Abstract
Objective
To determine whether increased gene expression of a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS-4) in laminae of horses with starch gruel– induced laminitis is accompanied by increased enzyme activity and substrate degradation.
Sample
Laminae from the forelimb hooves of 8 healthy horses and 17 horses with starch gruel–induced laminitis (6 at onset of fever, 6 at onset of Obel grade 1 lameness, and 5 at onset of Obel grade 3 lameness).
Procedures
Gene expression was determined by use of cDNA and real-time quantitative PCR assay. Protein expression and processing were determined via SDS-PAGE and quantitative western blotting. Protein distribution and abundance were determined via quantitative immunofluorescent staining.
Results
ADAMTS-4 gene expression was increased and that of versican decreased in laminitic laminae, compared with expression in healthy laminae. Catalytically active ADAMTS-4 also was increased in the tissue, as were ADAMTS-4–cleavage fragments of versican. Immunofluorescent analyses indicated that versican was depleted from the basal epithelia of laminae of horses at onset of Obel grade 3 lameness, compared with that in healthy laminae, and this was accompanied by regional separation of basal epithelial cells from the basement membrane. Aggrecan gene and protein expression were not significantly affected.
Conclusions and Clinical Relevance
Changes in gene and protein expression of ADAMTS-4 and versican in the basal epithelium of laminitic laminae indicated a fundamental change in the physiology of basal epithelial cells. This was accompanied by and may have caused detachment of these cells from the basement membrane.
The equine digital laminae connect the outer surface of the distal phalanx to the inner hoof wall and suspend the equine axial skeleton within the hoof capsule.1 In healthy horses, the 2-layer tissue supports the vertical load of the animals; resists force applied to the distal phalanx by the deep flexor tendon; accommodates compression and stretch deformation created by flexing, twisting, and tilting of the hoof capsule under various loading conditions; and absorbs a portion of the concussive shock imposed when the hoof strikes a solid surface. However, in a number of disease conditions, including retained fetal membranes and metritis, gram-negative pleuropneumonia, black walnut heartwood toxemia, strangulating intestinal obstruction, enterocolitis, and abnormal fermentation in the caudal part of the alimentary canal, the epidermal and dermal layers separate within regions of the digital laminae, which causes the distal phalanx to rotate and sink within the hoof capsule.2 This stretches laminar nerves and increases pressure on the solar soft tissues, which results in signs of severe pain and lameness. Approximately 1% of the horses in the United States are affected by laminitis, and targeted treatments (other than prolonged cooling of the affected limbs3) have not been developed.
Histologic analyses of laminae from laminitic horses have several pathological features that may contribute to their failure. These include increased apoptosis of basal epithelial cells4; reduced numbers of hemidesmosomes in basal epithelial cells, which results in diminished attachment to the basement membrane; and loss of anchoring filaments that attach the basement membrane to adjacent fibrillar collagen.5,6 In addition, inflammatory leukocytes infiltrate the dermal laminae of laminitic horses.7,8 Furthermore, we have detected regions within the secondary dermal laminae of laminitic horses that lack both cellular and extracellular matrix components, which is consistent with enzymatic degradation. Identifying physiologic changes in basal epithelial cells that result in detachment from the basement membrane or apoptosis as well as the enzymes that mediate damage to the basement membrane and its adjacent extracellular matrix are essential steps toward developing effective treatments.
In studies conducted by our research group7,9 and by other investigators,10 it has been reported that activities of pro–MMP-9, pro–MMP-2, and MMP-2 are increased in laminae of horses with naturally acquired laminitis and in some horses with experimentally induced laminitis. Invariably, the concentration of pro–MMP-9 in the laminae is related to the presence and the myeloperoxidase signature of inflammatory leukocytes,7,9 whereas expression of pro–MMP-2 and MMP-2 bears no relationship to the concentration of myeloperoxidase or to the activity of pro–MMP-9. This suggests that pro–MMP-2 is produced by a cell or cells other than inflammatory leukocytes.7 The regulatory propeptide of pro–MMP-9 typically blocks access to the active site, but conformational changes induced by binding of pro–MMP-9 to substrate or by oxidative modification of pro–MMP-9 can permit catalytic activity without proteolytic removal of the propeptide.11,12 However, although there is evidence of lipid peroxidation in laminitic laminae,13 there is no evidence that pro–MMP-9 is oxidized or active in the laminae. Indeed, there is little evidence of laminar degradation in horses with Obel grade 1 lameness, even when the laminae of these horses contain large numbers of infiltrative neutrophils and highly increased activities of pro–MMP-9.9
Increased activity of MMP-2 is a characteristic in the laminae of horses with chronic relapsing laminitis or starch gruel–induced laminitis at onset of Obel grade 3 lameness7; however, little or no MMP-2 is present at onset of Obel grade 1 lameness in horses with black walnut extract–induced lameness9 or, in our experience, starch gruel–induced lameness. Thus, MMP-2 may contribute to the deterioration of the laminae only in severe disease or may be induced as a component of a repair response. Furthermore, whereas the concentration of MMP-2 is substantially increased in the laminae of horses with starch gruel–induced laminitis at onset of Obel grade 3 lameness, compared with that in healthy horses, some horses develop starch gruel–induced Obel grade 3 lameness in the absence of increases in laminar MMP-2, and healthy horses occasionally can express a high activity of MMP-2 in the laminae without developing signs of lameness.7 Thus, metalloproteinases other than MMP-2 and MMP-9 are likely to contribute to degradation of the laminae in horses with laminitis.
Our laboratory group recently reported that the gene encoding ADAMTS-4 is expressed in the laminae of healthy horses and overexpressed in the laminae of horses with experimentally induced (black walnut heartwood toxemia and starch gruel overload) and naturally acquired laminitis.14 Furthermore, our laboratory group also reported that ADAMTS-4 protein is constitutively expressed in the laminae of healthy horses as a 51-kDa form.15 Catalytic activity was inferred by catalytic site neoepitope exposure, which is indicative of prior removal of the regulatory propeptide, and by the presence of ADAMTS-4 cleavage fragments of aggrecan and versican on the laminar tissues.15 Aggrecan and versican are large polysulfated proteoglycans and primary substrates of ADAMTS-4.16–18 Anionic groups on their GAG side chains carry with them positively charged counterions, such as Na+, which creates an osmotic gradient and draws water into the tissue. In combination with hyaluronan, the proteoglycans form hydrated gels that provide tissues with resistance to compression deformation.16,19,20 In addition, aggrecan and versican have signaling properties that affect cell proliferation, differentiation, adhesion, and intercellular communication17,21,22 and hence have additional, nonmechanical roles in regulating cell function.
In healthy horses, aggrecan and hyaluronan are found throughout the digital secondary epidermal laminae with their highest expression within the basal epithelial cell layer that abuts the basement membrane.15 Versican has a more restricted distribution than aggrecan and is localized solely to the basal epithelial cells. On the basis of their biochemical and biological properties and their tissue distribution, it is feasible that the proteoglycans affect development and maintenance of the basal epithelial cell layer as well as cushion basal epithelial cells against severe biomechanical stresses associated with their anatomic location. Therefore, elevated gene expression of ADAMTS-4 in laminitic laminae,14 if it is accompanied by elevated enzyme expression and degradation of its proteoglycan substrates, may play a critical role in failure of the laminae.
