic, severe AS with LV dysfunction (p = 0.51).
Conclusions
In severe AS, the prevalence of asymptomatic LV systolic dysfunction is 0.4%. Despite an asymptomatic clinical status, patients with severe AS and LV ejection fraction < 50% have a poor prognosis, with or without AVR.
Keywords: Aortic stenosis, valves, echocardiography, heart failure, valve surgery
INTRODUCTION
Management of the asymptomatic patient with severe aortic stenosis (AS) remains controversial. Patients who receive an aortic valve (AV) replacement (AVR) may have a better outcome (1–3), though this may be impacted by the selection of healthier patients for surgery. There is only one prospective study of early versus late surgical intervention in asymptomatic, severe AS (4) and there are no randomized controlled trials defining the optimal treatment strategy (5). According to ACC/AHA guidelines, left ventricular (LV) systolic dysfunction, defined as LV ejection fraction (LVEF) < 50%, is a class I(C) indication for AVR in severe, asymptomatic AS (6,7). This recommendation is based on very limited evidence.
It is unknown how often LV dysfunction develops in the absence of symptoms in patients with severe AS. This study was undertaken to determine the prevalence, clinical characteristics, and outcomes of asymptomatic LV dysfunction in patients with severe AS.
METHODS
Study Patients
A retrospective study of all adults ≥ 40 years with severe valvular AS by a comprehensive two-dimensional and Doppler transthoracic echocardiogram (TTE) from 1984 through 2010 was performed after Institutional Review Board approval. Severe AS was defined as a peak systolic velocity ≥ 4 m/s, mean gradient > 40 mm Hg, aortic valve area (AVA) < 1.0 cm2, or AVA index of < 0.6 cm2/m2 by Doppler echocardiography in combination with two-dimensional echocardiographic appearance of severe valvular AS (7). Only one of these Doppler criteria of severe AS was required for study inclusion, thus ensuring that patients with low-output, low-gradient severe AS were included. Those with LVEF < 50% on an initial qualifying TTE or subsequent follow-up TTE in the absence of prior AVR were reviewed for study inclusion.
Exclusion criteria included: (1) prior cardiac surgery, (2) multivalvular heart disease or greater than moderate aortic regurgitation, (3) prior valvuloplasty, (4) severe coronary artery disease by angiography (≥ 70% stenosis in ≥ 2 vessels), (5) prior myocardial infarction, (6) congenital heart disease other than bicuspid AV, (7) cardiomyopathy, (8) cardiac symptoms (presyncope, syncope, angina, exertional dyspnea, heart failure, or resuscitated sudden cardiac death), with or without stress testing. Stress testing was not routinely performed.
Control Patients
Following identification of asymptomatic patients with severe AS and LVEF < 50%, a 3:1 age/gender/date matched-control group of asymptomatic patients with severe AS meeting the same criteria except having LVEF ≥ 50% was performed.
Statistical analysis
Nominal variables are presented as absolute count and percentage of cohort. Continuous variables are presented as mean ± standard deviation. Categorical variables were compared between those with low LVEF and those with normal LVEF using chi-square tests. Continuous variables were compared between the groups using two sample t-tests or non-parametric Wilcoxon Rank Sum tests. Paired t-tests were used to compare changes in echo parameters over time. The Kaplan-Meier method was used to construct survival curves using time of death as event time and last known follow-up as censoring time. A time dependent analysis was conducted to determine the effect of AVR on survival. Cox-proportional hazards regression modeling was used to determine multivariate associations with survival and estimate hazard ratios.
RESULTS
Prevalence of asymptomatic LV dysfunction in severe AS
From 1984 through 2010, 9940 patients undergoing TTE and clinical evaluation at our institution had severe valvular AS; 1960 (20%) presented with LVEF < 50% and 7980 (80%) had LVEF ≥ 50%. Excluding those who underwent AVR prior to follow-up TTE (n = 2225), 59% (3388 of 5755) of patients had one or more follow-up TTEs performed: 14% (486 of 3388) of these patients developed LV dysfunction. Thus, a total of 2446 (24.6%) patients presented with (n = 1960) or developed (n = 486) LV systolic dysfunction. Only 43 (1.8%) of the 2446 patients had isolated severe AS and were asymptomatic, constituting 0.4% of all patients with severe AS.
