Summary
As impressive as the accomplishments of modern molecular biologists have been in finding genetic alterations that lengthen life in short-lived model organisms, they pale in comparison to the remarkable diversity of life spans produced by evolution. Some animal species are now firmly documented to live for more than four centuries and even some mammals, like the bowhead whale, appear to survive 200 years or more. Another group of species may not be as absolutely long-lived, but they are remarkably long-lived for their body size and metabolic rate. These species include a number of bats, some of which live for at least 40 years in the wild, as well as the naked mole-rat, which is the same size, but lives nearly 10 times as long as the laboratory mouse. Together these exceptionally long-lived organisms have important roles to play in our future understanding of the causal mechanisms and modulation of ageing. Bats and naked mole-rats in particular have already contributed in the following ways: (1) they have contributed to the abandonment of the rate-of-living theory and weakened enthusiasm for the oxidative stress hypothesis of ageing, (2) they have helped evaluate how the tumour-suppressing role of cellular senescence is affected by the evolution of diverse body sizes as well as diverse longevities, (3) they have shed light on the relationship between specific types of DNA repair and ageing, and (4) they have yielded insight into new processes, specifically the maintenance of the proteome and hypotheses concerning how evolution shapes ageing. The continuing acceleration of progress in genome sequencing and development of more and more cross-species investigatory techniques will facilitate even more contributions of these species in the near future.
Keywords: ageing, life span, animal model
Introduction
The essence of a comparative perspective in biology is the simultaneous perception of similarity and difference among individuals or species. Nothing could illustrate this better than the phenomenon of ageing. All mammals age in generally similar ways. That is, almost any casual observer would be able to distinguish an old horse from a young horse, an old dog from a young dog, or an old mouse from a young mouse. In each case, senescent decline would affect coat quality, gait, sensory acuity, muscle strength, cognitive prowess and a host of other phenotypes. Yet mammals exhibit clear differences in the ways they age as well. The most obvious of these differences is the rate at which ageing itself occurs. This is apparent even within a species. For instance, small dogs may display similar ageing phenotypes compared with large dogs, but in general they age more slowly, not only living longer but displaying a delay in a range of late-life pathologies (Bonnett et al., 2005; Miller and Austad, 2006). In man, it is clear that members of some families age more slowly than those in other families (Perls et al., 2002). Although the overall genetic heritability of human longevity is only about 25% (Herskind et al., 1996), siblings of centenarians are 8 – 17 times more likely to live to 100 years of age than the population as a whole (Perls et al., 2002). Put more vividly, if you want to become a healthy 80-year-old you need to live a healthy lifestyle, if you want to become a healthy 100-year-old, you need to inherit the right genes.
Modern molecular gerontology has illustrated this genetic reality again and again. Perhaps the most surprising laboratory finding in ageing research over the past 20 years is how easy it is to extend life by the alteration of single genes. Thus we now have more than 200 single gene manipulations that extend life in simple laboratory species such as worms and fruit flies, and more than 20 such manipulations that delay death and other symptoms of ageing in laboratory mice (Ladiges et al., 2009).
Yet these genetic effects observed within species pale beside the differences in ageing rate seen among species. A young mouse becomes an aged mouse in about 2 years, whereas it takes many decades for a young person to become an aged person. Some mammals may age even more slowly than people. For instance, reasonable age estimates for bowhead whales (Balaena mysticetus) from the racemization of eye lens proteins and the recovery of ancient harpoon tips from recently killed animals indicate they may live in excess of two centuries (George et al., 1999). Yet despite this enormous range of ageing rate among species, such variation has yet to be seriously exploited to try to understand mechanisms and methods of altering ageing itself.
For most of the history of ageing research, the conceptual focus has been on life span. How long do animals live? How might we make humans live longer? Most of us in the field understand that this is really a sort of shorthand which really means ‘how might we make people healthy for longer?’ It is self-evident that the preservation of life without the preservation of health is a profitless goal. If as many as 50% of people age 85 years or older suffer from disabling dementia (Ott et al., 1998), what benefit could be gleaned from further extending life if we did not simultaneously delay dementia? Thus current ageing research with animals has shifted its focus somewhat to the study of ‘health span’ (Kirkland and Peterson, 2009; Tatar, 2009; West and Bergman, 2009). Notably, the exceptionally long life spans such as that of the bowhead whale living amid the challenges of the natural world (e.g. predators, famine and diseases), suggests that virtually all of their bodily functions from brain to heart to immune system are exquisitely preserved. Thus, an investigation into species of exceptional longevity may yield clues as to how to enhance and extend human and animal health.
Methusaleh’s Zoo: Exceptionally Long-Lived Animals
Methusaleh was a biblical patriarch reputed to have lived 969 years, the most extreme claim for a human longevity record in existence. Thus, I term the group of animal species that display exceptional longevity ‘Methusaleh’s Zoo’. While the 211 year age estimate of bowhead whale longevity is extreme, it does not challenge the records of some of the world longest-lived animal species such as some of the bivalve molluscs (e.g. clams and oysters). For instance, a recent report used both radiocarbon dating and counting of shell growth rings to determine that a medium-size clam, the ocean quahog (Arctica islandica), had survived for over 400 years (Wanamaker et al., 2008), whereas radiocarbon dating alone suggests that a newly discovered species of deep sea oyster lived for over 500 years (Wisshak et al., 2009).
As impressive as these extreme longevities are, it is notable that they are found among poikilothermic animals living in cold environments. All processes of life from metabolism to the production of damaging reactive oxygen species (ROS), to DNA synthesis and repair, to rates of gene transcription and translation can be expected to be dramatically reduced in these species. Endothermic vertebrates (i.e. the mammals and birds), because they must maintain high body temperatures relative to poikilotherms, have special biological problems to contend with. In particular, they have extremely high energy demands compared with poikilotherms, potentially exposing them to enhanced oxidative damage among other hazards. In addition, their foraging demands are considerable and necessarily expose them to a variety of extrinsic dangers.
What is an exceptionally long-lived mammal? The question is not as straightforward as it might seem. Of course, one could focus exclusively on absolute longevity – how long a species has been documented to live. Doing so, man would be the second longest-lived mammal after the bowhead whale, although there is scant knowledge about longevity of other marine mammals, so some of those species could conceivably be longer-lived. On the other hand, one might plausibly consider longevity-for-body-size as a legitimate measure of exceptionally long life. The rationale for such an approach is that it is firmly established that larger mammal species generally live longer than smaller species (Sacher, 1959; Calder, 1986; Austad and Fischer, 1991). Longevity is not unique in this respect. A multitude of physiological variables, one might even say virtually all physiological variables, vary systematically with body size across species. Among these variables one finds rates of growth and development, heartbeats, metabolism, reproduction and respiration; details of body composition, muscle contraction velocity; as well as less obvious variables such as the number, size and longevity of various blood cell types (Calder, 1986; Promislow, 1991).
