Abstract
Objective
A novel mouse model with a specific genetic mutation in a G protein coupled receptor (GPCR) encoded by the oxgr1 gene results in a predisposition to spontaneous otitis media with effusion. As a primary component of interest in OME, mucin expression was examined in this model to assess expression as compared to wild type animals and suitability as a murine model of OME.
Method
Mutant (oxgr1−/−) and wild-type (oxgr1+/+) mice between ages of 2–5 months were examined by otoscopy and auditory brainstem response (ABR). Histology changes in the middle ear were evaluated. Expression of mucin genes in the middle ear epithelium was determined using RT-PCR and quantitative PCR.
Result
Otoscopic exam showed signs of inflammation in 82% of mutant mice. Significant elevated ABR thresholds were detected in mutant mice indicating hearing loss. Histology analysis of the middle ears demonstrated the presence of inflammatory cells, changes in the mucosal epithelium, and middle ear fluid. RT PCR using universal primers for bacterial 18s rRNA suggested the absence of bacteria in the middle ear. The knockout mice demonstrated expression of muc1, muc2, muc3, muc4, muc5AC, muc5B, muc9, muc10, muc13, muc15, muc16, muc18, muc19 and muc20. There was a trend of increase in muc5B and muc19 expression in the middle ear of the knockout mice compared to that of wild-type. There was no significant change in the level of muc2, and muc5AC was expressed at a level below the detection limit of quantification.
Conclusion
Development of a murine model with genetic defect has several attractive features. The rate of OME in these animals is high at 82%. It is clear that this OME is related to histopathologic changes in the middle ear epithelium of these knock-out mice. Induction of mucus effusion is evident though the viation in dysregulation of GFM does exist in this non-challenge study condition. The underlying cause of these differences between individual animal requires further investigation. Given this, the oxgr1−/− model is likely to be an ideal model to examine mucin regulation in MEE and potentially develop novel GPCR-specific targeted interventions to regulate these processes.
Keywords: oxgr1, knockout mice, otitis media, mucin
Introduction
Otitis media (OM) continues to be a prevalent and challenging disease process for children. OM affects the majority of children early in life and is responsible for billions of dollars in health care expenditures world-wide. In severe cases, OM leads to hearing loss, resulting in the potential for developmental delays and the need for surgical intervention. Progress has been made in the molecular understanding of the pathophysiology that takes place in OM [1–5] and in developing vaccines to limit the impact of this disease. However, numerous questions and challenges remain in understanding OM pathogenesis and developing novel solutions. One area of particular difficulty involves otitis media with effusion (OME). In this subset of OM, the persisting middle ear effusion (MEE) that develops can lead to longer term hearing loss and difficulties for children. Mucins are the primary components in the MEE of OME, and these mucins are responsible for this fluid’s viscosity and subsequent hearing loss [2]. In developing models for the study of acute otitis media (AOM) numerous animal models have proven efficacious. However, humans represent a unique species in which chronic OME develops in a subset of the population without obvious underlying genetic or anatomic deficits. Although studies have demonstrated genetic predisposition and genetic polymorphisms associated with OME; many patients with OME have no obvious genotypic defect [6]. In the primary animal models of OM; chinchilla, mouse and rat, OME does not develop in wild type animals. Thus, investigators have relied upon either creating anatomic changes, such as altering the Eustachian tube, or creating animals with a genetic defect predisposing them to OME [7–9]. Each of these approaches presents some difficulties in translating findings to human disease. However, they have provided robust systems for investigation and acceptable surrogates in studying the pathophysiology in OME.
This current investigation employed a novel mouse model with a specific genetic mutation in a G protein coupled receptor encoded by the oxgr1 gene resulting in a predisposition to spontaneous OME. As a primary component of interest in OME, mucin expression in this model was examined to assess expression, comparison to wild type animals and suitability for future experimentation using this murine model of OME.