The 43 patients included 38 patients with asymptomatic, severe AS and reduced LV function at the time of diagnosis, and 5 patients who developed asymptomatic LV dysfunction on follow-up TTE (range 6 to 44 months after presenting TTE).
Characteristics of asymptomatic patients with severe AS and LVEF < 50%
Of the 43 asymptomatic patients with reduced LV function, severe AS was identified in 40 (98%) by AVA index, 34 (79%) by AV peak velocity, 31 (76%) by AVA, and 25 (64%) by AV gradient. Four patients met severe AS criteria solely by AVA index. The clinical and TTE characteristics of the 43 patients compared with the age/gender/date matched-control group of asymptomatic patients with severe AS and preserved LV function are shown in Tables 1 and 2, respectively.
Table 1.
Baseline Characteristics of Asymptomatic Patients with Severe AS and LVEF < 50% (n = 43) versus Asymptomatic Patients with Severe AS and LVEF ≥ 50% (n = 122)
| Baseline and Clinical Characteristics |
Asymptomatic, severe AS with LVEF < 50% (n = 43) |
Asymptomatic, Severe AS with LVEF ≥ 50% (n = 122) |
P value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years, mean ± SD) | 73 ± 14 | 73 ± 13 | 0.941 |
| Male sex (n, %) | 30 (70%) | 86 (70%) | 0.929 |
| Body mass index (kg/m2) | 26.4 ± 3.9 | 27.7 ± 5.4 | 0.241 |
| Assisted living | 2 (4%) | 8 (7%) | 0.644 |
| Historical medical comorbidities | |||
| Systemic hypertension | 33 (78%) | 74 (61%) | 0.05 |
| Smoking history | 20 (47%) | 52 (43%) | 0.659 |
| Atrial fibrillation | 12 (28%) | 20 (16%) | 0.111 |
| Hyperlipidemia | 9 (21%) | 41 (34%) | 0.105 |
| Diabetes mellitus | 5 (12%) | 14 (11%) | 0.979 |
| Cardiac procedures | |||
| Percutaneous coronary intervention | 1 (2%) | 6 (5%) | 0.441 |
| Medical therapy | |||
| Antiplatelet | 16 (37%) | 49 (40%) | 0.733 |
| Diuretic* | 15 (35%) | 40 (33%) | 0.802 |
| Digoxin† | 10 (23%) | 22 (18%) | 0.463 |
| Angiotensin converting enzyme inhibitor or receptor blocker* | 10 (23%) | 24 (20%) | 0.621 |
| Calcium channel blocker* | 9 (21%) | 20 (16%) | 0.508 |
| Beta-blocker* | 7 (16%) | 22 (18%) | 0.709 |
| Coumadin | 6 (14%) | 7 (6%) | 0.103 |
| Statin | 4 (9%) | 21 (17%) | 0.194 |
| Nitrate | 0 (0%) | 5 (4%) | 0.08 |
| Electrocardiographic findings | |||
| LV hypertrophy | 14 (37%) | 29 (24%) | 0.120 |
| Left bundle branch block | 4 (11%) | 2 (2%) | 0.02 |
| Laboratory values | |||
| Hemoglobin (g/dL) | 13 ± 2 | 14 ± 2 | 0.768 |
| Creatinine clearance (mL/min) | 61 ± 28 | 54 ± 10 | 0.288 |
For treatment of hypertension.
For treatment of atrial fibrillation or flutter.
Table 2.