The purpose of the study reported here was to determine whether increased expression of the gene encoding ADAMTS-4 in laminitic laminae of horses is accompanied by an increase in the amount of ADAMTS-4 protein in the tissue, by an increase of fragments of aggrecan and versican-bearing AD-AMTS-4 cleavage neoepitopes, and by a decrease in expression of these proteoglycans within the laminae. We hypothesized that a dramatic change in AD-AMTS-4 substrate processing in the basal epithelium of laminitic laminae may compromise the function of basal epithelial cells and the association of epidermal and dermal layers of the laminae.
Materials and Methods
Sample
Laminae were obtained from the forelimb hooves of 25 horses (age, 3 to 12 years; body weight, 341 to 524 kg). Immediately after sample collection, horses were euthanized under protocols approved by the Institutional Animal Care and Use Committee of the University of the Missouri College of Veterinary Medicine.
Seventeen horses were administered carbohydrate gruel (85% cornstarch and 15% wood flour [17.6 g/kg]) via nasogastric tube to induce laminitis, and 8 horses were administered 6 L of deionized water, as described elsewhere.23,24 Complete physical examinations (consisting of measurement of rectal temperature, heart rate, and respiratory rate; auscultation of abdominal sounds and digital pulses; and evaluation with hoof testers) and gait evaluation (horses were walked and trotted in straight lines and walked in circles in each direction) were performed immediately prior to nasogastric tube intubation and at 2-hour intervals after administration of carbohydrate gruel or water. Anesthesia was induced in the 17 horses during development of laminitis that corresponded to onset of fever (rectal temperature ≥ 38.9°C [n = 6]), onset of Obel grade 1 lameness (constant shifting of weight among limbs with a short, bilaterally stilted gait during trotting [6]), or onset of Obel grade 3 lameness (reluctant to walk [5]).
Laminae were dissected from excised hoofs as described elsewhere.8 Samples of laminae were flash-frozen in liquid nitrogen for molecular and biochemical analyses. For immunofluorescent microscopic evaluation, segments were embedded in a commercial preparation of water-soluble glycols and resinsa and frozen over dry ice.
RNA extraction
The RNA was collected from 3 sections of dorsal lamina of each horse by means of an RNA extraction kit.b Briefly, flash-frozen tissue was pulverized in a prechilled (on dry ice) biopulverizer,c homogenized in the lysis buffer provided, and then passed through the column provided and washed accordingly. Purity and concentration of RNA were determined,d and extracted RNA was used for cDNA preparation only when the 260- to 280-nm absorbance ratio and the 260- to 230-nm absorbance ratio were approximately 2.0. Integrity of isolated RNA was confirmed via electrophoresis on a 1.0% agarose gel and staining with a proprietary polynucleotide gel stain.e
RT-qPCR assay
The cDNA was synthesized from isolated RNA with a cDNA synthesis kit.f Primer sets for equine ADAMTS-4, aggrecan and versican C-terminal domains, and GAPDH have been described elsewhere.15 Primer sets for versican G1, αGAG, and βGAG domains were used (Appendix). The RT-qPCR assays were conducted with a proprietary reaction mixture that contained a high-performance reverse transcriptase and reference dyesg; reactions were conducted in accordance with manufacturer’s instructions and with a thermal cycler.h Each sample was assayed in triplicate, and the mean cycle threshold values were calculated and analyzed by use of the comparative cycle threshold method (ie, ΔΔCT) of analysis.25 Each value was measured relative to GAPDH, and contamination and formation of primer dimers were monitored via a dissociation curve.
NP-40–soluble material
Approximately 0.35 g of snap-frozen tissue was pulverized in a prechilled (on dry ice) biopulverizer,c immediately homogenized in 10 mL of extraction buffer (50mM Tris [pH, 7.0], 150mM NaCl, 5mM EDTA, and 0.5% NP-40 containing 10μM E64, 1.5μM pepstatin A, and 1mM phenylmethanesulfonyl fluoride) on ice, and extracted overnight (approx 15 hours) at 4°C. The protein concentration was determined as described elsewhere.15
Guanidine hydrochloride–soluble material
Approximately 0.35 g of snap-frozen tissue was pulverized in a prechilled (on dry ice) biopulverizer,c homogenized for 30 seconds in 5 mL of cold (on ice) extraction buffer (0.1M PBS solution, 5mM iodoacetic acid, 0.1mM 4-[2-aminoethyl] benzenesulphonyl fluoride, 1% 3-[{cholamidopropyl}-dimethylammonio]-1-propane-sulfonate, 1 μg of pepstatin A/mL, 50mM sodium acetate, 5mM benzamidine hydrochloride hydrate, 5mM phenylmethylsulfonyl fluoride, 10mM N-ethylmaleimide, and 4M guanidine hydrochloride; pH, 7.6) supplemented with a proteinase inhibitor cocktail,i and extracted overnight in this buffer at 4°C. After overnight extraction, recovery of soluble material, processing to digest hyaluronan and remove chondroitin sulfate, and assay of solubilized protein were performed as described elsewhere.15
SDS-PAGE and western blotting
An aliquot (30 μg of protein content) of extract was boiled in reducing Laemmli sample bufferj for 5 minutes and subjected to SDS-PAGE in a 4% (wt/vol) polyacrylamide stacking gel with a 10% (wt/vol) polyacrylamide gel, as described elsewhere.19 Proteins were transferred to polyvinylidene fluoridek membranes via electroblotting. Each membrane was blocked by incubation with 5% dry milk in PBS solution with 0.05% Tween-20 for 1 hour, washed with PBS solution with 0.1% Tween-20 for 30 minutes, and then incubated with appropriate primary antibodies overnight at 4°C to detect polypeptides bearing the FASLSRFVET ADAMTS-4 catalytic site neoepitope,26,l ARGSVI aggrecan ADAMTS-4 cleavage neoepitope,27,m DPEAAE V0 and V1 ADAMTS-4 cleavage neoepitope,28,n or NIVSFE V0 and V2 ADAMTS-4 cleavage neoepitope29,o or β-actin.p Bound antibody was identified with appropriate secondary antibodies, as described elsewhere.15 Detection was performed via enhanced chemiluminescenceq with a gel imaging and documentation system,r and quantification was performed with associated software.s
Immunofluorescence analysis
Frozen sections (thickness, 10 μm) were cut from embeddeda tissues and affixed to treated glass slides.t Indirect immunofluorescent staining was performed as described elsewhere15 by means of a combination of antibodies that separately stained aggrecan,u versican,v or lamininw red; a commercial preparation of phalloidin-FITCx that stained actin green; and a fluorescent DNA-intercalating agent (ie, DAPI) that stained nuclei blue. All slides were evaluated by use of an inverted microscope with apotome gridy; UV, blue, and green excitation light; and 20× or 63× magnification of the objective.
Quantitation of tissue immunofluorescence
Mean fluorescence was calculated with commercially available software.z The basal epithelia of secondary epidermal lamellae were traced from the crypt to the tip, and mean fluorescence intensity values were calculated for the red-stained region in the evaluated area. Sixty measurements were obtained for each control horse (n = 6) and horses with Obel grade 1 (6) and Obel grade 3 (4) lameness.