Methods
Animals
Heterozygous (Oxgr1+/−) mice with mixed (C57BL/6J-Tyrc-Brd × 129Sv/EvBrd) genetic background generated by homologous recombination were developed by Lexicon Pharmaceuticals Incorporated (The Woodlands, TX). The homologous recombination removed the full length of Oxgr1 gene. The oxgr1 (NM_001001490) targeting vector was constructed from lambda POS system. The PCR generated selection cassette including flanking homology sequences of the specific gene was introduced into the mouse genomic clone by yeast recombination resulting in the full length removal of Oxgr1 gene. The linearized targeting vector was electroporated into 129Sv/EvBrd embryonic stem (ES) cells. To confirm Oxgr1 deletion, the select clones were analyzed by southern hybridization using specific external probes on both arms of homology. The targeted ES clones were injected into C57BL/6J-Tyrc-Brd blastocysts. The resulting chimeras were back crossed to C57BL/6J-Tyrc-Brd to generate F1 heterozygous offsprings. Heterozygous cross set up generated wild-type control (Oxgr1+/+), homozygous null (Oxgr1−/−) and heterozygous litter mates for the study population. Genotype was verified by PCR reaction of tail genomic DNA. Primer pairs UTT069-21 (5′-GAGCCATGATTGAGCCACTG-3′) and UTT069-25 (5′-CACCACTGGCATAGTAATGG-3′) generated a 294 bp Oxgr1 specific fragment which present in wild type but absent in mutant allele and UTT069-3 (5′-CAGAGCCATGCCTACGAG-3′) and GT (5′-CCCTAGGAATGCTCGTCAAGA-3′) amplified a 378 bp fragment specific to the selection cassette in mutant allele. The PCR amplicon from each allele was subjected to restriction analysis to confirm sequence specificity. In conjunction with the scientific team at Lexicon Pharmaceuticals the predictability of OME formation and suitability of this construct for the proposed experiments was determined and found acceptable. All animals were handled in accordance with the specific guidelines of the Institutional Animal Care and Use Committee at the Medical College of Wisconsin.
Auditory Brainstem Response and Otoscopy
Animals were housed in a pathogen-free environment and colonies were generated. Mixed gender adult animals were culled at 8–20 weeks for experimentation. A total of 12 wild type and 14 mutant mice were subjected to ABR threshold measurement performing at 4, 8, 12, 16, 24 and 32 kHz according to standard protocol [10]. All ears were examined by otoscopy following ABR for presence of middle ear fluid, inflammation, or infection.
Middle ear histopathology and OME pathogen detection
Five animals from each group were utilized for histology analysis of the middle ears. Animals were euthanized with an overdose of pentobarbital and intracardiac perfused with 10% buffered formalin. The intact bullas were removed bilaterally and immediately immersed in 10% buffered formalin. The resulting fixed bullas were decalcified, dehydrated and embedded in paraffin. Paraffin embedded bullas were sectioned horizontally at 5 microns and serially collected at every 50um. The sections were mounted on glass slides and stained with hematoxylin and eosin for histologic evaluation, and with the Periodic acid-Schiff (PAS) reaction to highlight mucus glycoprotein.
Despite housing in a pathogen-free environment, several steps were employed to ensure that the OME identified was related to spontaneous development of OME rather than pathogen-associated OME. Bulla sections were stained for bacterial contamination using Gram staining. Furthermore, polymerase chain reaction was utilized to amplify ribosomal RNA of both nonspecific bacteria and those prominent pathogens causing OME such as H influenzae, S pneumoniae and M catarrhallis. The specific primers and PCR conditions were as previously described [11].
Mucin gene expression in MEM
Following euthanasia, the temporal bone was harvested and homogenized in TriZol reagent (Invitrogen). The homogenate was prepared from each ear of the wild type and mutant mice. Total RNA was extracted following the manufacturer’s instructions. Genomic DNA was removed using RQ1 RNase-Free DNase (Promega). Two micrograms of purified total RNA was reverse transcribed using Superscript III First Strand Synthesis System (Invitrogen), following the manufacturer’s instructions. Specific mucin genes (muc1, muc2, muc3, muc4, muc5AC, muc5B, muc6, muc9, muc10, muc13, muc15, muc16, muc18, muc19 and muc20) were amplified with Platinum Blue PCR master mix (Invitrogen), each in 20ul reactions containing 0.2uM of each primer, 1.5mM MgCl2 and 1ul of cDNA template using MiniAmp (Biorad). Specific primers and PCR conditions utilized for mouse mucin genes were as previously described [12].