Echocardiographic Characteristics of Asymptomatic Patients with Severe AS and LVEF < 50% (n = 43) versus Asymptomatic Patients with Severe AS and LVEF ≥ 50% (n = 122)
| Echocardiographic Parameter |
Asymptomatic, severe AS with LVEF < 50% (n = 43) |
Asymptomatic, Severe AS with LVEF ≥ 50% (n = 122) |
P value |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 135 ± 20 | 140 ± 21 | 0.441 |
| LVEDD∥ (mm, mean ± SD) | 54 ± 7 | 47 ± 6 | < 0.001 |
| LV§ mass index (g/m2) | 145 ± 37 | 117 ± 40 | 0.0008 |
| Relative wall thickness | 0.46 ± 0.1 | 0.55 ± 0.12 | 0.003 |
| LVEF¶ (%) | 43 ± 6 | 64 ± 7 | < 0.001 |
| Stroke volume index (ml/m2) | 43 ± 11 | 48 ± 10 | 0.402 |
| Cardiac index (L/min/m2) | 3.1 ± 0.9 | 3.3 ± 0.7 | 0.258 |
| AVA† by continuity equation (cm2) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.911 |
| AVA index (cm2/m2) | 0.43 ± 0.1 | 0.44 ± 0.1 | 0.389 |
| AV* peak systolic velocity (m/s) | 4.1 ± 0.7 | 4.4 ± 0.7 | 0.02 |
| AV* mean gradient (mmHg) | 43 ± 14 | 48 ± 16 | 0.192 |
| LA end systolic dimension‡ (mm) | 44 ± 7 | 46 ± 8 | 0.957 |
| E/e’ ratio | 18 ± 8 | 16 ± 7 | 0.504 |
| Mitral deceleration time (ms) | 212 ± 88 | 256 ± 73 | < 0.001 |
| Right ventricular systolic pressure (mmHg) | 42 ± 13 | 42 ± 29 | 0.179 |
| Valvuloarterial impedance (mmHg·min/ml) | 4.6 ± 1.3 | 4.0 ± 0.9 | 0.137 |
AV = aortic valve;
AVA = aortic valve area;
LA = left atrial;
LV = Left ventricle;
LVEDD = left ventricular end diastolic dimension;
LVEF = left ventricular ejection fraction
Symptoms and Survival During Follow-up
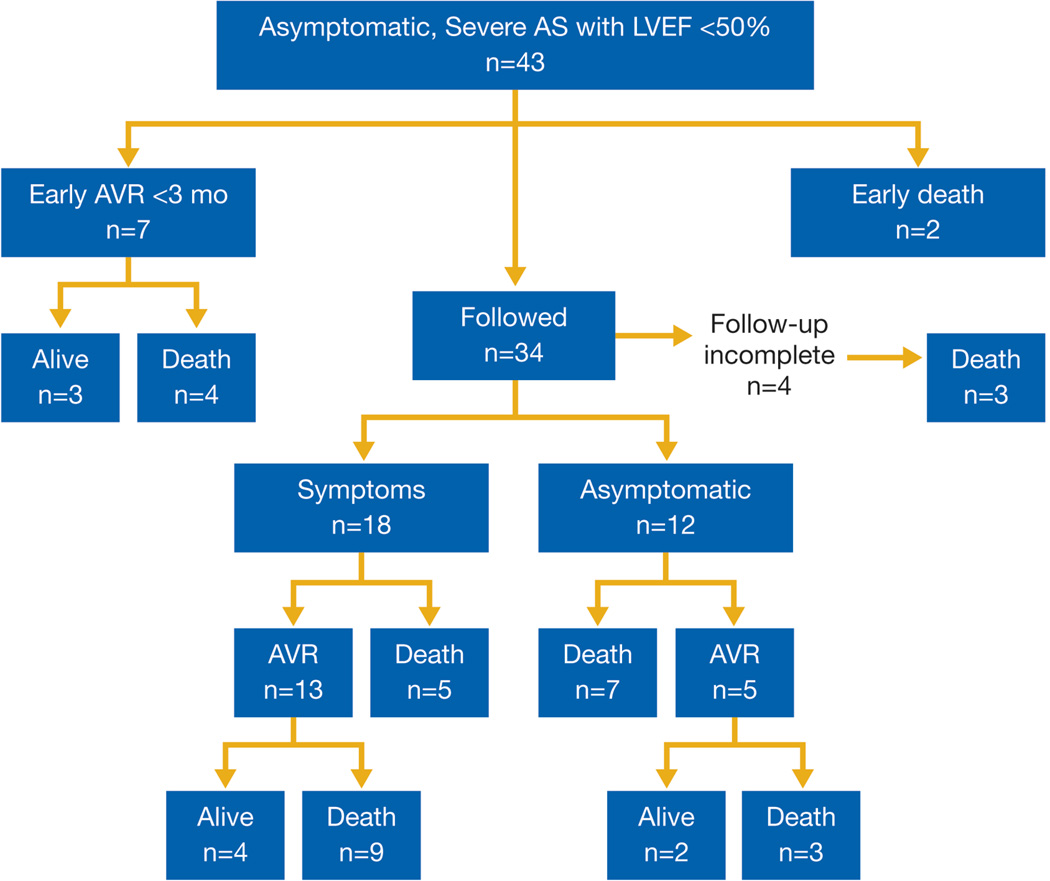
Figure 1 illustrates onset of symptoms and/or treatment with AVR in the 43 patients during 7.5 ± 6.7 years of follow-up. Eighteen patients developed cardiac symptoms, 12 remained asymptomatic until death, and 4 had indeterminate clinical status. Of those who developed symptoms, the average time from qualifying TTE to onset of symptoms was 21 ± 19 months (range 1 month to 5.6 years). Echocardiographic differences before and after onset of symptoms included AVA index (0.47 ± 0.13 vs. 0.41 ± 0.12 cm2/m2, p = 0.05), AV mean gradient (43 ± 10 mmHg vs. 56 ± 13 mmHg, p = 0.02), and left atrial dimension (44 ± 7 mm vs. 48 ± 7 mm, p = 0.02).