Statistical analysis
Statistical analyses of gene expression and western blot data were performed with commercial software.aa For comparisons of RT-qPCR and western blot data, normalized values for each horse were analyzed via a 1-way ANOVA with a Dunnett post-test. Pearson product moment correlation coefficient analyses with 2-tailed 95% confidence intervals were performed in pairs of expressed western blot bands. Values of P ≤ 0.05 were considered significant. Pearson r values can range from −1 to 1, and r2 values indicate (explained variance/[explained variance + unexplained variance]). Protein distribution analysis of immunofluorescence data was performed via a 2-way ANOVA with a Duncan posttest by use of a commercially available specialized statistical package.bb
Results
Gene expression
Analysis via RT-qPCR assay with GAPDH as the reference gene revealed that gene expression of ADAMTS-4 was significantly increased in the digital laminae of the 6 horses that developed Obel grade 1 lameness after receiving starch gruel, compared with that in the laminae of the 8 healthy control horses (Figure 1). There was a slight increase in gene expression of ADAMTS-4 in the digital laminae of horses at onset of fever (n = 6) and at onset of Obel grade 3 lameness (5). However, in these cases, the mean fold increase for ADAMTS-4 induction values were lower than that for the laminae of horses at onset of Obel grade 1 lameness. In addition, there was considerable sample variation and mean values did not differ significantly from that in laminae of healthy horses.
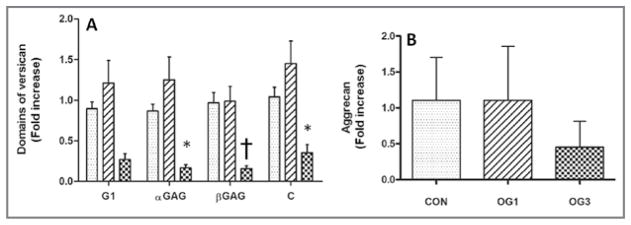
Figure 1.
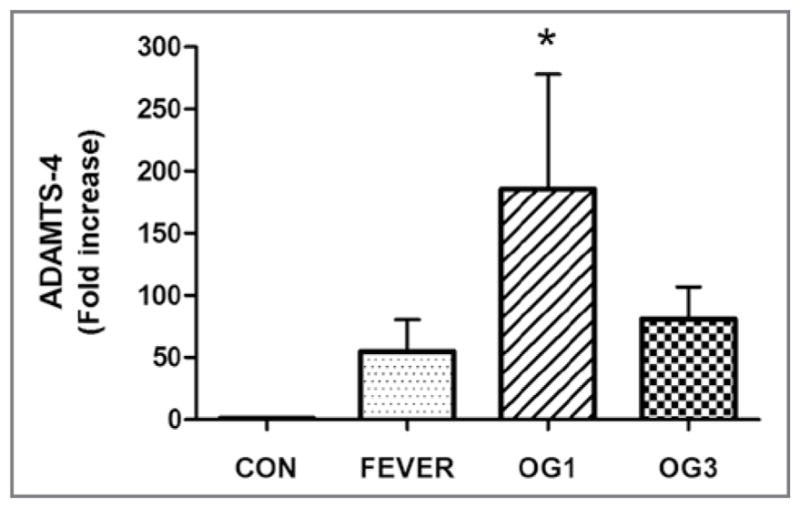
Mean ± SD fold increase in gene expression of ADAMTS-4 in healthy and laminitic equine digital laminae. The RNA was extracted from 3 sections of dorsal lamina obtained from 8 healthy control horses (CON), 6 horses during development of laminitis that corresponded to onset of fever (rectal temperature ≥ 38.9°C), 6 horses at onset of Obel grade 1 lameness (OG1), and 5 horses at onset of Obel grade 3 lameness (OG3) and reverse transcribed into cDNA. An RT-qPCR assay was performed in triplicate for each cDNA preparation with optimized gene-specific primers to analyze gene expression of ADAMTS-4 normalized on the basis of gene expression for GAPDH in corresponding samples. For each gene, primers were selected so that they did not amplify genomic DNA. *Value differs significantly (P < 0.05), compared with the value for the control horses, as determined from 95% confidence intervals of the treated samples via a 1-way ANOVA.
Versican
Analysis via RT-qPCR assay with GAPDH as the reference gene and primers specific for versican G1, αGAG, βGAG, and C-terminal domains revealed that expression of the gene encoding versican increased slightly for the 6 horses at onset of Obel grade 1 lameness but decreased (2.5-fold decrease) significantly (αGAG, P < 0.05; βGAG, P = 0.01; and C-terminal domain, P < 0.05) in the digital laminae of the 5 horses at onset of Obel grade 3 lameness, compared with that in laminae of the 8 healthy horses (Figure 2). The RT-qPCR analyses performed by use of primers specific for the G1 domain sequence of versican revealed a similar, although not significant, decrease in expression of versican in laminae of horses at onset of Obel grade 3 lameness, compared with that in laminae of the 8 healthy horses.
Figure 2.
Mean ± SD fold increase in gene expression of versican domains (A) and aggrecan (B) performed as described in Figure 1 in healthy and laminitic equine digital laminae for RNA extracted from 3 sections of dorsal lamina obtained from 8 healthy control horses (stippled bars), 6 horses at onset of Obel grade 1 lameness (diagonal-striped bars), and 5 horses at onset of Obel grade 3 lameness (crosshatched bars) and reverse transcribed into cDNA. *Value differs significantly (*P = 0.050; †P = 0.01), compared with the value for the control horses, as determined from 95% confidence intervals of the treated samples via a 1-way ANOVA.
Aggrecan
Analysis via RT-qPCR assay with GAPDH as the reference gene was conducted. It revealed that although mean fold expression of the gene encoding aggrecan was lower in the laminae of horses at onset of Obel grade 3 lameness, compared with that in laminae of the 8 healthy control horses (8), the values did not differ significantly (Figure 2).
Protein expression
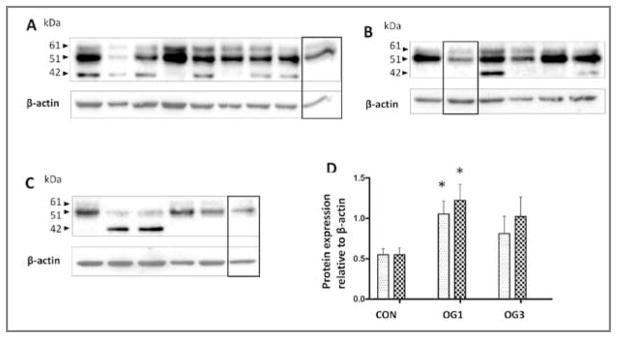
Analyses via SDS-PAGE and western blotting revealed that 2 ADAMTS-4 polypeptides of 61 and 51 kDa bearing the active site neopitope FASLSRFVET26 were significantly increased (approx 2-fold increase) in 0.5% NP-40 extracts of laminae of the 6 horses at onset of Obel grade 1 lameness relative to that in the laminae of the 8 healthy control horses (Figure 3). The 51-kDa form of ADAMTS-4 was more prevalent than the 61-kDa form in these and all other samples of laminae analyzed. In addition, β-actin, which is expected to remain constant in the tissue throughout development of laminitis (which was the case with gene expression of β-actin [data not shown]), was used as a loading control sample to normalize values, and a sample from the same protein extract was included in each blot to facilitate comparisons among blots. Gene expression for the 61- and 51-kDa forms of ADAMTS-4 did not differ significantly between horses at onset of Obel grade 3 and control horses, which mirrored results of gene expression for ADAMTS-4 (Figure 1).
Figure 3.