Quantification of gel forming mucin genes
Following demonstration of mucin gene expression as described above, quantitative PCR was performed to assess the expression of the gel-forming mucin genes: muc2, muc5AC, muc5B and muc19. TaqMan primer/probe sets were obtained commercially (Applied Biosystems) for mouse muc2 (P/N: Mm00458299_m1), muc5AC (P/N: 01276735_m1), muc5B (P/N: Mm00466407_m1), muc19 (P/N: Mm01306462_m1) and hprt (P/N: Mm03024075_m1). Two micrograms of total RNA from mouse middle ear was random primed and reverse transcribed using Superscript III First Strand Synthesis System (Invitrogen). Quantitative PCR reaction was performed using one-tenth of the cDNA reaction on a ViiA7 (Applied Biosystems). Each cDNA sample was analyzed in triplicates. The mRNA level of each target gene was normalized to Hprt gene. The relative fold change was then calculated using 2−ΔΔCt method [13].
Results
Genotypic screening of Oxgr1 knockout mice
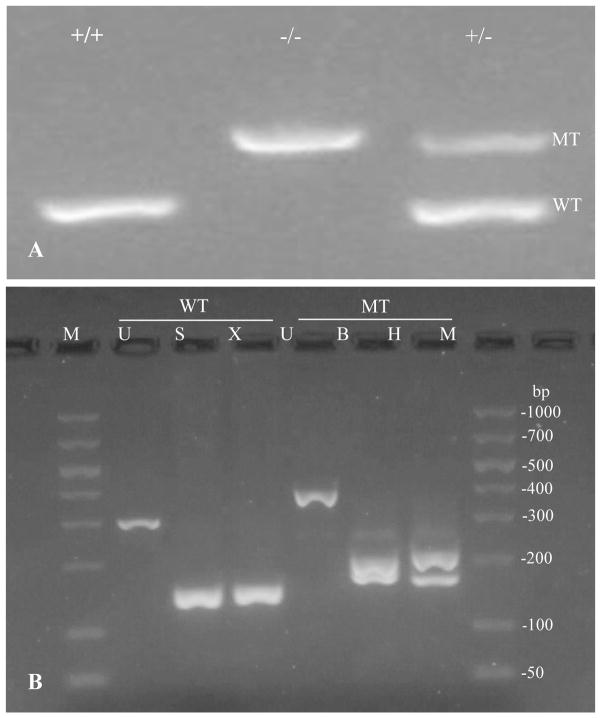
Cross set up between heterozygous (Oxgr1+/−) mice generated wild-type control, homozygous null (Oxgr1−/−) and heterozygous population for the study consistent with Mendelian ratios. The genotyping was confirmed by PCR amplification of the tail genomic DNA using specific primers for either the wild type or mutant allele. The specific primers 21 and 25 amplified 294 bp fragment of Oxgr1 coding region in the wild type but not mutant allele. The specific primers for mutant allele amplified a 378 bp fragment of the selection cassette that replaced the gene by homologous recombination (Figure 1).
Figure 1.
Genotype verification in litter mates by PCR amplification of tail genomic DNA. (A) Specific wild type allele primers generated a 294bp fragment in the homozygote wild type (oxgr1+/+) and heterozygotes (oxgr1+/−) animals. Specific mutant allele primers amplified a 378bp fragment of the selection cassette that generated homozygotes null (oxgr1−/−) via homologous recombination. (B) Restriction analysis of the amplified fragments from wild type and mutant alleles. The amplified wild type fragment yielded a 152 and 142bp in SmaI digestion, and a 150 and 144bp in XmaI digestion. The amplified mutant allele subjected to BamHI digestion resulted in 177 and 201bp, while HindIII reduced the fragment to 168 and 210bp. M; 50–1000bp DNA ladder, U; uncut, S; SmaI, X; XmaI, B; BamHI, and H; HindIII.