It was previously assumed that ageing and longevity were simply an inverse function of metabolic rate – the ‘rate-of-living’ theory (Pearl, 1928; Sacher, 1959). This idea makes certain intuitive sense. Larger animals tend to do everything slower, including have a slower basal metabolic rate. Therefore the speed, perhaps of critical biological processes like metabolism, might be fundamentally related to ageing. The hypothesis that ageing is due to the accumulation of cellular and molecular damage cause by ROS as a byproduct of metabolism provided a plausible mechanistic link between ageing and metabolism (Harman, 1956; Sohal, 1986).
In recent times the rate-of-living theory, as well as the mechanistic link between ROS production and longevity has not fared well because a mountain of evidence has accumulated against it (Speakman, 2005a; Buffenstein et al., 2008; Perez et al., 2009a). However, whether ROS production plays some role in ageing or not, the consistent relationship between species body size, longevity and a host of physiological variables remains. Thus, species that are exceptionally long-lived for their body size are of special gerontological interest.
A traditional method of assessing whether mammal species are long-lived for body size is to examine the residuals from the log-log plot of longevity versus body mass (Speakman, 2005b). This approach makes the implicit assumption that the true underlying relationship between these variables is linear throughout the mammalian size range. In fact, if one includes data from all mammal species with reasonable longevity information (more than 600 species), the positive relationship between body size and longevity disappears below a size threshold of about 1 kg (Austad and Fischer, 1991). This appears to be due to the fact that the longevity of small bats and marsupials is independent of body size. If one removes bats and marsupials from the data set, then longevity becomes a nicely linear function of body size throughout the mammal size range, even to the smallest species (Austad and Fischer, 1991). Note that because of fragmentary information on their longevity, marine mammals are not included in this analysis. Consequently, one reasonable body size standard against which to weigh mammalian longevity may be the ‘non-flying eutherian’ regression line; that is the line with bats and marsupials removed. Austad and Fischer (1991) defined longevity quotient (LQ) as the ratio of actual observed longevity to that predicted by the non-flying eutherian regression line. Excluding bats and marsupials mean LQ is 1.0 by definition. Using this standard, the longevity of bats is striking. Specifically, the 65 species of bats for which reasonable longevity data exist have a mean LQ of 3.52, meaning they live on average 3.5 times as long as an average non-bat, non-marsupial mammal. This is by far the highest among mammalian orders. Bat longevity seems particularly remarkable given that the majority of their longevity records come from wild populations, whereas records for virtually all other species come from captive, well-protected populations. On the low end, a number of marsupial, insectivore and rodent species have LQs of less than 0.5. Many of these data should be interpreted with caution however, because these short lives may stem from husbandry conditions that may not yet have been adequately worked out. The mammal with the smallest LQ and demonstrably good husbandry is the Norway (laboratory) rat, which lives only for 45% as long as an average mammal of its body size.
Man is an intriguing case. For most mammal species, complete life span data are available for a few hundred to perhaps a few thousand individuals. As the maximum longevity record for any species is likely to be partially a function of the number of individuals for which longevity information is available, human beings, for whom there are records for hundreds of millions of individuals, are difficult to compare fairly with other species. We know that about one in 10,000 people lives to 100 years of age (Perls et al., 2002), therefore if we assume that 100 years represents a comparable human sample to that of most species, then people live about 4.5 times as long as expected for their body size. Nineteen species are longer-lived than human beings by LQ. Eighteen of these are bats, one is the naked mole-rat (Heterocephalus glaber) with an LQ of 5.3. If we use for the human value the actual oldest known person with an authenticated birth record, then human maximum longevity is 122 years (Allard et al., 1998), which translates to a naked mole-rat-like LQ of 5.3.
A few species because of their availability for research as well as their exceptional longevity deserve special attention.
Naked Mole-Rats
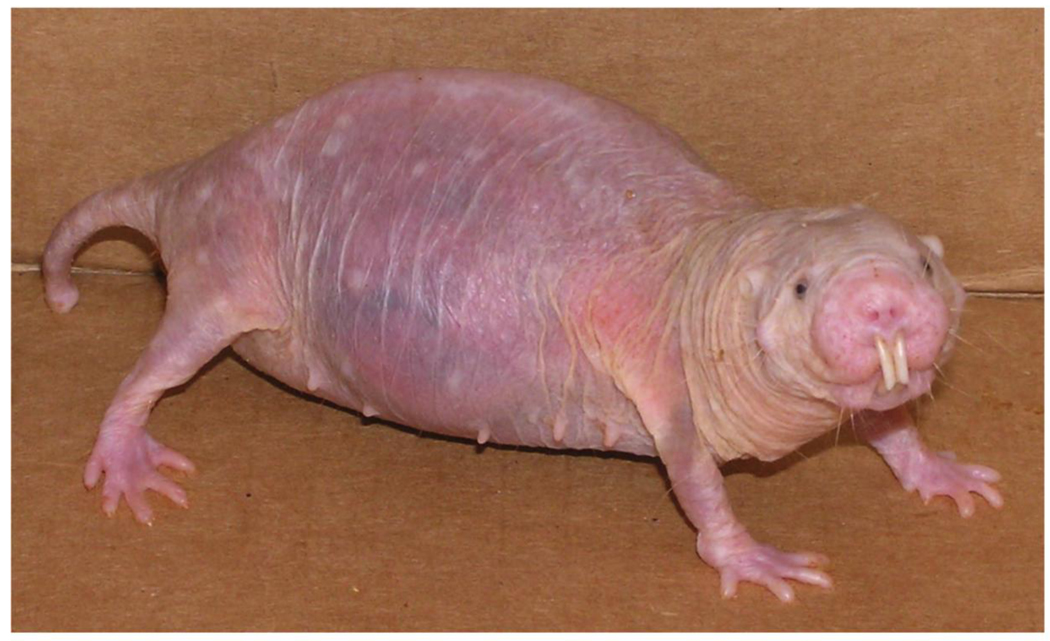
Naked mole-rats are subterranean, mouse-sized, eusocial rodents from arid equatorial East Africa (Fig. 1). They live in extensive sealed burrow systems in colonies of a few dozen to several hundred individuals and except when disposing of excavated soil through surface holes, they have no direct contact with the aboveground world. They are thus protected from many predators and live in a climatologically stable environment. Naked mole-rats eat mainly roots and other underground plant storage organs (Jarvis, 1991; Jarvis and Bennett, 1991).
Fig. 1.
Fifteen-year-old pregnant naked mole-rat. Photograph courtesy of R. Buffenstein.
Naked mole-rats originally came to broad attention in science when it was discovered that like certain insects such as ants, honeybees and termites, they were eusocial (Jarvis, 1981). That is, they live in colonies with overlapping generations in which one or a few individuals produce all the offspring and the rest serve as functionally sterile helpers that protect the colony and provide assistance for the reproductive female and her juvenile offspring. In the late 1980s and 1990s word began to circulate in zoological circles that naked mole-rats lived exceptionally long lives. Initially, it was thought that only the reproductive female (the ‘queen’) was long-lived as is the case in many eusocial insects. Indeed, this may be true in the wild, where queens have been reported to live at least 17 years compared with only approximately 4 years for non-breeders, however it is not true in the laboratory (Buffenstein, 2008).
