Abstract
Hypocretin (Hcrt) has been implicated in the control of motor activity and in respiration and cardiovascular changes. Loss of Hcrt in narcolepsy is linked to sleepiness and to cataplexy, a sudden loss of muscle tone which is triggered by sudden strong emotions. In the current study, we have compared the effects of treadmill running to yard play on cerebrospinal fluid (CSF) Hcrt level in normal dogs. We find that treadmill locomotion, at a wide range of speeds, does not increase Hcrt level beyond baseline, whereas yard play produces a substantial increase in Hcrt, even though both activities produce comparable increases in heart rate, respiration and body temperature. We conclude that motor and cardiovascular changes are not sufficient to elevate CSF levels of Hcrt and we hypothesize that the emotional aspects of yard play account for the observed increase in Hcrt.
Keywords: REM sleep, Breathing canine
Introduction
Loss of hypocretin (Hcrt) neurons in humans (Peyron et al., 2000; Thannickal et al., 2000), mice (Chemelli et al., 1999) and rats (Beuckmann et al., 2004; Zhang et al., 2007) or a mutation of the Hcrt-2 receptor in dogs (Lin et al., 1999) causes narcolepsy with cataplexy. Although the link between narcolepsy and hypocretin deficiency is well established, the normal function of hypocretin is unclear. We found that Hcrt levels in the cerebrospinal fluid (CSF) are greatly elevated in both narcoleptic and normal Doberman pinchers after their daily exercise/play period in a large yard (Wu et al., 2002) and in cats during active waking periods maintained by human interaction (Kiyashchenko et al., 2002). During these periods, the electroencephalogram was activated and the amplitude of the electromyogram (EMG) and locomotor activity was greatly increased relative to that in baseline waking periods. It is not clear, however, if it is the motor activity per se or other changes correlated with motor activity during active waking induced the increase in Hcrt level. Studies in rodents, mostly mice, have indicated that Hcrt is crucial for the regulation of respiration (Zhang et al., 2005; Zhang et al., 2009), coordination of respiration with behavior (Corcoran et al., 2010; Kuwaki, 2010) and for long term facilitation of respiration (Terada et al., 2008). These studies have used ICV injection of Hcrt, Hcrt receptor 1 antagonists and Hcrt ataxin mutant and Hcrt knock out (KO) mice.
Other studies in rodents have concluded that Hcrt neurons have a major role in the regulation of blood pressure (BP) and heart rate (HR). Intracisternal Hcrt administration increases HR and BP (Hirota et al., 2003; Jochem et al., 2006; Huang et al., 2010) and microinjections into the nucleus of the solitary tract elicit dose dependent changes in HR and BP. Stimulation of the hypothalamic perifornical region in Hcrt-ataxin mice with depleted Hcrt produced smaller and shorter lasting increases in HR and BP than in control mice (Zhang et al., 2006). Hcrt KO mice were found to have elevated BP during sleep but normal waking BP in one study (Bastianini et al., 2011) but lower BP in another study (Kayaba et al., 2003), and reduced BP and HR responsiveness to stress (Kuwaki, 2011). Ataxin-Hcrt mutant mice were found to have lower BP than control mice in all sleep and quiet waking states (Schwimmer et al., 2010). Another study found a short term, but no long term, role of Hcrt in BP regulation (Lin et al., 2002). Administration of a Hcrt receptor 1 antagonist was found to decrease BP and HR (Hirota et al., 2003; Guo et al., 2010). Hcrt-deficient human narcoleptics were found to have elevated HR and increased BP variability (Fronczek et al., 2008). HR has been found to greatly decrease in both canine and human cataplexy (Siegel et al., 1989; Donadio et al., 2008).
Hcrt has also been implicated in thermoregulation. Administration of an Hcrt-r1 antagonist increased brown adipose tissue temperature. Hcrt KO mice have elevated body temperature during sleep (Mochizuki et al., 2006). In apparent contrast, injection of Hcrt-1 into the diagonal band of Broca increases body temperature (Monda et al., 2004).