Oxgr1 knockout mice spontaneously develop otitis media with effusion
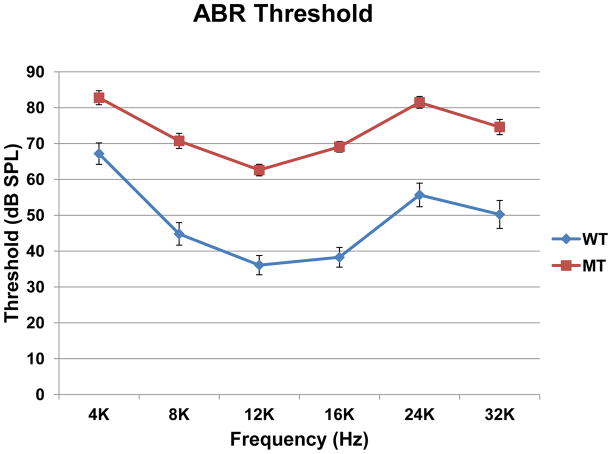
Otoscopic examination of 8–20 week old mutant mice revealed 25 of 34 ears (74%) were affected with middle ear fluid, middle ear air-fluid levels or bulging tympanic membranes. About 82% of animals had at least one affected ear. ABR thresholds measured on affected ears of these animals showed 20 to 30dB elevation across all frequencies (Figure 2). A Two Way Repeated Measures ANOVA showed a significant difference in hearing thresholds between the mutant and wild type mice (p <0.001).
Figure 2.
Comparison of auditory brainstem thresholds in affected ears of oxgr1−/−, as indicated by otoscopic exam, and wild type mice. Error bar indicated standard error of mean. Two Way Repeated Measures Analysis of Variance showed a significant hearing loss (p<0.001).
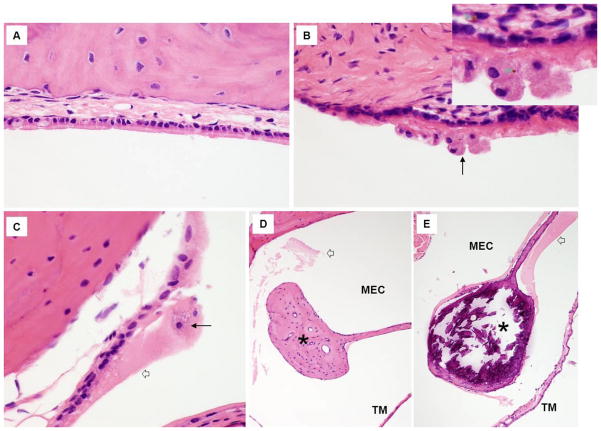
Histology analysis of middle ears from 5/5 mutant mice compared to wild type mice demonstrated chronic tissue injury with host response as evidenced by relatively abundant, densely eosinophillic/PAS-positive luminal fluid adherent to middle ear epithelial surfaces, entrapment of large foamy macrophages within the adherent fluid, focal deposition of hemosiderin within the macrophages and subepithelial stroma, and submucosal fibrosis (Figure 3). Acute inflammation changes were not present, and no statistically significant increase in middle ear goblet cell density in mutants was observed. Ossicles of the mutant animals were much more heavily mineralized than those of control wild types (Figure 3 E–F). These histopathological changes observed within the middle ears of the mutant mice would be predicted to negatively impact hearing. Attempts to amplify 16s ribosomal RNA of H influenza, S pneumoniae and M catarrhallis revealed negative results indicating the absence of these prominent OME causing pathogens. The negative result obtained from 16s ribosomal RNA universal primers with the detection limit of 12pg of bacterial genomic DNA, in addition, indicates the absence of other nonspecific bacteria. This result correlated with Gram staining of ME sections showing absence of both gram positive or gram negative bacteria. The oxgr1−/− mice universally develop spontaneous OME at a very high rate (82%) based on histologic evaluation and conductive hearing loss documented on ABR.
Figure 3.