Figure 1. Asymptomatic Adults with Severe AS and LVEF < 50% (n = 43).
During follow-up of 7.5 ± 6.7 years, 7 patients underwent early AVR and 2 of 43 patients died before scheduled follow-up. Of the remaining 34 patients, 18 developed symptoms, 12 remained asymptomatic and 4 had indeterminate symptom and surgical status.
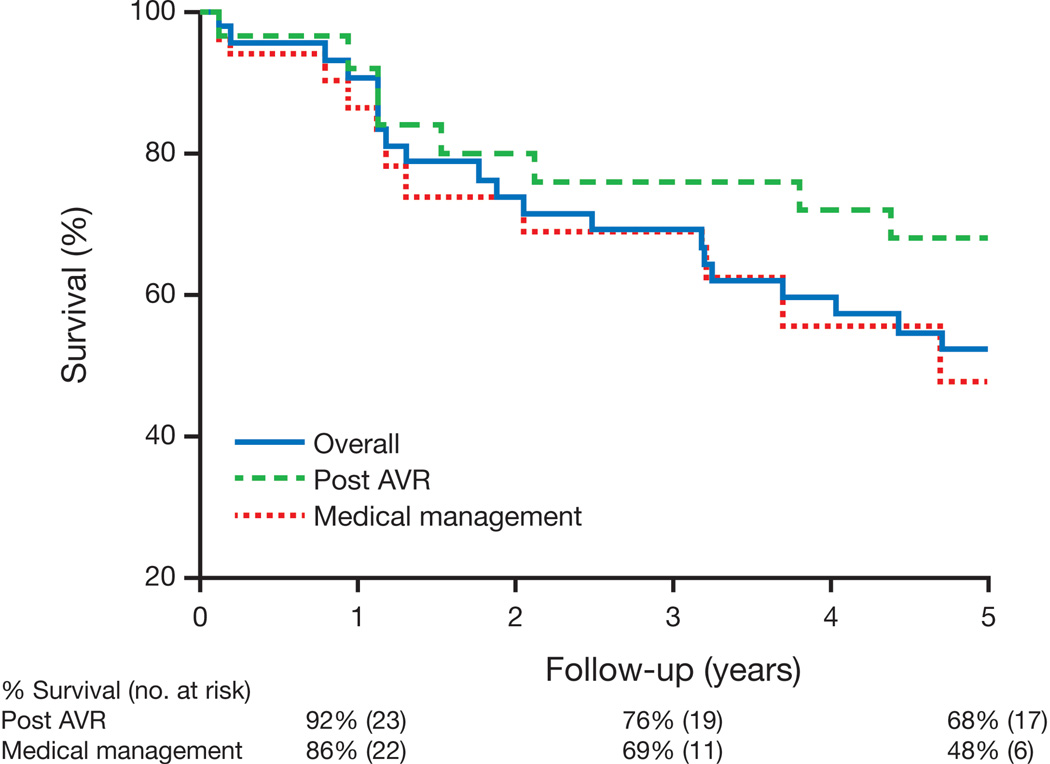
Vital status could be determined in 42 patients (Figure 1); 33 (79%) of 42 died. Five-year mortality in initially asymptomatic unoperated patients with severe AS and LV dysfunction was 48% (Figure 2). The cause of death was determined in 16 patients: 2 had sustained sudden, unexplained deaths within 6 weeks of their last clinical encounter without documented symptoms, 9 had cardiac death (6 heart failure, 1 myocardial infarction, 1 post AVR, and 1 during valvuloplasty) and 5 had noncardiac death.