Western blots of ADAMTS-4 immunoreactivity in 0.5% NP-40 extracts (30 μg of protein/lane) from laminae of 8 healthy control horses (A), 6 horses at onset of Obel grade 1 lameness (B), and 5 horses at onset of Obel grade 3 lameness (C). Samples were subjected to SDS-PAGE in 10% gels, polypeptides were transferred to polyvinylidine fluoride membrane, and blots were probed by use of an antibody against neoepitope FASLSRFVET exposed on the ADAMTS-4 catalytic domain after removal of the regulatory propeptide. Blots were stripped and probed with an antibody specific for β-actin, which was used as a loading control sample. The bands enclosed by the black box in each panel are from a common sample (from the Obel grade 1 lameness group) that was included in all gels and used for normalizing experimental variation. Mean ± SD intensity of chemiluminescence was quantified for the bands at 51 kDA (stippled bars) and 61 kDA (crosshatched bars) and statistically analyzed (D). *Value differs significantly (P < 0.05), compared with the corresponding value for the same band size in the control horses, as determined from 95% confidence intervals of the treated samples via a 1-way ANOVA.
V0 and V1 isoforms
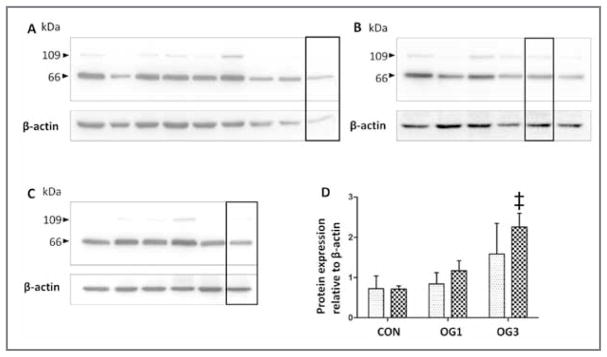
The ADAMTS-4 cleaves V0 and V1 isoforms within the βGAG domain, which yields 66- and 109-kDa G1-βGAG fragments with a characteristic C-terminal ADAMTS-4 DPEAAE cleavage neoepitope.28 Equine lamellar versican fragments bearing the DPEAAE neoepitope were fully extracted into 0.5% NP-40 (data not shown). Comparative analysis of 0.5% NP-40 extracts of laminae from healthy horses and from horses with starch gruel–induced laminitis indicated that the amount of the 66-kDa V0 and V1 ADAMTS-4 cleavage fragment was significantly (P = 0.001) increased (approx 2.5-fold increase) in the laminae of the 5 horses at onset of Obel grade 3 lameness, compared with that in the laminae of the 8 healthy control horses (Figure 4). The amount of the 109-kDa V0 and V1 ADAMTS-4 cleavage fragment was also increased (approx 2-fold increase) in horses at onset of Obel grade 3 lameness, compared with that in the control horses, although this was not a significant increase. However, paired analyses of 109- and 66-kDa ADAMTS-4 cleavage fragments of V0 and V1 revealed that these fragments were significantly, positively correlated (r = 0.70; P < 0.001), consistent with generation by the same process. Differential retention of the cleavage fragments in laminae or selective further degradation in the laminae may have accounted for unexplained variance in the abundance of each respective cleavage fragment.
Figure 4.
Western blots of versican V0 and V1 immunoreactivity in 0.5% NP-40 extracts (30 μg of protein/lane) from laminae of 8 healthy control horses (A), 6 horses at onset of Obel grade 1 lameness (B), and 5 horses at onset of Obel grade 3 lameness (C). Samples were subjected to SDS-PAGE in 10% gels, polypeptides were transferred to polyvinylidine fluoride membranes, and blots were probed by use of antibodies against the neoepitope DPEAAE of isoforms V0 and V1 generated by ADAMTS-4. Mean ± SD intensity of chemiluminescence was quantified for bands at 66 kDA (crosshatched bars) and 109 kDA (stippled bars) and statistically analyzed (D). ‡Value differs significantly (P < 0.001), compared with the corresponding value for the same band size in control horses, as determined from 95% confidence intervals of the treated samples via a 1-way ANOVA.
V0 and V2 isoforms
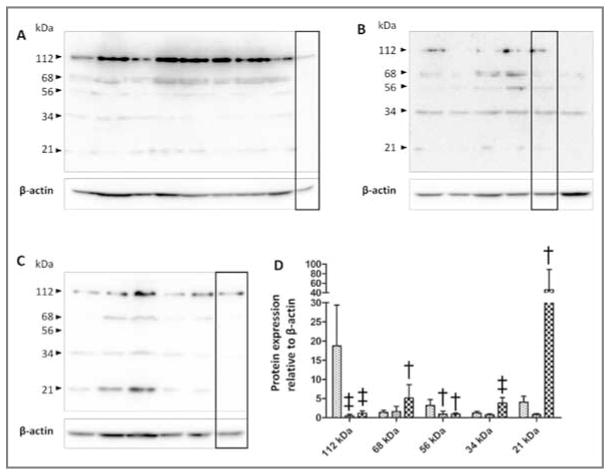
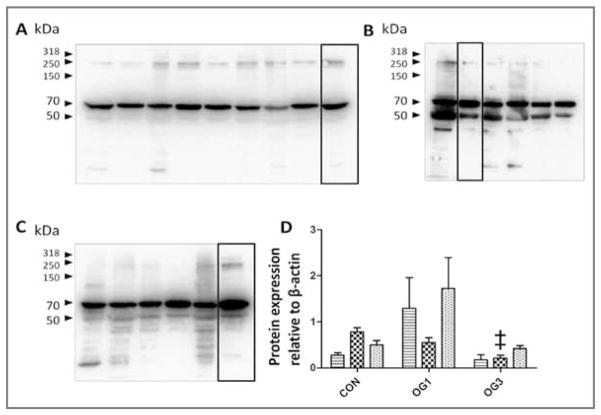
The V0 and V2 isoforms are constitutively cleaved by ADAMTS-4, which yields G1-αGAG fragments bearing a C-terminal ADAMTS-4 NIVSFE cleavage neoepitope.29 The NIVSFE-positive polypeptides of 112, 68, 56, 34, and 21 kDa were detected in extracts of laminae from healthy and laminitic horses (Figure 5). Comparative analysis of 0.5% NP-40 extracts of laminae from healthy horses and from horses with starch gruel–induced laminitis, which contained all extractable NIVSFE-neoepitope–bearing material, revealed that the presence of the 112-kDa polypeptide was significantly (P < 0.001) decreased (approx 8-fold decrease) in extracts of laminae from the 6 horses at onset of Obel grade 1 and the 5 horses at onset of Obel grade 3 lameness, compared with that in extracts of laminae from the 8 healthy control horses); this was paralleled by a significant (P = 0.01) decrease (approx 3-fold decrease) in the concentration of the 56-kDa NIVSFE-positive fragment. The concentrations of the 112- and 56-kDa fragments were significantly, positively correlated (r = 0.76; P < 0.001), consistent with their generation by the same process.
Figure 5.
Western blots of versican V0 and V2 immunoreactivity in 0.5% NP-40 protein extracts (30 μg of protein/lane) from laminae of 8 healthy control horses (stippled bars; A), 6 horses at onset of Obel grade 1 lameness (diagonal-striped bars; B), and 5 horses at onset of Obel grade 3 lameness (crosshatched bars; C). Samples were subjected to SDS-PAGE in 10% gels, polypeptides were transferred to polyvinylidine fluoride membranes, and blots were probed by use of antibodies against the neoepitope NIVSFE of V0 and V2 generated by ADAMTS-4. Mean ± SD intensity of chemiluminescence was quantified for the 3 groups of horses and statistically analyzed (D). Value differs significantly (†P < 0.01; ‡P < 0.001), compared with the corresponding value for the same band size in control horses, as determined from 95% confidence intervals of the treated samples via a 1-way ANOVA.