In the early 2000s, thanks primarily to the efforts of Rochelle Buffenstein, naked mole-rats came to the attention of the biogerontological research community (Buffenstein and Jarvis, 2002; O'Connor et al., 2002;). By then it had been established that in captivity, both breeding and non-breeding naked mole-rats were capable of living into their late 20s, nearly 10 times as long as similar-sized mice. These remarkable ages are not rare events. More than 40% of Buffenstein’s naked mole-rats captured in the wild or born in the first year of captivity were still alive 24 years later. From the perspective of comparative immune function, it may be notable that such exceptional longevity is found in a species living in large, dense social groups that were not maintained inside a pathogen barrier. Remarkably, mortality rate does not appear to increase with age as it does in virtually all other species at least up to the age of 24 years. Breeding females also show little sign of reproductive senescence, producing even larger litters (up to 29 pups) when old than when young. Perhaps most strikingly, naked mole-rats develop cancer rarely or perhaps not at all. Necropsy examinations of hundreds of animals that died in accidents or of natural causes in several colonies failed to turn up a single tumour (Buffenstein, 2008; P.W. Sherman, personal communication, 2009). A number of other age-related physiological changes that typically occur in laboratory rodents are forestalled in naked mole-rats. The state of current knowledge about the ageing biology of the naked mole-rat is reviewed in Buffenstein (2008).
Little Brown Bats
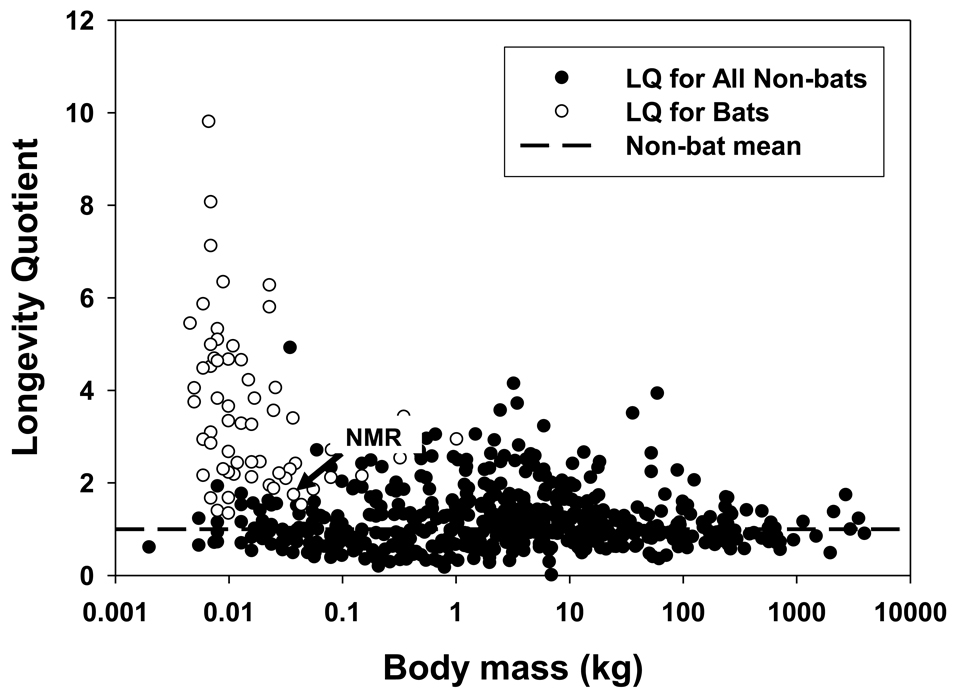
Bats of the genus Myotis, of which there are 87 species distributed world-wide except at polar and sub-polar latitudes, are colloquially known as little brown bats (Nowak, 1999). As a group, these species, which range in size from an average of less than 3 to more than 30 grams, are remarkably long-lived (Wilkinson and South, 2002). Of the 22 species which have been the subject of some long-term banding efforts, ten species are known to live at least 20 years, two additional species live at least 30 years, and one (Brandt’s bat; M. brandti) has survived up to at least 41 years (Wilkinson and South, 2002; Podlutsky et al., 2005). The LQ of Brandt’s bat is 9.8, the highest among mammals (Fig. 2). Exceptional longevity in bats is not confined to this genus, but is found in multiple evolutionary bat lineages (Wilkinson and South, 2002).
Fig. 2.
Longevity Quotient (actual longevity/expected longevity) for 630 mammalian species. NMR, naked mole-rat.
Several aspects of the remarkable longevity of bats should be noted. First, the existing data do not come from long-term studies designed to document bat longevity, but have been largely accidentally acquired. Thus many of these longevity records might be considerable underestimates of true longevity. Second, most of these records all come from wild populations, whereas longevity records for most other mammal species are from captive populations. Thus long-lived bats must preserve sufficient physiological capacity to meet the considerable demands of aerial foraging and prey capture as well as maintain their ability to escape predators and fight off infectious diseases. Finally, at least in the two species for which we have the greatest longevity records (M. lucifugus, 34 years; M. brandti, 41 years) all the longest-lived individuals appear to be males. In fact in Brandt’s bat, all 67 individuals documented to survive to at least age 20 years were males. Whether this represents a sex difference in fidelity to the hiberaculum where the bats were banded and later censused, or a real difference in longevity, is not yet clear. However, it would not be surprising if females are shorter-lived in nature given the energy demands of reproduction. Lactating M. lucifugus females, for instance, need to eat more than half their own body weight in prey each day (Anthony and Kunz, 1977).
Because of the lack of detailed demographic information, we do not know whether death rate increases with age in wild bat populations; nor do we know what might be the major causes of death. The fact that annual survival appears to fluctuate with food availability, at least in insect eating species, suggests that foraging success may be a significant contributor (Frick et al., 2009). Whether foraging success might decline with age is also unknown. Occasional deaths due to predation and environmental catastrophes are well-documented (Fenton and Barclay, 1980). Additionally, bats are known to harbour a wide variety of viruses, some related to emerging human diseases, but whether these pose a mortality risk for the bats is also unknown (Field, 2009; Misra et al., 2009). Recently, in the northeastern United States ‘white-nose syndrome’, named for the fungus that grows on the nose and wings of hibernating bats of several species, has killed up to 75% of the bats in some hibernacula and is estimated to have killed more than one million bats altogether since 2007 (Frick et al., 2009).
The exceptional longevity of many bat species has been known for some time (Bourliere, 1958; Herreid, 1964), however it was often dismissed as a trivial consequence of the habit of many bat species to enter daily torpor and/or seasonal hibernation. It was assumed that if one corrected for these periods of reduced metabolism, then bats were actually no longer-lived than other mammal species (Jürgens and Prothero, 1987). This is a version of the rate-of-living theory, which has now been thoroughly debunked as a general explanation for ageing as mentioned earlier. It should be noted that tropic bat species which do not hibernate are also exceptionally long-lived. Wilkinson and South (2002), in the most thorough analysis of bat longevity to date, compared hibernating and non-hibernating bats and calculated that hibernation accounted for possibly as much as 6 years of additional life in bats, still rendering them the most exceptionally long-lived group of mammals.