Figure 2. Unadjusted Survival in Asymptomatic Adults with Severe AS and LVEF < 50%.
During a median of 5.6 years follow-up, 33 of 42 patients died. After adjustment for age, gender, and time period (1984–1995 versus 1996–2010) of diagnosis, there was no survival benefit with AVR (p = 0.51).
AVR and Outcomes
AVR was performed in 25 (64%) of 39 patients in whom follow-up for AVR was available (Figure 1); concomitant coronary artery bypass grafting was performed in 13 (52%), involving a single vessel in 9 (70%). Seven asymptomatic patients underwent AVR < 3 months after their qualifying TTE, 13 developed symptoms prior to AVR and 5 asymptomatic patients underwent late AVR.
Post-operative survival is presented in Figure 2. There was a trend toward improved survival in patients undergoing AVR (HR 0.46, 95% CI 0.18–1.16, p = 0.10), which was not present after adjustment for age, gender, and time of diagnosis (1984–1995 versus 1996–2010) (HR 0.77, 95% CI 0.36–1.67, p = 0.51). Of the 25 patients who underwent AVR, LVEF improved in 17 (81%) of the 21 with post-AVR TTEs.
Among the 14 patients who did not undergo AVR, 7 refused AVR despite physician recommendations, 4 were advised against AVR by their physician due to comorbidities or discordant clinical and echocardiographic interpretations of AS severity, and 3 died while waiting for planned AVR.
DISCUSSION
Prevalence and incidence of asymptomatic LVEF < 50% in severe AS
In this retrospective study of 9940 adults with severe AS, 2403 (24%) patients had symptomatic LV dysfunction. Asymptomatic LV dysfunction was present in only 43 (0.4%) patients, including 38 who had LV dysfunction on presentation with severe AS, and 5 who developed it during follow-up.
Current ACC/AHA guidelines recommend annual TTE for patients with severe AS (7), allowing recognition of an asymptomatic decline in LV systolic function. Herein we show that although it is uncommon, patients can develop progressive decline in LV function despite remaining symptom-free.
Characteristics of patients with asymptomatic, severe AS and LVEF < 50%
AVA index < 0.6 cm2/m2 was present in 98% of our cohort. While parameters of AV mean gradient and maximal velocity are impacted by LV function and may create confusion in grading AS severity, the AVA index establishes AS severity relatively independent of flow (8).
Compared to asymptomatic adults with severe AS and LVEF ≥ 50%, those with reduced LV function were more likely to have a history of hypertension and echocardiographic evidence of eccentric LV hypertrophic remodeling. While our study was not powered to evaluate predictors of LV systolic dysfunction, hypertension may be a poor prognostic factor in severe AS. Pellikka et al and Hachicha et al, in independent studies of asymptomatic patients with severe AS and normal LV function, showed LV hypertrophy and increased valvuloarterial impedance, respectively, to be independent predictors of mortality (9,10).
Outcomes with asymptomatic LV dysfunction and severe AS
The compensated clinical status and independent functional status of asymptomatic patients with severe AS and decreased LV systolic function provide false reassurance for the underlying risk of morbidity and mortality. Within our study group, 53% (18 of 34) of unoperated patients developed symptoms within 2 years of their qualifying TTE; this rate was higher that reported by Pellikka et al (9) in patients with asymptomatic AS and any LVEF where 33% developed symptoms at 2 years.
The 5-year mortality rate for our asymptomatic adults with severe AS and LV systolic dysfunction was substantial at 48%. While AVR has previously been shown to be beneficial in low-risk elderly patients with severe AS and in symptomatic patients with LV dysfunction (11–13), our study isolates, for the first time, asymptomatic patients with reduced LV function. Although LVEF improved after AVR in these patients, survival was similar in patients with and without AVR after adjustment for age, gender, and study date.