The decrease in the concentration of the 112- and 56-kDa V0 and V2 fragments bearing the ADAMTS-4 cleavage neoepitope NIVSFE in laminitic laminae was accompanied by significant increases in the concentration of the 68-kDA (approx 2.5-fold increase; P = 0.01), 34-kDA (approx 3-fold increase; P < 0.001), and 21-kDa (approx 9-fold increase; P = 0.01) polypeptides bearing the NIVSFE neoepitope. These polypeptides had positive correlations (n = 19) for fragments of 68 vs 34 kDa (r = 0.47; P < 0.05), 68 vs 21 kDa (r = 0.79; P < 0.001), and 34 vs 21 kDa (r = 0.73; P < 0.001]), which is consistent with their generation by the same process.
Correlation analysis of 66-kDa DPEAAE–positive and 34- and 21-kDa NIVSFE–positive versican fragments
The concentration of the 66-kDa ADAMTS-4 cleavage fragment of V0 and V1 increased in laminitic laminae, compared with that in healthy laminae (Figure 4). Similarly, the concentrations of the 34- and 21-kDa ADAMTS-4 fragments also increased in laminitic laminae, compared with those in healthy laminae (Figure 5). A paired comparison of these data revealed a significant strong positive correlation (r = 0.77; P < 0.001) between the concentrations of the 66-kDa V0 and V1 fragment and the 34-kDa V0 and V2 fragment and a significant but weaker correlation (r = 0.53; P = 0.02) between the 66- and 21-kDa putative V0 and V2 fragment, which was consistent with their generation by cleavage of the V0 and V1 and the V0 and V2 isoforms by AD-AMTS-4. Unexplained variance may have resulted from differential synthesis of V0, V1, and V2 relative to ADAMTS-4 or from differential fragment stability or laminar retention in vivo.
Aggrecan
The ADAMTS-4 cleavage fragments of aggrecan could be extracted from laminae with 4M guanidine hydrochloride but not with 0.5% NP-40. Extracted material was digested with chondroitinase ABC to remove chondroitin sulfate GAGs. Chondroitin sulfate GAG–free aggrecan fragments bearing the AR-GSVI27 ADAMTS-4 cleavage neoepitope had molecular weights of 250, 70, and 50 kDa (Figure 6). Relative amounts of the 250- and 50-kDa polypeptides were similar in extracts of laminae from the 8 healthy control horses, the 6 horses at onset of Obel grade 1 lameness, and the 5 horses at onset of Obel grade 3 lameness. Detection of the 70-kDa polypeptide was significantly (P < 0.001) decreased (approx 2.5-fold decrease) in laminae from horses at onset of Obel grade 3 lameness relative to that in extracts of laminae from the healthy control horses. For these analyses, a common sample was included in each gel and used to normalize data. Actin was not detected in the preparations (except for its inclusion as a loading control sample), but an equivalent amount of total protein was loaded in each lane.
Figure 6.
Western blots of immunoreactivity of aggrecan in hyaluronidase- and chondroitinase ABC–digested 4M guanidine hydrochloride extracts (30 μg of protein/lane) from laminae of 8 healthy control horses (A), 6 horses at onset of Obel grade 1 lameness (B), and 5 horses at onset of Obel grade 3 lameness (C). Samples were subjected to SDS-PAGE in 10% gels, polypeptides were transferred to polyvinylidine fluoride membranes, and blots were probed by use of antibodies against the aggrecan neoepitope ARGSVIL generated by ADAMTS-4. Mean ± SD intensity of chemiluminescence was quantified for bands at 50 kDA (stippled bars), 70 kDA (crosshatched bars), and 250 kDA (horizontal-striped bars) and statistically analyzed (D). ‡Value differs significantly (P < 0.001), compared with the corresponding value for same band size in control horses, as determined from 95% confidence intervals of the treated samples via a 1-way ANOVA.
Protein distribution
Indirect immunofluorescence evaluation was performed on thin frozen sections of laminae from healthy horses and horses with starch gruel–induced laminitis to determine whether increased gene and protein expression of ADAMTS-4 affected the distribution of aggrecan or versican. In these sections, nuclei were stained blue with DAPI, and aggrecan or versican were stained red by use of specific antibodies (Figure 7). Representative images were obtained from sections of laminae from a control horse, a horse at onset of Obel grade 1 lameness, and a horse at onset of Obel grade 3 lameness. Within each field, basal epithelial cells were examined to determine the mean intensity of red fluorescence, and 60 fields were analyzed for each section from each sample of laminae. Results obtained for samples from horses at onset of Obel grade 1 and onset of Obel grade 3 lameness were compared with those for samples from control laminae.
Figure 7.
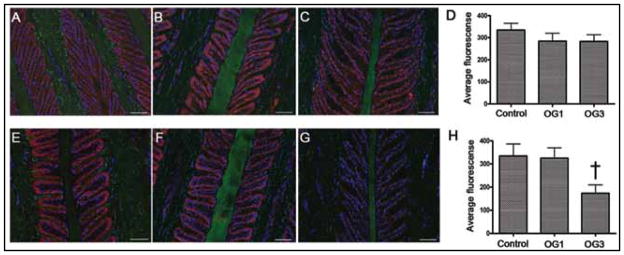
Photomicrographs depicting the distribution of aggrecan and versican in thin sections of frozen laminae from a representative healthy horse (A and E), a representative horse at onset of Obel grade 1 lameness (B and F), and a representative horse at onset of Obel grade 3 lameness (C and G). Sections were stained (red) with antibodies against aggrecan (A, B, and C) and versican (E, F, and G). Auto-fluorescent laminae components (putatively collagen and keratin; green) were stained with a fluorescent dye, and nuclei (blue) were stained with DAPI. Bar = 50 μm. Mean ± SD fluorescence of aggrecan (D) and versican (H) was quantified and statistically analyzed by use of a 2-way ANOVA and the Duncan posttest; the analyses included data for only 4 horses at onset of Obel grade 3 lameness. †Value differs significantly (P = 0.01) from the value for the control healthy horses. See Figures 1 and 2 for remainder of key.
Distributions of aggrecan and versican were the same in the laminae of control and laminitic horses (Figure 7). In all samples, aggrecan was detected only in the secondary epidermal laminae and was most highly expressed in the basal epithelial cells. In all samples, versican was detected only in basal epithelial cells of the secondary epidermal laminae. The intensity of ag-grecan staining in basal epithelial cells of the secondary epidermal laminae was not affected by the development of laminitis, as indicated by quantification for specific staining. In contrast, the concentration of versican was significantly lower (approx 3-fold lower) in the laminae of horses at onset of Obel grade 3 lameness than in the laminae of healthy horses (P = 0.01) and the laminae of horses at onset of Obel grade 1 lameness (P < 0.05). The concentration of versican in laminae of horses at onset of Obel grade 1 lameness did not differ significantly from that in laminae of healthy control horses.