Mechanisms and Hypotheses
Virtually everything we know about the modulation of ageing (and we know a lot) comes from experiments and observations on traditional short-lived laboratory species such as fruit flies, the nematode Caenorhabditis elegans and mice that are demonstrably unsuccessful at combating basic ageing processes. As exciting as our success in lengthening life in these species has been to date, there is no guarantee that the same processes and mechanisms that modulate ageing in these very short-lived organisms will also do so for long-lived creatures such as human beings. By extension then, exceptionally long-lived species (Methusaleh’s Zoo) may be more than a collection of zoological curiosities. They serve as exemplars of the one or many ways that evolution has found to design animals such that they resist ageing exquisitely well. As the biochemist Leslie Orgel was fond of saying, ‘Evolution is cleverer than you are’. Methusaleh’s Zoo thus may be particularly informative concerning proposed mechanisms that underlie slow ageing. Below I discuss a few hypotheses and mechanisms for which long-lived organisms have contributed significantly to their current interpretation.
Oxidative Stress
The naked mole-rat has helped re-shape thinking about the role of oxidative stress in ageing. Almost a default hypothesis for years – assumed to be true even in the absence of compelling evidence – was the oxidative stress theory of ageing. This intuitively reasonable hypothesis, a lineal descendent of the rate-of-living theory, holds that because of an imbalance between the production of damaging ROS and body’s ability to prevent or repair such damage, oxidative damage to macromolecules will accumulate with age thus leading to tissue degradation which is likely to contribute to ageing. Several lines of evidence are consistent with this hypothesis. First, oxidative damage to tissues does increase with age in many laboratory animals (Hamilton et al., 2001; Ward et al., 2005). Second, some life-extending treatments, in particular dietary restriction, reduce both ROS production and oxidative tissue damage. Third, some genetic manipulations that extend life in model laboratory organisms also reduce oxidative damage (Perez et al., 2009a). Even from a comparative perspective, long-lived species have been reported to exhibit lower ROS production than short-lived species (Ku and Sohal, 1993; Brunet-Rossinni, 2004; Lambert et al., 2007).
On the other hand, a surprising corpus of experimental data is not consistent with predictions of the oxidative stress hypothesis. For instance, feeding animals various antioxidants has had no consistent life-extending effect (Strong et al., 2008). Moreover, although genetically manipulating some cellular antioxidant defences has had longevity-modulating effects in fruit flies (Landis and Tower, 2005), such effects have for the most part not been observed in mice (Perez et al., 2009a). Occasionally, the diminishment of antioxidant defenses has even led to a small increase in longevity (Ran et al., 2007).
These various experimental results might be explained away by claims that they were performed in highly artificial environments with highly artificial animals (i.e. inbred domesticated flies or mice) or that the relationship between dietary or genetic manipulations of antioxidant levels has an uncertain relationship to actual oxidative tissue damage. This latter point is critical. Surprisingly few studies which seek to critically evaluate the oxidative stress hypothesis have actually measured oxidative damage to tissues, instead assessing only ROS production and/or antioxidant activity (Ku and Sohal, 1993; Brunet-Rossinni, 2004; Lambert et al., 2007). This is understandable to the extent that sensitive, accurate and reproducible measurement of oxidative damage to macromolecules continues to be technically demanding (Hamilton et al., 2001; Chaudhuri et al., 2006; Roberts and Milne, 2008). Yet, there are many reasons that measuring ROS production and antioxidant defenses by themselves will not necessarily be a good predictor of actual tissue damage. For instance, there are multiple reactive oxygen (and nitrogen) species, a bewildering diversity of antioxidant defense mechanisms, the timing and cellular localization of both will affect tissue damage, and the inherent repair capacity of various species and tissues are also likely to vary. Thus it is critical for rigorously evaluating the hypothesis to assess actual tissue damage and discover whether it is indeed inversely related to longevity.
By this criterion, a comparative perspective utilizing exceptionally long-lived species presents considerable difficulty for the oxidative stress hypothesis. For instance, greater oxidative damage to DNA was observed in brain and heart (but not liver) from a bird species (budgerigar, maximum longevity 21 years) compared with much shorter-lived laboratory mice and rats (maximum longevity 4 years) (Hamilton et al., 2001). Additionally, naked mole-rats when young display higher oxidation of proteins, lipids and nucleic acids in several tissues compared with young mice, despite their nearly 10-fold longer lives (Andziak and Buffenstein, 2006; Andziak et al., 2006). These results should not be interpreted to suggest that longer-lived species always display higher levels of tissue oxidation than shorter-lived species. For instance, some (but not all) long-lived bat species exhibit significantly lower protein oxidation than shorter-lived mice (Salmon et al., 2005). However, it seems clear that there is no consistent pattern between species longevity and tissue oxidation.
Is the oxidative stress hypothesis of ageing dead then? Perhaps not, but its breathing is shallow and stertorous. It is conceivable that reduced oxidative stress in some critical but non-obvious tissues or at some specific developmental phase enhances longevity and improves health or that absolute tissue oxidation level is not as important as whether it changes with age or how tissues respond to acute oxidative stress. For instance, superoxide production in vascular endothelium increases rapidly with age in mice but not in naked mole-rats (Ungvari et al., 2008). Without doubt, oxidative stress has been implicated in a host of late-life diseases. However as a general explanation for the rate of ageing or its modulation, there is little at this point to recommend it.
Cellular Senescence
Species from Methusaleh’s Zoo also contributed to our understanding of cellular senescence. As has been known for nearly 50 years, most normal somatic cells in man and numerous other species have a finite replicative capacity (Hayflick, 1998). That is, after a certain number of cell divisions, they reach a state (now called cellular or replicative senescence) in which they become permanently incapable of further division. Evolutionarily, cellular senescence is thought to be an adaptation to suppress tumour growth (Beausejour and Campisi, 2006). From the time of its discovery, the relationship of cellular senescence to organismal ageing has been debated with extreme positions represented by the contentions that: (1) it has little or nothing to do with organismal ageing, or (2) it is a near complete model of organismal ageing (Masoro, 1985; Fossel, 2002).
Perhaps the most compelling argument that cellular senescence bore a close relationship to organismal ageing was the report that cultured cells (fibroblasts) from long-lived mammalian species displayed greater replicative capacity than those from short-lived species (Röhme, 1981). This claim is worth considering in some detail.