A limitation of these studies is that many involved manipulation of the Hcrt system using systemic, ICV or local injection of Hcrt agonists or antagonists. Such manipulations are not likely to mimic the normal pattern of synaptic release of Hcrt. Another approach employed, was the analysis of cardio-respiratory changes in Hcrt KO mice or, in the case of humans, narcoleptics with a longterm absence of Hcrt. In these conditions, brain reorganization in response to the absence of Hcrt during development or after degenerative changes may alter cardiovascular and respiratory regulation. None of these studies monitored the levels of Hcrt in normal animals to provide a direct measurement of its release during behaviors that alter respiration or HR.
To clarify the underlying relations between exercise, emotion, respiration, cardiovascular changes and Hcrt elevation, we measured CSF Hcrt-1 level in normal Doberman pincher dogs allowed to play in a yard or trained to exercise on a treadmill. We varied the speed of the treadmill from zero m/min to the maximum rate that each dog was capable of following, to see if Hcrt-1 level changes with the intensity of locomotion. We measured the respiration, HR, BP and body temperature changes.
Methods
All procedures were approved by the Institutional Animal Care and Use Committees of the University of California, Los Angeles and the Veterans Administration Greater Los Angeles Healthcare System.
Treadmill exercise
Four normal Doberman pinschers, all male, between 2 and 4.4 years old, were used. The large cisterna magna of the dog makes it easy to extract sufficient volumes of CSF for radioimmunoassay. Furthermore, dogs can be readily trained to run at high speeds on a treadmill. The dogs were trained in steps to first walk and then run on a treadmill (Jog A Dog, Ottawa Lake, MI, USA). The maximum speed each dog was capable of walking or running was determined. The treadmill had a speed adjustable from 0 to 268 m/min.
The experiments were done between 8:00 AM and 1:00 PM. Each dog was run at the same time of a day within this period. First, they were taken to a cage (3 m × 3 m × 3 m) one hour before the start of treadmill procedure. Then, they were placed on a treadmill. On experimental days the dogs were subjected to one of four conditions: 1) standing on the treadmill for 30 min without walking or running, 2) walking for 30 min at a speed of 25 m/min, 3) walking at an average speed of 65 m/min, alternating between 50 and 80 m/min every min for 30 min, and 4) brisk walking and running at an average speed of 105 m/min for 30 min, alternating the speed among 80, 105 and 135 m/min every min). We varied running speeds within each speed group because we found that dogs performed more consistently if we varied treadmill speeds, whereas some balked and got off the treadmill when the higher speeds were maintained for as long as 30 min.
CSF was extracted on average 45 min after the end of each exercise condition (range 30–60 min). Three replications were done for each condition. Respiration was measured by visual observation of the chest and mouth. Rectal temperature, HR, BP and respiration were measured two times at a 2.5 min interval immediately before and after all conditions and the averages of the two replications for each subject were used for analysis. BP and HR were measured using an oscillometric non-invasive BP monitor (SurgiVet Model V60046, SurgiVet Inc. Waukesha, WI, USA) with a cuff on the limb. Systolic, diastolic and mean arterial pressure (MAP) measurements were recorded.
Yard play
Four normal Doberman pinschers, three male and one female, age between 2.4 and 5 years old, were used. Dogs were brought to a cage (3 m × 3 m × 3 m) in the laboratory one hour before the yard play/exercise. Then they were released and encouraged to run and play in an outdoor exercise area (26.7 m × 17.7 m) for 30 min with two other dogs. The exercise period occurred between 8:00 AM and 11:00 AM. No food was available during exercise or treadmill studies. The animals were brought back to the laboratory after exercise for CSF collection. For the baseline, no-exercise, trials the animals remained in the cage in a quiet waking state without the intervening yard activity. The animals were continuously monitored to assure that they remained awake for the entire 1.5 h period in all baseline and exercise conditions. Each dog was tested on one condition a week.
CSF collection
Two ml of cerebrospinal fluid (CSF) was collected from the cisterna magna using a 22 G spinal needle while the animal was under thiopental anesthesia (12.5 mg/kg, IV). The samples were frozen immediately on dry ice and stored in a −20°C freezer until analysis.
Hypocretin assay
The procedures for the assay for hypocretin have been described previously (Wu et al., 2002). Samples (0.5 ml) were acidified with 1% trifluoracetic acid (TFA) and loaded onto a C18 SEP-Column (Waters, Milford, MA). The peptide was eluted with 1% TFA/40% acetonitrile. The eluant was then dried and resuspended in radioimmunoassay (RIA) buffer. The solid-phase RIA (Maidment and Evans, 1991) provided an IC50 of 2–3 fmol and a limit of detection of ~0.1 fmol. The Hcrt-1, iodinated Hcrt-1, and Hcrt-1 antiserum were obtained from Phoenix Pharmaceuticals (Cat. #RK-003-30; Belmont, CA).