Laminin and actin
Immunofluorescence analyses were performed on thin frozen sections of laminae from healthy horses and horses with starch gruel– induced laminitis to examine the relationship between the basement membrane and basal epithelial cells. Representative results from a control horse, a horse at onset of Obel grade 1 lameness, and a horse at onset of Obel grade 3 lameness were consistent with histochemical observations reported by other investigators.30 Briefly, basal epithelial cells in laminae of horses at onset of Obel grade 3 lameness appeared flattened and spread out on the basement membrane, compared with the appearance of those of healthy horses (Figure 8). In addition, in some locations, the basement membrane pulled free of the tips of secondary epidermal laminae and also separated from basal epithelial cells in crypt regions of the secondary epidermal laminae.
Figure 8.
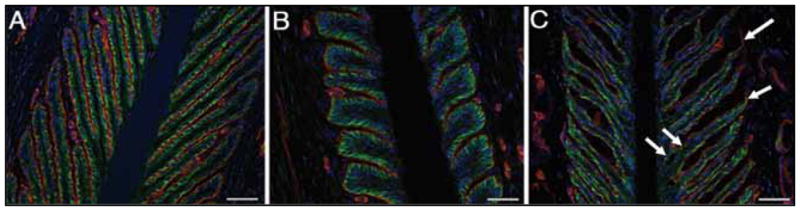
Photomicrographs of thin sections of laminae from a representative healthy horse (A), a representative horse at onset of Obel grade 1 lameness (B), and a representative horse at onset of Obel grade 3 lameness (C). Tissue sections were stained with an antibody against laminin (red) to enable evaluation of the basement membrane and phalloidin-FITC to enable evaluation of intracellular cortical actin (green). Nuclei (blue) were stained with DAPI. Notice the arrows marking detachment of basement membrane laminin from basal epithelial cells in the section of laminae collected at onset of Obel grade 3 lameness (C). Bar = 50 μm.
Discussion
Starch gruel–induced laminitis causes an increase in gene expression of ADAMTS-4 in the laminae of the forelimb hooves of horses.23,24 Results of the study reported here confirmed those of another study.14 In addition, we found that the increase in gene expression of ADAMTS-4 was accompanied by an increase in activity of ADAMTS-4, a decrease in gene expression of versican that occurs between Obel grade 1 and Obel grade 3 lameness, an increase in concentrations of low–molecular weight ADAMTS-4 cleavage fragments of V0 and V1 and V0 and V2 in the tissue, depletion of versican from basal epithelial cells and regional separation of basal epithelial cells from the basement membrane. Although a direct pathophysiologic consequence of versican loss cannot be established from the present study, this loss, together with changes in gene expression of ADAMTS-4 and versican, indicated a global change in the physiology of basal epithelial cells that may have contributed to detachment of the epithelial cells from the basement membrane and the development of severe lameness. This conclusion is consistent with results of another study6 in which there was a reduction in desmosomal attachments of basal epithelial cells to adjacent basement membrane in laminitic tissue.
A moderate but significant positive correlation was detected between expression of genes encoding versican and aggrecan in laminae. However, results of analyses of other tissues indicate that expression of these genes is not consistent with a straightforward program of coregulation. For example, gene expression of both aggrecan and versican is increased in porcine chondrocytes subjected to microgravity,31 whereas in ruptured calcaneal tendons, gene expression of aggrecan is increased and that of versican is decreased.32 Furthermore, exposure of equine articular cartilage to interleukin-1β elicits increases in gene expression of versican but decreases in gene expression of aggrecan.33
Gene expression of both ADAMTS-4 and versican decreased in the laminae of horses at onset of Obel grade 3 lameness, relative to that in horses at onset of Obel grade 1 lameness, although the decreases were to differing degrees. Nevertheless, there was a moderate positive correlation between expression of genes encoding ADAMTS-4 and versican, which is consistent with results in other tissues. For example, incubation with transforming growth factor-β1 causes upregulation of the gene expression of versican in human skin34 and bronchial epithelial cells,35 upregulation of gene expression of ADAMTS-4 in cultured human tendon cells,36 and upregulation of expression of both genes in prostatic stromal cells.37 Additional analysis of signaling pathways that regulate expression of genes encoding ADAMTS-4, versican, and aggrecan in laminar basal epithelium is dependent on the development of appropriate cell lines.
The increase in ADAMTS-4 in laminae of horses with starch gruel–induced laminitis was accompanied by a significant increase in V0 and V1 and V0 and V2 fragments bearing ADAMTS-4 cleavage neoepitopes. Although aggrecan is a recognized substrate of ADAMTS-418 and is present together with ADAMTS-4 and versican in basal epithelial cells of the laminae,15 the relative amount of aggrecan fragments bearing ADAMTS-4 cleavage neoepitopes did not increase in laminitic laminae. The most likely interpretation of these data is that aggrecan and versican are differentially cleaved by ADAMTS-4 in laminitic laminae. Differential cleavage of versican versus aggrecan might result from differential specificity of the 51-kDa truncated form of ADAMTS-4, which is the predominant form in laminae for these substrates, from unequal partition of ADAMTS-4 into distinct aggrecan- and versican-rich compartments, or both. In this regard, C-terminal auto-proteolytic truncation of ADAMTS-4, which results in generation of the approximately 51-kDa form, can reduce the specificity of ADAMTS-4 for sulfated GAGs38 and alter the substrate range of ADAMTS.39–41
Concentrations of 68-, 34-, and 21-kDa V0 and V2 cleavage fragments bearing the ADAMTS-4 cleavage neoepitope NIVSFE were dramatically increased in the laminae of horses at onset of Obel grade 1 and onset of Obel grade 3 lameness, compared with concentrations in laminae of healthy horses. Cleavage of both V0 and V2 isoforms by ADAMTS-4 takes place within the αGAG domain15,29 and is expected to yield a G1-αGAG fragment that corresponds to the 68-kDa fragment described in the present study. Additional cleavage of this fragment is required to yield the 34- and 21-kDa fragments. It remains to be determined whether C-terminal truncation of equine ADAMTS-4 provides it with this additional catalytic activity or whether other proteases are involved. It also remains to be determined whether the low–molecular weight V0 and V1 and V0 and V2 fragments, which were found in the present study to accumulate in laminitic laminae and to be soluble, are secreted and have biological activity that contributes to the pathogenesis of laminitis.
Retention of aggrecan and loss of versican from basal epithelial cells during laminitis is not consistent with the simple idea that cleavage of polysulfated proteoglycans by ADAMTS-4 removes a protective gel layer, thus increasing the vulnerability of basal epithelial cells to compression and concussion forces. Of the 2 large polysulfated proteoglycans, aggrecan is the one most heavily substituted with chondroitin sulfate18,19,42 and consequently would be expected to have the greater impact on retention of water in the tissue. In addition to its role as a biological cushion, versican can regulate a wide range of cell functions, including proliferation, migration, and death. Of particular interest is the ability of recombinant V1 to induce mesenchymal-to-epithelial cell transition of the NIH3T3 fibroblast cell line and to regulate expression of cadherins and connexins that increase cell adhesion and communication through gap junctions.22,43 Also of interest is the ability of recombinant V2 to inhibit cell proliferation.44 If V1 and V2 isoforms exert these regulatory functions in basal epithelial cells of the equine digital laminae, their depletion through reduced gene expression and ADAMTS-4 cleavage might lead to functional modification of the basal epithelial cells. Furthermore, differential depletion of V1 and V2 isoforms in laminae, which can be inferred from the greater accumulation of V0 and V2, compared with that of V0 and V1 versican ADAMTS-4 cleavage fragments, would be expected22,43,44 to favor an increase in proliferation of basal epithelial cells combined with some loss of phenotype.