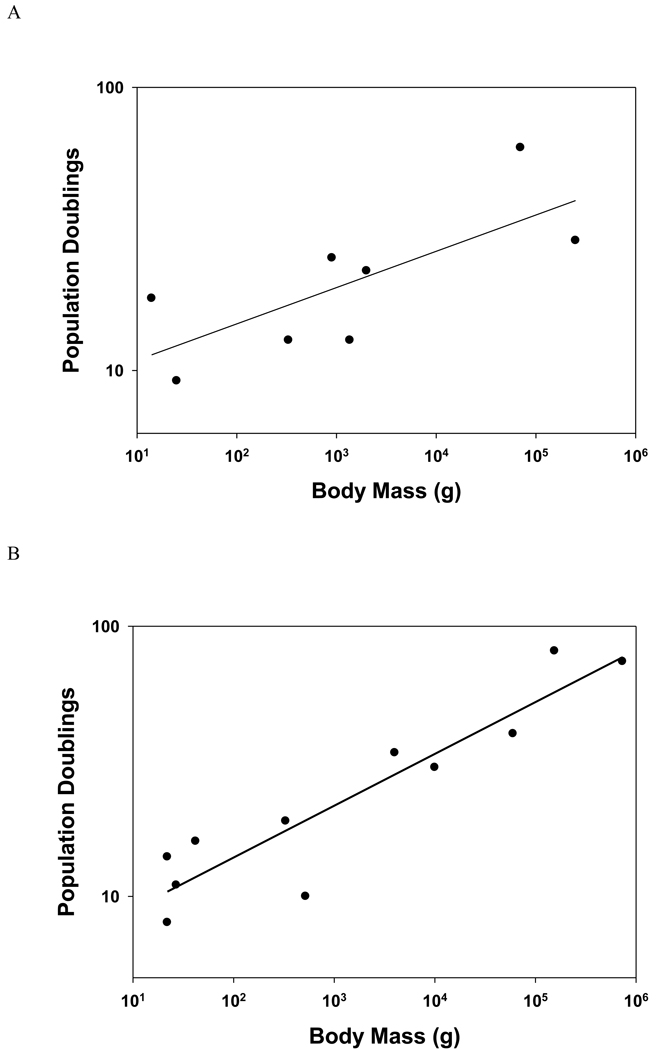
Röhme assessed the number of population doublings (a measure of replicative capacity) undergone by cultured cells from eight mammalian species and found a strong positive correlation (r = 0.95) with species life span. This finding was particularly surprising in that the fibroblasts from different species came from different tissues (skin, lung, testis or whole embryo) and from animals of different developmental stages (fetal, adult or of unknown age), factors that are known to affect replicative capacity. One potentially significant confounding factor as in all comparative studies was that these eight species differed dramatically in body size as well as longevity, the smallest being a mouse, the largest a horse. It is conceivable that since all mammals develop from a single cell, the fertilized egg, to their full-grown adult size, that replicative capacity would be expected to correlate with adult body size because more cell divisions are necessary during growth and there will be a greater need for replacement of highly proliferative tissues such as haematopoietic and endothelial cells even in adulthood. In Röhme’s study, replicative capacity was also significantly related to species body size (Fig. 3).
Fig. 3.
Relationship between body size and replicative capacity. (A) These variables are significantly correlated (r = 0.73, P = 0.041) in this study from Röhme (1981) (B) These variables are highly significantly correlated (r = 0.93, P < 0.001) in this study from Lorenzini et al. (2005).
To try to untangle the relationship of replicative capacity, longevity and body size, Lorenzini et al. (2005) assessed replicative capacity of fibroblasts from 11 carefully selected mammal species. These authors’ conceptual approach was to use a range of species which differed substantially in both size and longevity, yet utilizing some species that were large but comparatively short-lived like cows, and others which were small yet long-lived, like naked mole-rats and bats (Lorenzini et al., 2005). In this selection of species, replicative capacity turned out to be highly correlated with body size (P < 0.001) but only marginally correlated with longevity (P = 0.051). Using partial correlation analysis to control for the effect of body size, they found that even the marginal relationship of replicative capacity with longevity disappeared (P = 0.71), whereas when controlling for the impact of longevity by the same statistical technique, replicative capacity remained highly correlated with body size (P = 0.001). Thus it appears that the major factor influencing cellular replicative capacity is body size.
One of the mechanisms by which replicative capacity is limited is the erosion of telomeres, which are repetitive DNA sequences at the ends of linear chromosomes. Each time a cell divides its telomeres shorten a little. When they reach a critically short length, cells perceive this as DNA damage and permanently halt division of that cell (Shay and Wright, 2007). But some cells (e.g. germ cells) need to maintain their ability to divide. They avoid the telomere shortening problem by activating an enzyme, telomerase, which has the capacity to prevent telomere shortening. One of the ways in which cancers avoid the telomere shortening problem is by reactivating telomerase in cells in which it is normally turned off (Shay and Wright, 2006).
Human beings and mice differ dramatically in telomere length and in the extent to which they actively express telomerase in adulthood. People have short telomeres (~ 10kb) and telomerase is turned off in most somatic cells. By contrast, mice have long telomeres (20 – 50 kb) and express telomerase in most cells in most tissues. One explanation for this difference had been that because mice are short-lived compared with people, they require less rigorous defenses against cancer. Mice simply do not survive long enough (in the wild) for exquisite tumour control to be evolutionarily advantageous.
To test this idea, Seluanov et al. (2007) examined telomere length and telomerase activity in somatic tissues of 15 species of rodents, ranging in body size from 20 – 55,000 grams and in longevity from 4 – 28 years (naked mole-rats). They observed no relationship between longevity or body size and telomere length. Moreover, they found that telomerase activity apparently co-evolved with body size rather than with longevity, as smaller species regardless of their life spans exhibited high telomerase activity in somatic tissues and larger species regardless of their life spans suppressed it (Seluanov et al., 2007). These results suggest that because larger animals consist of greater numbers of somatic cells, each of which has the potential to develop into a fatal tumour, body size may represent a greater tumour risk than longevity.
Genome Maintenance
A related explanatory hypothesis for differences in ageing rate, that is both plausible and intuitively satisfying, has been called the genome maintenance hypothesis (Vijg, 2007). According to this notion, ageing results from the accumulation of somatic damage to DNA thus interfering with cellular function and dysregulating proliferative homeostasis. Supporting evidence comes primarily from mice genetically altered to have defective DNA repair pathways. These mice are invariably short-lived and exhibit some signs of accelerated ageing (Hasty et al., 2003). However considerable controversy surrounds the issue of whether short-lived mice represent accelerated ageing versus relatively uninformative specific pathology (Hasty and Vijg, 2004; Miller, 2004).
According to the genome maintenance hypothesis, species differences in ageing rate arise either from a superior ability to resist DNA damage or a greater speed and/or efficiency of DNA repair. In fact, a handful of papers reported in the 1970s and 1980s that longer-lived species had superior DNA repair rates (Hart and Setlow, 1975; Francis et al., 1981; Hall et al., 1984). We now know that DNA has multiple independent but overlapping repair pathways depending on the type of DNA damage (Friedberg et al., 2005). These early studies primarily assessed what is now called nucleotide excision repair (NER), which is a response to the formation of bulky DNA adducts and intrastrand cross-links induced experimentally by ultraviolet radiation and certain chemotherapeutic agents. A considerably more common type of DNA damage is thought to be single-strand breaks induced by ROS. Researchers have estimated that single-strand breaks occur in vivo up to 55,000 times per cell per day (Tice and Setlow, 1985). Single-strand breaks are typically repaired by a somewhat different process called base excision repair (BER).