Histology of the middle ear from oxgr1−/− mice compared to wild type mice. (A,D) H&E staining of middle ears from wild type (WT) mice revealed no significant in inflammatory or chronic reactive changes. In A, notice the intact ciliated epithelium overlaying delicate lamina propria abutting bone. In D, notice the normally scant, wispy eosinophillic luminal material (open white arrow) that has survived histology processing and the lightly mineralized ossicular bone (black asterisk). The tympanic membrane (TM) is thin and delicate. (B, C, E) H&E staining of middle ear from mutant mice demonstrate evidence of chronic tissue injury and host response. In B and C, notice clusters of large foamy macrophages (long black arrows) adherent to injured ciliated epithelium and set within abundant densely eosinophillic fluid (C and E, open white arrow). Focal deposits of golden brown hemosiderin are present within the macrophages and subepithelial stroma (B, see small green arrows in enlarged inset). The submucosa (B) is fibrotic. Ossicular bone (black asterisks) with the middle ear cavity (MEC) is much more heavily mineralized in the mutant mice (E) than in the wild type control (D).
Mucin gene expression in MEM of oxgr1 knock-out mice
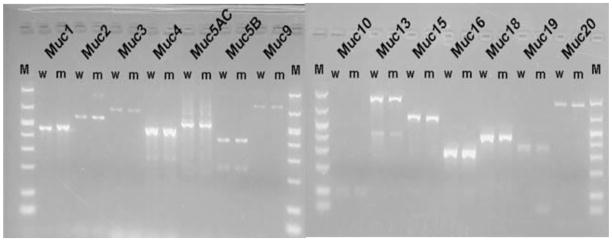
To investigate the impact of oxgr1 deficiency on expression of middle ear mucin gene expression, conventional RT PCR was used to verify this expression in mouse middle ear mucosa. From previous experiments, full characterization of mouse middle ear epithelial mucin gene expression has been determined in an immortalized cell-culture model [12]. These experiments demonstrated expression of muc1, muc2, muc3, muc4, muc5AC, muc5B, muc9, muc10, muc13, muc15, muc16, muc18, muc19 and muc20. Results from this current investigation demonstrated expression of each of these mucin genes (Figure 4) representing 100% correlation of the oxgr1−/− model to our previous in vitro mouse middle ear cell-culture model as well as correlation to human in vivo and in vitro models developed in our laboratory [2,12]
Figure 4.
Conventional RT PCR identified mucin genes expressed in middle ear mucosa of wild type (w) and mutant (m) mice. This expression pattern was 100% correlated to the mouse and human middle ear epithelium culture models.
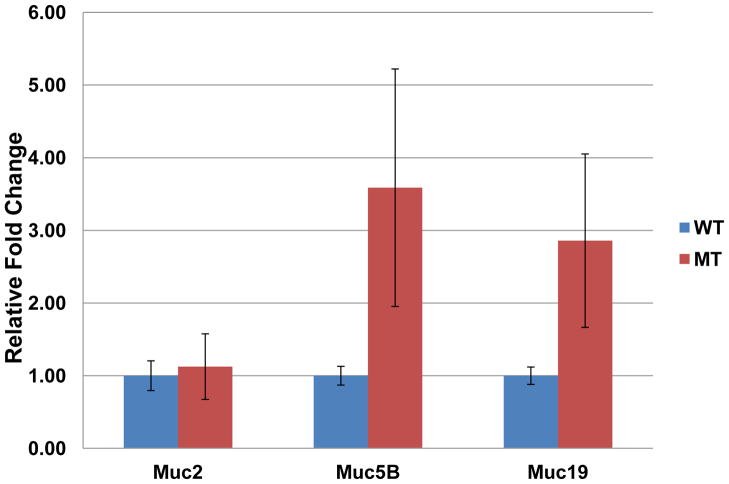
The relative expression of mRNA levels of each of the 4 gel-forming mucin genes in MEM of mutant mice was assessed using quantitative PCR (Figure 5). The mRNA level of muc2 was comparable between the mutant samples and wild type samples (t-test p-value = 0.920). The expression level of muc5AC mRNA in both groups of animals was below the detection limit of quantification, with the threshold cycles higher than 40, most likely due to the low number of goblet cells present in mouse middle ear. A trend of increase in muc5B level was detected in mutant animals though not significant (Mann-Whitney Rank Sum p-value = 0.381). A similar trend was observed in muc19 mRNA level (t-test p-value = 0.368).
Figure 5.