We propose that versican plays a key role in regulating physiologic properties of basal epithelial cells in the digital laminae of horses. Furthermore, we propose that depletion of versican through cleavage by ADAMTS-4 and downregulation of gene expression in laminitic laminae is responsible for the observed changes in basal epithelial cells and their detachment from the basement membrane, which contribute to separation of the laminae at the epidermal-dermal junction.
Acknowledgments
Supported by the Morris Animal Foundation (D08EQ-054) and the USDA National Research Initiative Cooperative State Research, Education, and Extension Service (35204-18313). Dr. Black was supported by the USDA Cooperative State Research, Education, and Extension Service (MAS00907). Ms. Pawlak was supported by a Lotta M. Crabtree Fellowship in Agriculture. Dr. Alfandari was supported by the National Institutes of Health (DE016289).
The authors thank Dr. Andria Cogswell for assistance with collection of laminae, Dr. Wesley Autio for assistance with the statistical analysis, and Drs. Hannah Galantino-Homer and Baixiang Zou for technical assistance.
ABBREVIATIONS
- ADAMTS-4
A disintegrin and metalloproteinase with thrombospondin motifs-4
- DAPI
4′,6-diamidino-2-phenylindole
- FITC
Fluorescein isothiocyanate
- GAG
Glycosaminoglycan
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- MMP
Matrix metalloproteinase
- NP-40
Tergitol-type nonyl phenoxylpolyethoxylethanol
- qPCR
Quantitative PCR
- RT
Real-time
Appendix
Primer sequences used in qPCR evaluation of gene expression of versican in laminae of healthy horses and horses with gruel starch–induced laminitis.
| Domain | Sequence | GenBank accession No. | Amplicon length (bp) | Primer efficiency (%) | R2 |
|---|---|---|---|---|---|
| G1 | F: 53-ttatgaagatgggtttgagcag-333 R: 53-agtttcatgaggagaacggaat-33 |
100065275 | 151 | 101 | 0.984 |
| αGAG | F: 53-acacttcccatacggtttctct-333 R: 53-aggagaaatagtcgcttcaagg-33 |
100065275 | 152 | 108 | 1.000 |
| βGAG | F: 53-gttttcagcacatctcttgagg-333 R: 53-tttctggtagcagatgctgaat-33 |
100065275 | 179 | 101 | 0.999 |
| C | F: 53-cctgcaattaccatctcaccta-333 R: 53-cagggagttgatttcataacga-33 |
100065275 | 122 | 92.1 | 0.988 |
F = Forward. R = Reverse.
Footnotes
Tissue-Tek OCT, Sakura Finetek USA Inc, Torrance, Calif.
Stratagene Absolutely RNA kit, Stratagene, La Jolla, Calif.
Biospec Products Inc, Bartlesville, Okla.
NanoDrop 1000, Thermo Scientific, Wilmington, Del.
SYBRSafe DNA gel stain, Molecular Probes, Eugene, Ore.
Quanta q Script cDNA synthesis kit, Quanta BioSciences, Gaithersburg, Md.
SYBR Premix Ex Taq, Applied Biosystems, Foster City, Calif.
Stratagene MX 3005p, Stratagene, La Jolla, Calif.
Sigma Fast protease inhibitor cocktail, Sigma-Aldrich, St Louis, Mo.
Laemmli reducing sample buffer, Bio Rad Life Sciences, Hercules, Calif.
Millipore, Billerica, Mass.
Provided by Drs. M. D. Tortorella and Dr. A. M. Malfait, Pfizer Global Research and Development, St Louis, Mo.
No. ab3773, AbCam, Cambridge, Mass.
No. ab19345, AbCam, Cambridge, Mass.
No. ab28671, AbCam, Cambridge, Mass.
No. ab8226, AbCam, Cambridge, Mass.
ECL, Bio Rad Life Sciences, Hercules, Calif.
G : Box, Syngene, Frederick, Md.
Gene Tools, Syngene, Frederick, Md.
Fisher Superfrost Plus, Fisher Scientific, Fair Lawn, NJ.
No. ab16320, AbCam, Cambridge, Mass.
No. 26706, Santa Cruz Biotechnologies, Santa Cruz, Calif.
No. ab11575, AbCam, Cambridge, Mass.
Phalloidin-FITC, Sigma-Aldrich, St Louis, Mo.
Zeiss MOT200 with Zeiss apotome, Carl Zeiss MicroImaging Inc, Thornwood, NY.
Axiovision, version 4.6.3.0, Carl Zeiss MicroImaging Inc, Thornwood, NY.
Prism, version 5, GraphPad Software Inc, San Diego, Calif.
ProcGLM, SAS, version 9.2, SAS Institute Inc, Cary, NC.
Presented in part at the American Association of Equine Practitioners Foundation Equine Laminitis Workshop, West Palm Beach, Fla, November 2009, and at the Conference for Research Workers in Animal Diseases, Chicago, November 2010.
References
- 1.Pollitt C. The anatomy and physiology of the suspensory apparatus of the distal phalanx. Vet Clin North Am Equine Pract. 2010;26:29–49. doi: 10.1016/j.cveq.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Eades SC. Overview of what we know about the pathophysiology of laminitis. J Equine Vet Sci. 2010;30:83–86. [Google Scholar]
- 3.van Eps AW. Therapeutic hypothermia (cryotherapy) to prevent and treat acute laminitis. Vet Clin North Am Equine Pract. 2010;26:125–133. doi: 10.1016/j.cveq.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Faleiros RR, Stokes AM, Eades SC, et al. Assessment of apoptosis in epidermal lamellar cells in clinically normal horses and those with laminitis. Am J Vet Res. 2004;65:578–585. doi: 10.2460/ajvr.2004.65.578. [DOI] [PubMed] [Google Scholar]
- 5.French KR, Pollitt CC. Equine laminitis: congenital, hemidesmosomal plectin deficiency in a Quarter Horse foal. Equine Vet J. 2004;36:299–303. doi: 10.2746/0425164044877206. [DOI] [PubMed] [Google Scholar]
- 6.French KR, Pollitt CC. Equine laminitis: loss of hemidesmosomes in hoof secondary epidermal lamellae correlates to dose in an oligofructose induction model: an ultrastructural study. Equine Vet J. 2004;36:230–235. doi: 10.2746/0425164044877125. [DOI] [PubMed] [Google Scholar]
- 7.Loftus JP, Johnson PJ, Belknap JK, et al. Leukocyte-derived and endogenous matrix metalloproteinases in the lamellae of horses with naturally acquired and experimentally induced laminitis. Vet Immunol Immunopathol. 2009;129:221–230. doi: 10.1016/j.vetimm.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Faleiros RR, Johnson PJ, Nuovo GJ, et al. Laminar leukocyte accumulation in horses with carbohydrate overload-induced laminitis. J Vet Intern Med. 2011;25:107–115. doi: 10.1111/j.1939-1676.2010.0650.x. [DOI] [PubMed] [Google Scholar]
- 9.Loftus JP, Belknap JK, Black SJ. Matrix metalloproteinase-9 in laminae of black walnut extract treated horses correlates with neutrophils abundance. Vet Immunol Immunopathol. 2006;113:267–276. doi: 10.1016/j.vetimm.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Mungall BA, Pollitt CC. Zymographic analysis of equine laminitis. Histochem Cell Biol. 1999;112:467–472. doi: 10.1007/s004180050430. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto T, Akaike T, Sawa T, et al. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 12.Bannikov GA, Karelina TV, Collier IE, et al. Substrate binding of gelatinase B induces its enzymatic activity in the presence of intact propeptide. J Biol Chem. 2002;277:16022–16027. doi: 10.1074/jbc.M110931200. [DOI] [PubMed] [Google Scholar]