My laboratory investigated both NER and BER in fibroblasts from six carefully-chosen mammalian species. Two of these species were bats, the little brown bat which as previously noted lives up to 34 years and the Brazilian free-tailed bat (Tadarida brasiliensis) which lives at least 12 years (Wilkinson and South, 2002); two were rodents, the deer mouse (Peromyscus maniculatus) which lives more than 8 years in the laboratory and the shorter-lived laboratory mouse (maximum longevity 4 years); and two were primates, man and common marmosets (Callithrix jacchus), a 400 gram species which lives up to 16 years. We induced damage with either UV (NER) or γ-irradiation (BER) and assessed damage and the rate of repair via the alkaline comet assay which detects DNA strand breaks and abasic sites (Olive and Banath, 2006). In the comet assay, the percentage of DNA in the comet’s ‘tail’ is directly related to the amount of damage.
In response to the same UV exposure, there was no clear relationship between species longevity and the amount of DNA damage induced or the rate of repair. Primates had the lowest rates of damage and speed of repair, although there was no difference in either maximum DNA or rate of repair between the relatively short-lived marmoset and human cells. Rodent cells experienced the most damage and never completely repaired it, but the longer-lived deer mouse cells exhibited greater damage than the shorter-lived laboratory mouse. Bats were intermediate in both aspects (Podlutsky et al., unpublished observations). There appeared to be no coherent relationship between resistance to UV-induced DNA damaged or the rate of repair in these species.
For BER the data are somewhat more consistent with the hypothesis. In response to 3 Gy irradiation, rodent DNA (both species combined) was the most damaged and slowest to repair. Within the rodents, long-lived deer mouse cells also repaired more rapidly than those from the shorter-lived laboratory mouse. DNA from both primate species was less damaged and repaired faster than DNA from either of the two rodent species. Moreover, the marmoset cells exhibited more damage and slower repair than the human cells. The least damage and fastest repair was by the two bat species, although the shorter-lived free-tailed bat was not different in this regard to the longer-lived little brown bat (Podlutsky, et al., unpublished). This latter finding brings up a weakness with some of the bat longevity data. Because most longevity information on bats is accidentally acquired, it is difficult to assert with confidence that one species is exceptionally long-lived compared with another. The recorded difference could as easily be due to some combination of less banding, less recapture success or effort or a shorter follow-up period than to real demographic differences. Free-tailed bats, because they live in colonies of as many as millions of individuals, and because they do not reliably return to the same roosts year after year, are notoriously difficult to recapture after banding. Thus although the recorded longevity difference between the two bat species we investigated was substantial, it may be more apparent than real. Still, given these preliminary data, the idea that BER is a critical contributor to mammalian longevity deserves further investigation.
Protein Stability
A relatively new hypothesis about the modulation of ageing is that a major contributing factor might be generalized proteome maintenance. Because proteins are critical for both maintenance of cell structure and biochemical integrity and also require precise folding which can be disrupted by a variety of processes, an attractive idea is that species that are better at maintaining the structural integrity of their proteins might age more slowly. Well-maintained proteins are, after all, fundamental to virtually every cellular process including DNA repair.
Two studies recently addressed this issue from a comparative perspective using a combination of long- and short-lived species. In the first, protein oxidation state, protein stability in response to unfolding stress and protein turnover were measured in naked mole-rats compared with mice. As previously mentioned, young naked mole-rats have greater overall protein oxidation as measured by carbonylation in several tissues compared with mice. However, unlike mice, the level of protein oxidation does not increase with age in naked mole-rats (Perez et al., 2009b). Perhaps more interestingly, liver proteins from naked mole-rats display less unfolding when subjected to pH (urea) stress than do proteins from mice. In addition, whereas the fraction of damaged proteins as measured by their ubiquitination is high and increases with age in mice, there is no age-related change in naked mole-rats. This enhanced stability may be at least partially due to a higher turnover rate of damaged proteins as proteasome activity is higher in naked mole-rats than mice (Perez et al., 2009b).
Long-lived bats also exhibit enhanced protein stability compared with mice, although they achieve this stability with some intriguing differences compared with naked mole rats. In these studies two bat species (Brazilian free-tailed bat, T. brasiliensis; cave myotis, Myotis velifer), each of which is documented to live at least 12 years in the wild, were compared with house mice recently derived from wild populations. One bat species (T. brasiliensis) exhibited significantly lower basal protein carbonylation than mice, whereas the other did not. However, when stressed either with γ-irradiation (whole animals) or treatment with iron ascorbate (liver homogenates), both bat species displayed significantly less protein oxidation than mice (Salmon et al., 2009). Both bat species also showed less global protein unfolding in response to urea stress compared with mice. Although data are not available to compare changes with age among these species, the amount of damaged proteins as measured by their ubiquitination was significantly lower in both bat species than in young mice. In contrast to the naked mole-rat which had higher apparent protein turnover than mice as indicated by greater proteasome activity, both bat species had dramatically lower proteasome activity than did mice. Thus although bats and naked mole-rats both appear to possess enhanced protein stability in the face of unfolding stress, they seem to achieve this stability by different means. These results in sum emphasize that comparative studies of ageing can lead to new hypotheses, but can also address the possible diversity of mechanisms for achieving the same end.
Conclusions
Short-lived, genetically tractable, model organisms have contributed greatly to our recent understanding of how the ageing process might be modulated, yet there are unique lessons to be learned by expanding the research bestiary to include species that are exceptionally long-lived. Some informative species are likely to be absolutely long-lived, surviving centuries, but others of equal interest are long-lived for their body size and metabolism. These species have already contributed to the rigorous testing of broad hypotheses about ageing and helped develop new hypotheses. However with the dramatic acceleration in our genome sequencing capability, it is likely that new investigatory tools for these species of exceptional gerontological interest will be developed at an accelerating pace. The role of Methusaleh’s Zoo in ageing research is likely to blossom in the near future.
Acknowledgments
My research on the comparative biology of ageing has been supported grants from the US National Institute on Aging (R01 AG022873 and K07 AG025063), the Paul Glenn Foundation for Medical Research, the National Academies Keck Futures Initiative and the San Antonio Area Foundation. I am grateful to them.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The author was an invited speaker at the Merial European Comparative Vaccinology Symposium and received travel expenses and an honorarium for this presentation.