Data analysis
All data are presented as mean ± SEM. CSF Hcrt-1 levels after the treadmill conditions were compared with their corresponding values taken under baseline conditions. The dependent t-test was used to test for statistical analysis. Because all three measures of BP are highly correlated, only the analysis of MAP is presented. Data from the treadmill experiments were subjected to either t-test with Bonferroni correction or analysis of variance (ANOVA) followed by Tukey post-hoc comparisons. All samples used in Fig. 1A were analyzed together. Similarly all samples used in Fig. 1B were analyzed together. All RIA analyses were done blind to the animals exercise/play condition.
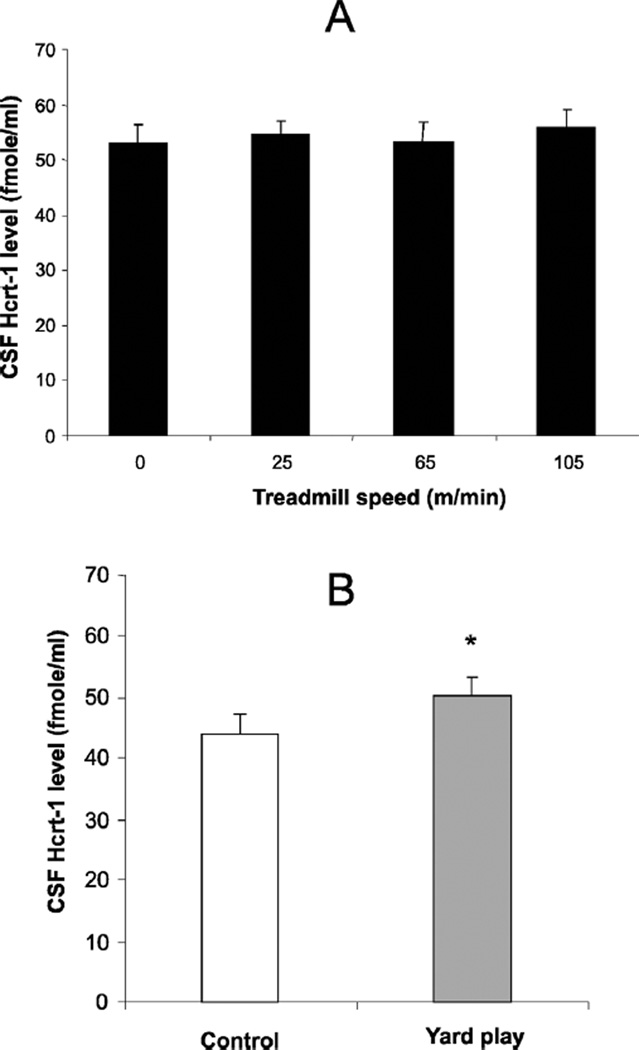
Fig. 1.
(A) CSF Hcrt-1 levels were not significantly elevated after maximal speed treadmill exercise (105 m/min) compared to level when standing without moving on the treadmill (0) or at intermediate levels of exercise. (B). Hcrt levels were significantly elevated after 30 min of yard play. *P < 0.02, t-test comparing to control condition.