Relative quantification of gel forming mucin genes in the temporal bone of oxgr1−/− mutant demonstrated comparable level of muc2. The mRNA of muc5AC was observed at lower level than the detection limit with cycler thresholds higher than 40 on both wild type and mutant. A trend of increasing in muc5B and muc19 level was observed in mutant mice.
Discussion
Mucins continue to be an important area of focus in research directed at the physiology and pathophysiology of respiratory epithelium, and particularly this epithelium in the middle ear. Mucins are necessary to mechanically protect the underlying middle ear epithelium (MEE), to assist with the processing of antigens, and to provide trapping and clearance of invading pathogens. However, in too much abundance or in the incorrect composition of mucins there exists stasis of secretions and eventual hearing loss. Given their secretory nature, high viscosity and up-regulation during periods of inflammation such as OM, the gel-forming mucins MUC2, MUC5AC, MUC5B and MUC19 have been a primary area of interest [14–16]. However, we have previously demonstrated that a wide variety of mucins are produced in the MEE [2] and that experimental models should recognize these other mucins as possibly important in the overall regulation of MEE mucosal immunity, protection and function. Given this, the initial experiments utilizing the oxgr1 knock-out mouse (oxgr1−/−) required validation of an appropriate expression of mucin genes in the middle ear as compared to other mouse and human models employed in our laboratory. As demonstrated in Figure 4, the expression of each of the gel-forming mucins muc2, muc5AC, muc5B and muc19 in the oxgr1−/− model correlates with the other models for OM which have been investigated in our laboratory. This includes human in vivo and in vitro models, mouse in vitro models and chinchilla in vivo and in vitro models. [2,3,12]. In addition, the profile of non-gel-forming mucins expression in the oxgr1−/− model correlates well with previously described human, murine and chinchilla models [2,3,12,17]. Given these findings, the oxgr1−/− model appears to be an appropriate murine model of spontaneous OME development for future investigation of MEE mucin physiology and pathophysiology.
The development of the oxgr1−/− model for the study of OM has a number of critically important aspects. First, efforts to identify or create an animal model of chronic OME have primarily relied on creating anatomic changes to the ET [7, 18] or murine models with evident craniofacial deficits [9,19]. Although these methods have merit and have proven as viable models for investigation, permanently altering the ET presents some limitations in experimental design. An additional model of chronic OME has been developed and relies on an abnormality in the toll-like 4 receptor [8]. However, this abnormality creates a significant systemic abnormality for these mice with a complete inability to clear Gram-negative bacteria, again presenting some limitations in experimental design. The oxgr1−/− model avoids each of these difficulties by focusing on a novel knock-out model in the G protein-coupled receptor (GPCR) family.
The GPCR superfamily is comprised of cell surface proteins containing seven transmembrane domains and the receptor ability to interact with G protein. The family was classified based on phylogenetic analysis of their sequence structure into 5 groups; Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2, and Secretin [20]. Oxgr1 encodes oxoglutarate (alpha-ketoglutarate) receptor 1, a GPCR in Rhodopsin group. Very little is known about its biological functions. The physiological ligand for OXGR1, alpha ketoglutarate (2-oxoglutarate), was reported to involve in regulation of VEGF, an important inducer of angiogenesis and vascular permeability [21]. Increased production of VEGF in response to bacterial components and hypoxia resulted in increase of vascular permeability of middle ear mucosa and production of middle ear effusion. These contributed significantly to pathogenesis of the disease both in experimental animals and patients with OME [22–23]. This should warrant an investigation on whether OME associated VEGF induction is regulated through oxgr1. In addition, members of GPRC family have been linked to numerous biologic signaling pathways; a number of which involve important inflammatory events [24–25]. Some preliminary work would suggest that these GPCRs have an important regulatory function in epithelial mucosal integrity, mucin regulation and inflammatory pathways [26].
Development of a murine model with this particular genetic defect has several attractive features. First, the rate of development of OME in these animals is high at 82%. Additionally, it is clear from the initial work presented here that this OME is related to histopathologic changes in the middle ear of these mice. Induction of mucus effusion is evident though the variation in dysregulation of GFM does exist in this non-challenge study condition. The underlying cause of these differences between individual animal requires further investigation. Given this, the oxgr1−/− model is likely to be an ideal model to examine mucin regulation in MEE and potentially develop novel GPCR-specific targeted interventions to regulate these processes.