Limitations
Given the retrospective method and small sample size, caution must be exercised in the conclusions drawn. Patients were deemed asymptomatic by review of medical history alone; exercise testing was not required. Coronary angiography was not performed in all patients and 52% who underwent AVR had concomitant coronary artery bypass. Similarly, hypertension was a frequent comorbidity and the combination of hypertension with severe AS may have adversely impacted outcome. Serial TTEs were not performed in all asymptomatic patients during follow-up. Newer methods for detecting LV dysfunction, including strain rate imaging (14), were not available.
Novel study conclusions
LV dysfunction may occur in patients with severe AS in the absence of symptoms. However, it is uncommon: our study defines the prevalence of asymptomatic LV dysfunction occurring in patients with severe valvular AS at 0.4%. Although 20% of patients with severe AS had LV dysfunction at presentation, the majority were symptomatic. While infrequently encountered, the asymptomatic patient with severe AS and LV dysfunction has a high risk of mortality, even with AVR.
Current ACC/AHA guidelines offer limited recommendations regarding management of asymptomatic patients with severe AS. We show that the Class I(c) indication for AVR in asymptomatic, severe AS, namely development of LVEF < 50%, is rarely applicable. However, mortality for these patients is high – regardless of whether medical or surgical management is employed. Further efforts are needed to define the optimal time for AVR.
ACKNOWLEDGEMENTS
Funding: Cardiovascular Research Division, Mayo Clinic, Rochester, MN 55905
This publication was supported by NIH/NCRR CTSA Grant Number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
ABBREVIATIONS
- ACC
American College of Cardiology
- AHA
American Heart Association
- AS
Aortic stenosis
- AV
Aortic valve
- AVA
Aortic valve area
- AVR
Aortic valve replacement
- LV
Left ventricle
- LVEF
Left ventricular ejection fraction
- TTE
Transthoracic echocardiogram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Industry relationships: None
REFERENCES
- 1.Brown ML, Pellikka PA, Schaff HV, et al. The benefits of early valve replacement in asymptomatic patients with severe aortic stenosis. J Thorac Cardiovasc Surg. 2008;135:308–315. doi: 10.1016/j.jtcvs.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 2.Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg. 2008;135:1270–1278. doi: 10.1016/j.jtcvs.2007.12.042. discussion 1278–9. [DOI] [PubMed] [Google Scholar]
- 3.Pai RG, Kapoor N, Bansal RC, Varadarajan P. Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg. 2006;82:2116–2122. doi: 10.1016/j.athoracsur.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 4.Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation. 2010;121:1502–1509. doi: 10.1161/CIRCULATIONAHA.109.909903. [DOI] [PubMed] [Google Scholar]
- 5.Owen A, Henein MY. Challenges in the management of severe asymptomatic aortic stenosis. Eur J Cardiothorac Surg. 2011;40:848–850. doi: 10.1016/j.ejcts.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 7.Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. quiz 101–2. [DOI] [PubMed] [Google Scholar]
- 9.Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111:3290–3295. doi: 10.1161/CIRCULATIONAHA.104.495903. [DOI] [PubMed] [Google Scholar]
- 10.Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. 2009;54:1003–1011. doi: 10.1016/j.jacc.2009.04.079. [DOI] [PubMed] [Google Scholar]
- 11.Ashikhmina EA, Schaff HV, Dearani JA, et al. Aortic Valve Replacement in the Elderly: Determinants of Late Outcome. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.110.987560. [DOI] [PubMed] [Google Scholar]
- 12.Chiappini B, Camurri N, Loforte A, Di Marco L, Di Bartolomeo R, Marinelli G. Outcome after aortic valve replacement in octogenarians. Ann Thorac Surg. 2004;78:85–89. doi: 10.1016/j.athoracsur.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 13.Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction:result of aortic valve replacement in 52 patients. Circulation. 2000;101:1940–1946. doi: 10.1161/01.cir.101.16.1940. [DOI] [PubMed] [Google Scholar]
- 14.Adda J, Mielot C, Giorgi R, et al. Low-Flow, Low-Gradient Severe Aortic Stenosis Despite Normal Ejection Fraction Is Associated With Severe Left Ventricular Dysfunction as Assessed by Speckle-Tracking Echocardiography: A Multicenter Study. Circ Cardiovasc Imaging. 2012;5:27–35. doi: 10.1161/CIRCIMAGING.111.967554. [DOI] [PubMed] [Google Scholar]