Results
Treadmill and yard play exercise
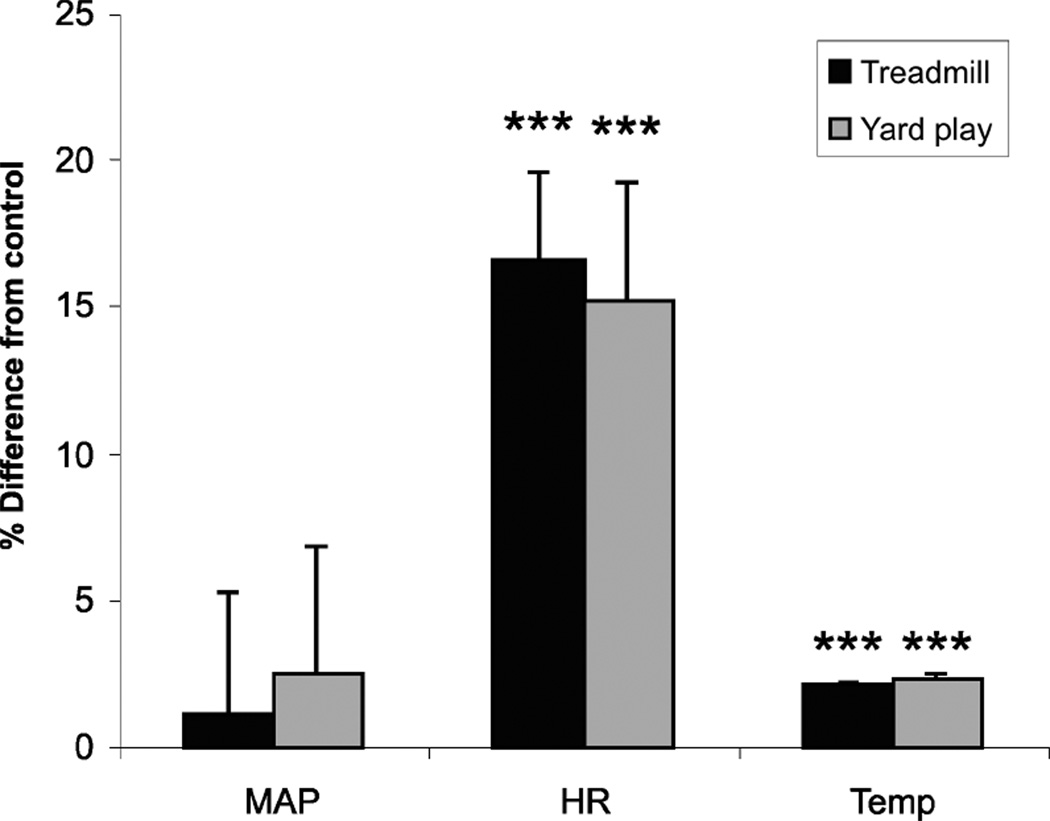
There was no significant change in CSF Hcrt-1 levels after treadmill exercise across different speeds as compared to the no-exercise control condition. The mean level in the exercise conditions were within 2.7 ± 0.9% of baseline levels (Fig. 1A). On the other hand, 30 min of playing in the yard significantly increased Hcrt levels in the CSF by 17% as compared to the baseline condition (Fig. 1B). At these animals’ maximum treadmill speeds, HR and body temperature were significantly elevated but BP was not significantly changed (Fig. 2). Similarly, both HR and temperature were significantly elevated after 30 min of yard play, but BP did not change significantly (Fig. 2). Respiration rate, which was 37 breaths/minute after being on the treadmill at 0 m/min for 30 min, increased by 9% after 30 min in the 25 m/min condition, 48% after the 65 m/min condition and 293% after the 105 m/min condition.
Fig. 2.
Exercise and play conditions significantly increased heart rate and body temperature in both treadmill and yard play conditions, but only increased Hcrt level in the yard play condition (Fig. 1). Percent changes of the mean arterial blood pressure (MAP), heart rate (HR) and core body temperature (Temp) from control condition after 30 min of treadmill exercise at individual animal’s maximum speed (Treadmill) or after 30 min of play in the yard (Yard play). ***P < 0.0001, t-test. Baseline BP (mmHg) was 117.9 ± 5.6 for treadmill, 120.4 ± 3.1 for yard play; HR (beats/min) baseline was 107.8 ± 5.0 for treadmill, 115.5 ± 3.3 for yard, Temp (°C) baseline was 38.3 ± 0.1 for treadmill, 38.4 ± 0.1 for yard play.
Discussion
In a previous study we reported that Hcrt-1 levels in dogs were significantly increased by yard play (Wu et al., 2002), suggesting that the hypocretin system might be activated by motor activity. In the present study, however, we found that intense locomotor activity alone on a treadmill did not change CSF Hcrt-1 level compared to the control condition of simply standing on the treadmill, despite major changes in activity level, respiration, HR and body temperature. Clearly, changes in Hcrt release, as assessed by CSF level, are not required to mediate these phenomena. This contrasts with prior studies implicating Hcrt in mediating cardiovascular changes. However, these prior conclusions were all based on manipulations of the Hcrt system or examination of mutants rather than recordings of Hcrt release. Our results do not rule out the possibility of smaller changes that might not be detectable in the CSF. But they indicate that the magnitude of any such changes are significantly less than those produced by yard play. Furthermore our results show that great differences in locomotor speed, and the associated correlated changes in respiratory and cardiovascular activity during treadmill locomotion, produce no detectable change in CSF Hcrt level.