Acknowledgments
This work was supported by NIH grant NIDCD: DC007903 (JEK), and also supported in part through funding provided by the Department of Otolaryngology and Communication Sciences, Medical College of Wisconsin.
Footnotes
Conflict of interest statement
None of the authors of this manuscript have any financial or non-financial competing interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kerschner JE, Horsey E, Ahmed A, Erbe C, Khampang P, Cioffi J, Hu FZ, Post JC, Ehrlich GD. Gene expression differences in infected and noninfected middle ear complementary DNA libraries. Arch Otolaryngol Head Neck Surg. 2009 Jan;135(1):33–9. doi: 10.1001/archoto.2008.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerschner JE. Mucin gene expression in human middle ear epithelium. Laryngoscope. 2007 Sep;117(9):1666–76. doi: 10.1097/MLG.0b013e31806db531. [DOI] [PubMed] [Google Scholar]
- 3.Kerschner JE, Meyer TK, Burrows A. Chinchilla middle ear epithelial mucin gene expression in response to inflammatory cytokines. Arch Otolaryngol Head Neck Surg. 2004 Oct;130(10):1163–7. doi: 10.1001/archotol.130.10.1163. [DOI] [PubMed] [Google Scholar]
- 4.Kerschner JE, Yang C, Burrows A, Cioffi JA. Signaling pathways in interleukin-1beta-mediated middle ear mucin secretion. Laryngoscope. 2006 Feb;116(2):207–11. doi: 10.1097/01.mlg.0000191467.63650.9e. [DOI] [PubMed] [Google Scholar]
- 5.Smirnova MG, Kiselev SL, Gnuchev NV, Birchall JP, Pearson JP. Role of the pro-inflammatory cytokines tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and interleukin-8 in the pathogenesis of the otitis media with effusion. Eur Cytokine Netw. 2002 Apr-Jun;13(2):161–72. [PubMed] [Google Scholar]
- 6.Ubell ML, Khampang P, Kerschner JE. Mucin gene polymorphisms in otitis media patients. Laryngoscope. 2010 Jan;120(1):132–8. doi: 10.1002/lary.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song JJ, Kown SK, Kim EJ, Lee YS, Kim BY, Chae SW. Mucosal expression of ENaC and AQP in experimental otitis media induced by Eustachian tube obstruction. Int J Pediatr Otorhinolaryngol. 2009 Nov;73(11):1589–93. doi: 10.1016/j.ijporl.2009.08.011. Epub 2009 Sep 3. [DOI] [PubMed] [Google Scholar]
- 8.MacArthur CJ, Hefeneider SH, Kempton JB, Trune DR. C3H/HeJ mouse model for spontaneous chronic otitis media. Laryngoscope. 2006 Jul;116(7):1071–9. doi: 10.1097/01.mlg.0000224527.41288.c4. [DOI] [PubMed] [Google Scholar]
- 9.Depreux FF, Darrow K, Conner DA, Eavey RD, Liberman MC, Seidman CE, Seidman JG. Eya4-deficient mice are a model for heritable otitis media. J Clin Invest. 2008 Feb;118(2):651–8. doi: 10.1172/JCI32899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willott JF. Measurement of the Auditory Brainstem Response (ABR) to Study Auditory Sensitivity in Mice. Current Protocols in Neurosciences. 2006:8.21B.1–8.21B.12. doi: 10.1002/0471142301.ns0821bs34. [DOI] [PubMed] [Google Scholar]
- 11.Gok U, Bulut Y, Keles E, Yalcin S, Doymaz MZ. Bacteriological and PCR analysis of clinical material aspirated from otitis media with effusions. Int J Pediatr Otorhinolaryngol. 2001 Jul 30;60(1):49–54. doi: 10.1016/s0165-5876(01)00510-9. [DOI] [PubMed] [Google Scholar]