- 13.Yin C, Pettigrew A, Loftus JP, et al. Tissue concentrations of 4-HNE in the black walnut extract model of laminitis: indication of oxidant stress in affected laminae. Vet Immunol Immunopathol. 2009;129:211–215. doi: 10.1016/j.vetimm.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Coyne MJ, Cousin H, Loftus JP, et al. Cloning and expression of ADAM-related metalloproteases in equine laminitis. Vet Immunol Immunopathol. 2009;129:231–241. doi: 10.1016/j.vetimm.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawlak E, Wang L, Johnson PJ, et al. Distribution and processing of a disintegrin and metalloproteinase with thrombospondin motifs-4, aggrecan, versican, and hyaluronan in equine digital laminae. Am J Vet Res. 2012;73:xxx–xxx. doi: 10.2460/ajvr.73.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roughley PJ, Melching LI, Heathfield TF, et al. The structure and degradation of aggrecan in human intervertebral disc. Eur Spine J. 2006;15(suppl 3):S326–S332. doi: 10.1007/s00586-006-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricciardelli C, Sakko AJ, Ween MP, et al. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009;28:233–245. doi: 10.1007/s10555-009-9182-y. [DOI] [PubMed] [Google Scholar]
- 18.Salter RC, Ashlin TG, Kwan AP, et al. ADAMTS proteases: key roles in atherosclerosis? J Mol Med. 2010;88:1203–1211. doi: 10.1007/s00109-010-0654-x. [DOI] [PubMed] [Google Scholar]
- 19.Kiani C, Chen L, Wu YJ, et al. Structure and function of aggrecan. Cell Res. 2002;12:19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 20.Miwa HE, Gerken TA, Huynh TD, et al. Mammalian expression of full-length bovine aggrecan and link protein: formation of recombinant proteoglycan aggregates and analysis of proteolytic cleavage by ADAMTS-4 and MMP-13. Biochim Biophys Acta. 2006;1760:472–486. doi: 10.1016/j.bbagen.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 22.Sheng W, Wang G, La Pierre DP, et al. Versican mediates mesenchymal-epithelial transition. Mol Biol Cell. 2006;17:2009–2020. doi: 10.1091/mbc.E05-10-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garner HE, Coffman JR, Hahn AW, et al. Equine laminitis of alimentary origin: an experimental model. Am J Vet Res. 1975;36:441–444. [PubMed] [Google Scholar]
- 24.Johnson PJ, Kreeger JM, Keeler M, et al. Serum markers of lamellar basement membrane degradation and lamellar histopathological changes in horses affected with laminitis. Equine Vet J. 2000;32:462–468. doi: 10.2746/042516400777584695. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Tortorella MD, Arner EC, Hills R, et al. ADAMTS-4 (aggrecanase-1): N-terminal activation mechanisms. Arch Biochem Biophys. 2005;444:34–44. doi: 10.1016/j.abb.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Sandy JD, Neame PJ, Boynton RE, et al. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991;266:8683–8685. [PubMed] [Google Scholar]
- 28.Sandy JD, Westling J, Kenagy RD, et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- 29.Westling J, Gottschall PE, Thompson VP, et al. ADAMTS4 (aggrecanase-1) cleaves human brain versican V2 at Glu405-Gln406 to generate glial hyaluronate binding protein. Biochem J. 2004;377:787–795. doi: 10.1042/BJ20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollitt CC, Visser MB. Carbohydrate alimentary overload lamintis. Vet Clin North Am Equine Pract. 2010;26:65–78. doi: 10.1016/j.cveq.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Stamenkovic V, Keller G, Nesic D, et al. Neocartilage formation in 1g, stimulated, and microgravity environments: implication for tissue engineering. Tissue Eng Part A. 2010;16:1729–1736. doi: 10.1089/ten.tea.2008.0624. [DOI] [PubMed] [Google Scholar]
- 32.Corps AN, Robinson AH, Movin T, et al. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology (Oxford) 2006;45:291–294. doi: 10.1093/rheumatology/kei152. [DOI] [PubMed] [Google Scholar]
- 33.Ley C, Svala E, Nilton A, et al. Effects of high mobility group box protein-1, interleukin-1β, and interleukin-6 on cartilage matrix metabolism in three-dimensional equine chondrocyte cultures. Connect Tissue Res. 2011;52:290–300. doi: 10.3109/03008207.2010.523803. [DOI] [PubMed] [Google Scholar]
- 34.Kahari VM, Larjava H, Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-β1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem. 1991;266:10608–10615. [PubMed] [Google Scholar]
- 35.Kamitani S, Yamauchi Y, Kawasaki S, et al. Simultaneous stimulation with TGF-β1 and TNF-α induces epithelial mesenchymal transition in bronchial epithelial cells. Int Arch Allergy Immunol. 2010;155:119–128. doi: 10.1159/000318854. [DOI] [PubMed] [Google Scholar]
- 36.Corps AN, Jones GC, Harrall RL, et al. The regulation of aggrecanase ADAMTS-4 expression in human Achilles tendon and tendon-derived cells. Matrix Biol. 2008;27:393–401. doi: 10.1016/j.matbio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cross NA, Chandrasekharan S, Jokonya N, et al. The expression and regulation of ADAMTS-1, -4, -5, -9, and -15 and TIMP-3 by TGF-β1 in prostate cells: relevance to the accumulation of versican. Prostate. 2005;63:269–275. doi: 10.1002/pros.20182. [DOI] [PubMed] [Google Scholar]
- 38.Flannery CR, Zeng W, Corcoran C, et al. Autocatalytic cleavage of ADAMTS-4 (aggrecanase-1) reveals multiple glycosaminoglycan-binding sites. J Biol Chem. 2002;277:42775–42780. doi: 10.1074/jbc.M205309200. [DOI] [PubMed] [Google Scholar]
- 39.Kashiwagi M, Enghild JJ, Gendron C, et al. Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem. 2004;279:10109–10119. doi: 10.1074/jbc.M312123200. [DOI] [PubMed] [Google Scholar]
- 40.Gendron C, Kashiwagi M, Lim NH, et al. Proteolytic activities of human ADAMTS-5: comparative studies with ADAMTS-4. J Biol Chem. 2007;282:18294–18306. doi: 10.1074/jbc.M701523200. [DOI] [PubMed] [Google Scholar]
- 41.Porter S, Clark IM, Kevorkian L, et al. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenagy RD, Plaas AH, Wight TN. Versican degradation and vascular disease. Trends Cardiovasc Med. 2006;16:209–215. doi: 10.1016/j.tcm.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng W, Dong H, Lee DY, et al. Versican modulates gap junction intercellular communication. J Cell Physiol. 2007;211:213–219. doi: 10.1002/jcp.20921. [DOI] [PubMed] [Google Scholar]
- 44.Sheng W, Wang G, Wang Y, et al. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol Biol Cell. 2005;16:1330–1340. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]