References
- Allard M, Lebre V, Robine J-M. Jeanne Calment: from Van Gogh's Time to Ours. New York: WH Freeman & Co.; 1998. [Google Scholar]
- Andziak B, Buffenstein R. Disparate patterns of age-related changes in lipid peroxidation in long-lived naked mole-rats and shorter-lived mice. Aging Cell. 2006;5:525–532. doi: 10.1111/j.1474-9726.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Andziak B, O'Connor TP, Qi W, DeWaal EM, Pierce A, et al. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Anthony ELP, Kunz TH. Feeding strategies of the little brown bat, Myotis lucifugus, in southern New Hampshire. Ecology. 1977;58:775–786. [Google Scholar]
- Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. Journal of Gerontology. 1991;46:B47–B53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- Beausejour CM, Campisi J. Ageing: balancing regeneration and cancer. Nature. 2006;443:404–405. doi: 10.1038/nature05221. [DOI] [PubMed] [Google Scholar]
- Bonnett BN, Egenvall A, Hedhammar A, Olson P. Mortality in over 350,000 insured Swedish dogs from 1995–2000: I. Breed-, gender-, age- and cause-specific rates. Acta Veterinaria Scandinavica. 2005;46:105–120. doi: 10.1186/1751-0147-46-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourliere F. The comparative biology of aging. Journal of Gerontology. 1958;13:16–24. [Google Scholar]
- Brunet-Rossinni AK. Reduced free-radical production and extreme longevity in the little brown bat (Myotis lucifugus) versus two non-flying mammals. Mechanisms in Ageing and Development. 2004;125:11–20. doi: 10.1016/j.mad.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. Journal of Comparative Physiology [B] 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Edrey YH, Yang T, Mele J. The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. Age (Dordrecht) 2008;30:99–109. doi: 10.1007/s11357-008-9058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R, Jarvis JU. The naked mole rat - a new record for the oldest living rodent. Science of Aging Knowledge Environment. 2002:e7. doi: 10.1126/sageke.2002.21.pe7. [DOI] [PubMed] [Google Scholar]
- Calder WAI. Size, Function and Life History. New York: Dover, Mineola; 1986. [Google Scholar]
- Chaudhuri AR, de Waal EM, Pierce A, Van RH, Ward WF, et al. Detection of protein carbonyls in aging liver tissue: a fluorescence-based proteomic approach. Mechanisms in Ageing and Development. 2006;127:849–861. doi: 10.1016/j.mad.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Fenton MB, Barclay MR. Myotis lucifugus. Mammalian Species. 1980;142:1–8. [Google Scholar]
- Field HE. Bats and emerging zoonoses: henipaviruses and SARS. Zoonoses and Public Health. 2009;56:278–284. doi: 10.1111/j.1863-2378.2008.01218.x. [DOI] [PubMed] [Google Scholar]
- Fossel M. Cell senescence in human aging and disease. Annals of the New York Academy of Science. 2002;959:14–23. doi: 10.1111/j.1749-6632.2002.tb02078.x. [DOI] [PubMed] [Google Scholar]
- Francis AA, Lee WH, Regan JD. The relationship of DNA excision repair of ultraviolet-induced lesions to the maximum life span of mammals. Mechanisms in Ageing and Development. 1981;16:181–189. doi: 10.1016/0047-6374(81)90094-4. [DOI] [PubMed] [Google Scholar]
- Frick WF, Reynolds DS, Kunz TH. Influence of climate and reproductive timing on demography of little brown myotis Myotis lucifugus. Journal of Animal Ecology. 2009 doi: 10.1111/j.1365-2656.2009.01615.x. doi: 10.1111/j.1365- 2656.01615.x. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, et al. DNA Repair and Mutagenesis. Washington DC: ASM Press; 2005. [Google Scholar]
- George JC, Bada J, Zeh J, Scott L, Brown SE, et al. Age and growth estimates of bowhead whales (Balaena mysticetus) via aspartic acid racemization. Canadian Journal of Zoology. 1999;77:571–580. [Google Scholar]
- Hall KY, Hart RW, Benirschke AK, Walford RL. Correlation between ultraviolet-induced DNA repair in primate lymphocytes and fibroblasts and species maximum achievable life span. Mechanisms in Ageing and Development. 1984;24:163–173. doi: 10.1016/0047-6374(84)90068-x. [DOI] [PubMed] [Google Scholar]
- Hamilton ML, Guo Z, Fuller CD, Van RH, Ward WF, et al. A reliable assessment of 8-oxo-2- deoxyguanosine levels in nuclear and mitochondrial DNA using the sodium iodide method to isolate DNA. Nucleic Acids Research. 2001;29:2117–2126. doi: 10.1093/nar/29.10.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hart RW, Setlow RB. DNA repair and life span of mammals. Basic Life Sciences. 1975;5B:801–804. doi: 10.1007/978-1-4684-2898-8_59. [DOI] [PubMed] [Google Scholar]
- Hasty P, Campisi J, Hoeijmakers J, Van SH, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–1359. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- Hasty P, Vijg J. Accelerating aging by mouse reverse genetics: a rational approach to understanding longevity. Aging Cell. 2004;3:55–65. doi: 10.1111/j.1474-9728.2004.00082.x. [DOI] [PubMed] [Google Scholar]
- Hayflick L. A brief history of the mortality and immortality of cultured cells. Keio Journal of Medicine. 1998;47:174–182. doi: 10.2302/kjm.47.174. [DOI] [PubMed] [Google Scholar]
- Herreid CF. Bat longevity and metabolic rate. Experimental Gerontology. 1964;1:1–9. [Google Scholar]
- Herskind AM, McGue M, Holm NV, Sorensen TI, Harvald B, et al. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Human Genetics. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- Jarvis JU. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- Jarvis JUM. Reproduction of naked mole-rats. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton, New Jersey: Princeton University Press; 1991. pp. 384–425. [Google Scholar]
- Jarvis JU, Bennett N. Ecology and behavior of the family Bathyergidae. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton, New Jersey: Princeton University Press; 1991. pp. 66–96. [Google Scholar]
- Jürgens KD, Prothero J. Scaling of maximal lifespan in bats. Comparative Biochemistry and Physiology. 1987;88A:361–367. doi: 10.1016/0300-9629(87)90498-1. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Peterson C. Healthspan, translation, and new outcomes for animal studies of aging. Journal of Gerontology A: Biological Science and Medical Science. 2009;64:209–212. doi: 10.1093/gerona/gln063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku HH, Sohal RS. Comparison of mitochondrial pro-oxidant generation and anti-oxidant defenses between rat and pigeon: possible basis of variation in longevity and metabolic potential. Mechanisms of Ageing and Development. 1993;72:67–76. doi: 10.1016/0047-6374(93)90132-b. [DOI] [PubMed] [Google Scholar]
- Ladiges W, Van RH, Strong R, Ikeno Y, Treuting P, et al. Lifespan extension in genetically modified mice. Aging Cell. 2009 doi: 10.1111/j.1474-9726.2009.00491.x. in press. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, et al. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mechanisms of Ageing and Development. 2005;126:365–379. doi: 10.1016/j.mad.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lorenzini A, Tresini M, Austad SN, Cristofalo VJ. Cellular replicative capacity correlates primarily with species body mass not longevity. Mechanisms in Ageing and Development. 2005;126:1130–1133. doi: 10.1016/j.mad.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. A discussion of the aging process: current theories. Drug Nutrition Interaction. 1985;4:35–41. [PubMed] [Google Scholar]