- 12.Kerschner JE, Lin J, Tsushiya K, Khampang P. Mucin gene expression and mouse middle ear epithelium. Int J Pediatr Otorhinolaryngol. 2010 Aug;74(8):864–868. doi: 10.1016/j.ijporl.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Samuel EA, Burrows A, Kerschner JE. Cytokine regulation of mucin secretion in a human middle ear epithelial model. Cytokine. 2008 Jan;41(1):38–43. doi: 10.1016/j.cyto.2007.10.009. Epub 2007 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glycoconj J. MUC5AC expression in human middle ear epithelium of patients with otitis media. Arch Otolaryngol Head Neck Surg. 2010 Aug;136(8):819–24. doi: 10.1001/archoto.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerschner JE, Khampang P, Erbe CB, Kolker A, Cioffi JA. Mucin gene 19 (MUC19) expression and response to inflammatory cytokines in middle ear epithelium. Glycoconj J. 2009 Dec;26(9):1275–84. doi: 10.1007/s10719-009-9245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerschner JE, Khampang P, Samuels T. Extending the chinchilla middle ear epithelial model for mucin gene investigation. Int J Pediatr Otorhinolaryngol. 2010 Sep;74(9):980–5. doi: 10.1016/j.ijporl.2010.05.009. Epub 2010 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piltcher OB, Swarts JD, Magnuson K, Alper CM, Doyle WJ, Hebda PA. A rat model of otitis media with effusion caused by eustachian tube obstruction with and without Streptococcus pneumoniae infection: methods and disease course. Otolaryngol Head Neck Surg. 2002 May;126(5):490–8. doi: 10.1067/mhn.2002.124935. [DOI] [PubMed] [Google Scholar]
- 19.Tateossian H, Hardisty-Hughes RE, Morse S, Romero MR, Hilton H, Dean C, Brown SD. Regulation of TGF-beta signalling by Fbxo11, the gene mutated in the Jeff otitis media mouse mutant. Pathogenetics. 2009 Jul 6;2(1):5. doi: 10.1186/1755-8417-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003 Jun;63(6):1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Imagawa S, Obara N, Suzuki N, Takahashi S, Nagasawa T, Yamamoto M. 2-Oxoglutarate downregulates expression of vascular endothelial growth factor and erythropoietin through decreasing hypoxia-inducible factor-1alpha and inhibits angiogenesis. J Cell Physiol. 2006 Nov;209(2):333–40. doi: 10.1002/jcp.20733. [DOI] [PubMed] [Google Scholar]
- 22.Sekiyama K, Ohori J, Matsune S, Kurono Y. The role of vascular endothelial growth factor in pediatric otitis media with effusion. Auris Nasus Larynx. 2011 Jun;38(3):319–24. doi: 10.1016/j.anl.2010.10.008. Epub 2011 Jan 11. [DOI] [PubMed] [Google Scholar]
- 23.Kim TH, Chae SW, Kim HJ, Jung HH. Effect of recombinant vascular endothelial growth factor on experimental otitis media with effusion. Acta Otolaryngol. 2005 Mar;125(3):256–9. doi: 10.1080/00016480410024677. [DOI] [PubMed] [Google Scholar]
- 24.Yona S, Lin HH, Dri P, Davies JQ, Hayhoe RP, Lewis SM, Heinsbroek SE, Brown KA, Perretti M, Hamann J, Treacher DF, Gordon S, Stacey M. Ligation of the adhesion-GPCR EMR2 regulates human neutrophil function. FASEB J. 2008 Mar;22(3):741–51. doi: 10.1096/fj.07-9435com. Epub 2007 Oct 10. [DOI] [PubMed] [Google Scholar]
- 25.Chang W, Chen J, Schlueter CF, Hoyle GW. Common pathways for activation of proinflammatory gene expression by G protein-coupled receptors in primary lung epithelial and endothelial cells. Exp Lung Res. 2009 May;35(4):324–43. doi: 10.1080/01902140802712738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanida S, Kataoka H, Mizoshita T, Shimura T, Kamiya T, Joh T. Intranuclear translocation signaling of HB-EGF carboxy-terminal fragment and mucosal defense through cell proliferation and migration in digestive tracts. Digestion. 2010;82(3):145–9. doi: 10.1159/000310903. Epub 2010 Jun 25. [DOI] [PubMed] [Google Scholar]