The emotional excitement that comes with social interaction with other dogs in the yard contrasts with the monotonous physical movement on the treadmill. Hypocretin neurons are heavily innervated by the amygdala and cholinergic arousal-related basal forebrain (Sakurai et al., 2005; Yoshida et al., 2006). They are activated by anticipation of positive reinforcements, such as food and opiate drugs (Boutrel et al., 2005; Harris et al., 2005; Mileykovskiy et al., 2005; Borgland et al., 2006), and during spontaneous wheel running (Anaclet et al., 2009; Furlong et al., 2009).
Heightened, and most often positive, emotions trigger cataplexy in Hcrt-deficient narcoleptic humans and animals (Guilleminault, 1976; Lin et al., 1999; Mitler et al., 1976). In humans, laughter is the most common trigger. During the yard play period, the dogs were free to engage in any activity, including digging holes in the grass, playing with toys and interacting with other dogs. When cages were opened for the daily exercise period, the dogs would run to the yard. In contrast, they generally had to be coaxed to go to the treadmill room. This behavior suggests that they enjoyed the free play more than the treadmill locomotion. However, once they were on the treadmill, they ran and elevated autonomic measures to levels comparable to those achieved during the yard play.
In conclusion, the present experiments show that motor activity and the associated autonomic and respiratory changes do not alter Hcrt level. We hypothesize that activity during positive emotions, of the sort that the dogs experienced during play behavior, is responsible for the increased Hcrt release we observed.
Acknowledgments
Supported by MH64109, NS14610 and the Medical Research Service of the Department of Veterans Affairs
References
- Anaclet C, Parmentier R, Ouk K, Guidon G, Buda C, Sastre JP, Akaoka H, Sergeeva OA, Yanagisawa M, Ohtsu H, Franco P, Haas H, Lin JS. Orexin/hypocretin and histamine: Distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J. Neurosci. 2009;29:14423–14438. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianini S, Silvani A, Berteotti C, Elghozi JL, Franzini C, Lenzi P, Lo MV, Zoccoli G. Sleep related changes in blood pressure in hypocretin-deficient narcoleptic mice. Sleep. 2011;34:213–218. doi: 10.1093/sleep/34.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckmann CT, Sinton CM, Williams SC, Richardson JA, Hammer RE, Sakurai T, Yanagisawa M. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J. Neurosci. 2004;24:4469–4477. doi: 10.1523/JNEUROSCI.5560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc. Natl. Acad. Sci. USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Corcoran A, Richerson G, Harris M. Modulation of respiratory activity by hypocretin-1 (orexin A) in situ and in vitro. Adv. Exp. Med. Biol. 2010;669:109–113. doi: 10.1007/978-1-4419-5692-7_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio V, Plazzi G, Vandi S, Franceschini C, Karlsson T, Montagna P, Vetrugno R, Bugiardini E, Mignot E, Liguori R. Sympathetic and cardiovascular activity during cataplexy in narcolepsy. J. Sleep Res. 2008;17:458–463. doi: 10.1111/j.1365-2869.2008.00682.x. [DOI] [PubMed] [Google Scholar]
- Fronczek R, Overeem S, Reijntjes R, Lammers GJ, van Dijk JG, Pijl H. Increased heart rate variability but normal resting metabolic rate in hypocretin/orexin-deficient human narcolepsy. J. Clin. Sleep Med. 2008;4:248–254. [PMC free article] [PubMed] [Google Scholar]
- Furlong TM, Vianna DML, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur. J. Neurosci. 2009;30:1603–1614. doi: 10.1111/j.1460-9568.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- Guilleminault C. Cataplexy. In: Guilleminault C, Dement WC, Passouant P, editors. Narcolepsy. New York: Spectrum; 1976. pp. 125–143. [Google Scholar]
- Guo BQ, Jia M, Liu JX, Zhang Z. Cardiovascular effect of intracerebroventricular injection of orexin-1 receptor antagonist in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2010;26:278–283. [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hirota K, Kushikata T, Kudo M, Kudo T, Smart D, Matsuki A. Effects of central hypocretin-1 administration on hemodynamic responses in young-adult and middle-aged rats. Brain Res. 2003;981:143–150. doi: 10.1016/s0006-8993(03)03002-6. [DOI] [PubMed] [Google Scholar]
- Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J. Pharmacol. Exp. Ther. 2010;334:522–529. doi: 10.1124/jpet.110.167791. [DOI] [PubMed] [Google Scholar]
- Jochem J, Zwirska-Korczala K, Zabielski R, Kato I, Kuwahara A. Cardiovascular effects of centrally acting orexin A in haemorrhage-shocked rats. J. Physiol. Pharmacol. 2006;57(Suppl 11):115–124. [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R581–R593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J. Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki T. Hypothalamic modulation of breathing. Adv. Exp. Med. Biol. 2010;669:243–247. doi: 10.1007/978-1-4419-5692-7_49. [DOI] [PubMed] [Google Scholar]
- Kuwaki T. Orexin links emotional stress to autonomic functions. Auton. Neurosci. 2011;161:20–27. doi: 10.1016/j.autneu.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Kadotani H, Rogers W, Lin X, Qui X, de Jong P, Nishino S, Mignot E. The REM sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lin Y, Matsumura K, Tsuchihashi T, Abe I, Iida M. Chronic central infusion of orexin-A increases arterial pressure in rats. Brain Res. Bull. 2002;57:619–622. doi: 10.1016/s0361-9230(01)00756-0. [DOI] [PubMed] [Google Scholar]
- Maidment NT, Evans CJ. Measurement of extra-cellular neuropeptides in the brain: Microdialysis linked to solid phase radioimmunoassays with subfemptomole limits of detection. In: Robinson TE, Justice JB, editors. Microdialysis in the Neurosciences. Amsterdam: Elsevier; 1991. pp. 275–303. [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/ orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler MM, Soave O, Dement WC. Narcolepsy in seven dogs. J. Am. Vet. Med. Ass. 1976;168:1036–1038. [PubMed] [Google Scholar]
- Mochizuki T, Klerman EB, Sakurai T, Scammell TE. Elevated body temperature during sleep in orexin knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R533–R540. doi: 10.1152/ajpregu.00887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monda M, Viggiano A, Viggiano A, Fuccio F, De Luca V. Injection of orexin A into the diagonal band of Broca induces sympathetic and hyperthermic reactions. Brain Res. 2004;1018:265–271. doi: 10.1016/j.brainres.2004.05.084. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Schwimmer H, Stauss HM, Abboud F, Nishino S, Mignot E, Zeitzer JM. Effects of sleep on the cardiovascular and thermoregulatory systems: a possible role for hypocretins. J. Appl. Physiol. 2010;109:1053–1063. doi: 10.1152/japplphysiol.00516.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Tomaszewski KS, Fahringer H, Cave G, Kilduff T, Dement C. Heart rate and blood pressure changes during sleep-waking cycles and cataplexy in narcoleptic dogs. Am. J. Physiol. 1989;256:H111–H119. doi: 10.1152/ajpheart.1989.256.1.H111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J. Appl. Physiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, John J, Maidment N, Lam HA, Siegel JM. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R1079–R1086. doi: 10.1152/ajpregu.00207.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J. Comp. Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci. Lett. 2005;385:131–136. doi: 10.1016/j.neulet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sakurai T, Fukuda Y, Kuwaki T. Orexin neuron-mediated skeletal muscle vasodilation and shift of baroreflex during defense response in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1654–R1663. doi: 10.1152/ajpregu.00704.2005. [DOI] [PubMed] [Google Scholar]
- Zhang S, Lin L, Kaur S, Thankachan S, Blanco-Centurion C, Yanagisawa M, Mignot E, Shiromani PJ. The development of hypocretin (OREXIN) deficiency in hypocretin/ataxin-3 transgenic rats. Neuroscience. 2007;148:34–43. doi: 10.1016/j.neuroscience.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhang N, Sakurai T, Kuwaki T. Orexin neurons in the hypothalamus mediate cardiorespiratory responses induced by disinhibition of the amygdala and bed nucleus of the stria terminalis. Brain Res. 2009;1262:25–37. doi: 10.1016/j.brainres.2009.01.022. [DOI] [PubMed] [Google Scholar]