- Miller RA. 'Accelerated aging': a primrose path to insight? Aging Cell. 2004;3:47–51. doi: 10.1111/j.1474-9728.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- Miller RA, Austad SN. Growth and aging: why do big dogs die young? In: Masoro EJ, Austad SN, editors. Handbook of the Biology of Aging. San Diego: Academic Press; 2006. pp. 512–533. [Google Scholar]
- Misra V, Dumonceaux T, Dubois J, Willis C, Nadin-Davis S, et al. Detection of polyoma and corona viruses in bats of Canada. Journal of General Virology. 2009;90:2015–2022. doi: 10.1099/vir.0.010694-0. [DOI] [PubMed] [Google Scholar]
- Nowak RM. Walker's Mammals of the World. Baltimore: Johns Hopkins University Press; 1999. [Google Scholar]
- O'Connor TP, Lee A, Jarvis JU, Buffenstein R. Prolonged longevity in naked mole-rats: age-related changes in metabolism, body composition and gastrointestinal function. Comparative Biochemistry and Physiology A: Molecular Integrated Physiology. 2002;133:835–842. doi: 10.1016/s1095-6433(02)00198-8. [DOI] [PubMed] [Google Scholar]
- Olive PL, Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nature Protocols. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- Ott A, Breteler MM, Van HF, Stijnen T, Hofman A. Incidence and risk of dementia. The Rotterdam study. American Journal of Epidemiology. 1998;147:574–580. doi: 10.1093/oxfordjournals.aje.a009489. [DOI] [PubMed] [Google Scholar]
- Pearl R. The Rate of Living. New York: AA Knopf; 1928. [Google Scholar]
- Perez VI, Bokov A, Remmen HV, Mele J, Ran Q, et al. Is the oxidative stress theory of aging dead? Biochimica et Biophysica Acta. 2009a;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proceedings of the National Academy of Sciences USA. 2009b doi: 10.1073/pnas.0809620106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perls TT, Wilmoth J, Levenson R, Drinkwater M, Cohen M, et al. Life-long sustained mortality advantage of siblings of centenarians. Proceedings of the National Academy of Sciences USA. 2002;99:8442–8447. doi: 10.1073/pnas.122587599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlutsky AJ, Khritankov AM, Ovodov ND, Austad SN. A new field record for bat longevity. Journal of Gerontology A: Biological Science and Medical Science. 2005;60:1366–1368. doi: 10.1093/gerona/60.11.1366. [DOI] [PubMed] [Google Scholar]
- Promislow DEL. The evolution of mammalian blood parameters: patterns and their interpretation. Physiological Zoology. 1991;64:393–431. [Google Scholar]
- Ran Q, Liang H, Ikeno Y, Qi W, Prolla TA, et al. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. Journal of Gerontology A: Biological Science and Medical Science. 2007;62:932–942. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, Milne GL. Isoprostanes. Journal of Lipid Research. 2008;50:S219–S223. doi: 10.1194/jlr.R800037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhme D. Evidence for a relationship between longevity of mammalian species and life spans of normal fibroblasts in vitro and erythrocytes in vivo. Proceedings of the National Academy of Sciences USA. 1981;78:5009–5013. doi: 10.1073/pnas.78.8.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher GA. Relation of life span to brain weight and body weight in mammals. In: Wolstenholme GEW, O'Connor M, editors. CIBA Foundation Colloquia on Ageing. London: Churchill; 1959. pp. 115–113. [Google Scholar]
- Salmon AB, Leonard S, Masamsetti V, Pierce A, Podlutsky AJ, et al. The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB Journal. 2009;23:2317–2326. doi: 10.1096/fj.08-122523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, et al. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. American Journal of Physiology, Endocrinology and Metabolism. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Seluanov A, Chen Z, Hine C, Sasahara TH, Ribeiro AA, et al. Telomerase activity coevolves with body mass not lifespan. Aging Cell. 2007;6:45–52. doi: 10.1111/j.1474-9726.2006.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Telomerase therapeutics for cancer: challenges and new directions. Nature Reviews Drug Discovery. 2006;5:577–584. doi: 10.1038/nrd2081. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Hallmarks of telomeres in ageing research. Journal of Pathology. 2007;211:114–123. doi: 10.1002/path.2090. [DOI] [PubMed] [Google Scholar]
- Sohal RS. The rate of living theory: a contemporary interpretation. In: Collatz K-G, Sohal RS, editors. Insect Aging. Berlin: Springer-Verlag; 1986. pp. 23–44. [Google Scholar]
- Speakman JR. Body size, energy metabolism and lifespan. Journal of Experimental Biology. 2005a;208:1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Correlations between physiology and lifespan - two widely ignored problems with comparative studies. Aging Cell. 2005b;4:167–175. doi: 10.1111/j.1474-9726.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M. Can we develop genetically tractable models to assess healthspan (rather than life span) in animal models? Journal of Gerontology A: Biological Science and Medical Science. 2009;64:161–163. doi: 10.1093/gerona/gln067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice RR, Setlow RB. Handbook of the Biology of Aging. San Diego: Academic Press; 1985. [Google Scholar]
- Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, et al. Oxidative stress in vascular senescence: lessons from successfully aging species. Frontiers in Bioscience. 2008;13:5056–5070. doi: 10.2741/3064. [DOI] [PubMed] [Google Scholar]
- Vijg J. Aging of the Genome. New York: Oxford University Press; 2007. [Google Scholar]
- Wanamaker AD, Jr, Heinemeier J, Scourse JD, Richardson CA, Butler PG, et al. Very long-lived molluscs confirm 17th century AD tephra-based radiocarbon reservoir ages for north Icelandic shelf waters. Radiocarbon. 2008;50:1–14. [Google Scholar]
- Ward WF, Qi W, Van RH, Zackert WE, Roberts LJ, et al. Effects of age and caloric restriction on lipid peroxidation: measurement of oxidative stress by F2-isoprostane levels. Journal of Gerontology A: Biological Science and Medical Science. 2005;60:847–851. doi: 10.1093/gerona/60.7.847. [DOI] [PubMed] [Google Scholar]
- West GB, Bergman A. Toward a systems biology framework for understanding aging and health span. Journal of Gerontology A: Biological Science and Medical Science. 2009;64:205–208. doi: 10.1093/gerona/gln066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, South JM. Life history, ecology and longevity in bats. Aging Cell. 2002;1:124–131. doi: 10.1046/j.1474-9728.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- Wisshak M, López Correa M, Gofas S, Salas C, Taviani M, et al. Shell architecture, element composition, and stable isotope signature of the giant deep-sea oyster Neopycnodonte zibrowii sp. n. from the NE Atlantic. Deep-Sea Research. 2009;56:374–407. [Google Scholar]