Abstract
Background
We sought to examine how patients’ treatment decisions incorporate potentially conflicting information from standard clinical indicators (e.g., tumor size) and genomic tests for breast cancer recurrence risk.
Methods
Participants were 77 early stage breast cancer survivors who previously received genomic testing. They read six hypothetical vignettes that varied recurrence risk indicated by standard tests (low or high risk) coupled with the genomic test (low, intermediate or high risk). For each vignette, women reported their perceived recurrence risk and treatment preferences.
Results
Test results indicating high recurrence risk increased perception of risk and preference for chemotherapy (p<.001 for all). Perceived risk explained (i.e., mediated) the effect of test results on chemotherapy preferences. When test results conflicted, women gave more weight to genomic over standard test results.
Discussion
Hypothetical genomic test results had the intended effect of influencing women’s perceptions of recurrence risk and interest in chemotherapy.
Keywords: Risk Recurrence, Genomics, Oncotype DX, Chemotherapy, Patient Decision Making
As genomic testing becomes more common in clinical care for cancer (Morris & Carey, 2007), understanding how patients use information from these tests has increased in importance. One such genomic test, Oncotype DX, predicts 10 year risk of distant recurrence among early stage breast cancer patients (estrogen-receptor positive). The test examines the activity of 21 genes in breast tumors (Paik et al., 2004; Sparano et al., 2008) and offers a more precise risk estimate than from standard methods for assessing recurrence risk (such as tumor size and grade)(Albain et al., 2010; Albain et al., 2010; Dowsett et al., 2010; Habel et al., 2006; Paik et al., 2006). Test results can help women and their doctors make decisions about whether to include chemotherapy in their treatment plans (Gianni et al., 2005). Women with high recurrence risk may opt for more aggressive treatment (i.e., adjuvant chemotherapy), whereas those with low recurrence risk may forgo such treatment and its potential side effects. The American Society of Clinical Oncology recently endorsed Oncotype DX as part of their clinical guidelines (Harris et al., 2007).
While genomic tests provide potentially useful information for guiding treatment decisions (Lo et al., 2010; Oratz et al., 2007; Oratz et al., 2011; Tzeng et al., 2010), their results can conflict with information from standard clinical indicators of recurrence risk (which we will call “standard tests”, for the sake of simplicity). Oncotype DX reports a low risk result for between 33% and 50% of women with early stage breast cancer whose standard test results suggest are at high risk for recurrence (Paik, 2007; Van De Vijver et al., 2002). Recategorizing from high to low risk is likely more common in the US where different clinical criteria are used to determine risk compared to the St. Gallen approach commonly used in Europe (Van De Vijver et al., 2002).
Our previous research, which used hypothetical recurrence risk information to understand how breast cancer patients incorporate potentially conflicting test results, suggested that some would be reluctant to forgo chemotherapy if genomic tests indicated low risk and standard tests indicated high risk(Brewer et al., 2009). This finding suggested that one of the main promises of genomic testing, allowing many women to safely forgo unnecessary treatment, would not be fulfilled. However, previous research predated common clinical use, and patient familiarity with genomics and these assays’ findings could differ today (O’Neill et al., 2007). Furthermore, the previous study did not assess the role of perceived risk in these treatment decisions. Presumably, high recurrence risk test results influence patients’ beliefs about recurrence, which then alter treatment choices (Brewer et al., 2007), but this hypothesis has never been tested.
The present study extends our previous work by examining effects of hypothetical genomic and standard test results among a sample of patients who received the Oncotype DX test as part of clinical care for early-stage breast cancer. We hypothesized that results indicating higher risk of recurrence would increase women’s interest in chemotherapy. Furthermore, we hypothesized that this association would be due to increases in women’s estimates of their chances of having breast cancer return (i.e., mediation). Given that genomic testing is performed in hormone receptor-positive breast cancer, and the results assume receipt of adjuvant antiestrogen therapy, testing cannot meaningfully inform hormone therapy decisions. Thus, we hypothesized that standard, but not genomic tests, indicating higher risk would increase interest in hormone therapy. Finally, we hypothesized that information from genomic tests would have a stronger impact on women’s treatment preferences when results conflicted with the standard tests, because of a growing emphasis on genomic medicine in clinical practice and the media (Guttmacher et al., 2002) and patients’ strong interest in testing (O’Neill et al., 2007).
METHODS
Participants and Procedure
Eligible participants were women previously treated for early-stage breast cancer at the University of North Carolina Breast Center (Chapel Hill, NC), whose medical records indicated they received the Oncotype DX test between when it first became available in June 2004 through October 2008. Women eligible for Oncotype DX had stage I or II, node-negative, hormone-receptor positive breast cancer; we also included one woman with node-positive breast cancer, because the test validation includes some women with node-positive breast cancers. Exclusion criteria were not being English-speaking or under age 18.
Researchers mailed study questionnaires between December 2008 and February 2009. Women could complete a HIPAA authorization form to allow a review of their medical records of cancer diagnosis and treatment. Women received a $15 incentive and up to two follow-up letters to maximize response rates. The institutional review board of the University of North Carolina approved the study protocol and materials.
Measures
We used hypothetical vignettes to assess potential responses to genomic and standard testing for breast cancer recurrence risk. Use of hypothetical vignettes is a well-accepted methodology for understanding response to new cancer testing when information on actual behavior is not available (Brewer et al., 2009; Lerman et al., 2000) or not easily recalled.
Standard and genomic test results
The study used a 2 × 3 within-subjects design that varied hypothetical breast cancer recurrence risk from standard tests (low or high risk) combined with those from genomic tests (low, intermediate, or high risk). Thus, all participants received six vignettes: (1) standard and genomic tests both showed low recurrence risk, (2) both tests showed high risk, (3) standard showed high risk and genomic showed intermediate risk, (4) standard showed high risk and genomic showed low risk, (5) standard showed low risk and genomic showed intermediate risk, and (6) standard showed low risk and genomic showed high risk. We presented these six vignettes as a grid where the first column presented the standard test result and the second column presented the corresponding genomic test result. Researchers randomly assigned participants to receive the vignettes in one of four counterbalanced orders. One order started with the vignette that had low genomic and low standard recurrence risk results (low-low), followed by low-intermediate, low-high, high-low, high-intermediate, and high-high. A second order started with the vignette that had low genomic and high standard recurrence risk results (low-high), followed by low-intermediate, low-low, high-high, high-intermediate, and high-low. The third and fourth orders were the reverse of the first and second orders, respectively
Perceived risk and treatment
For each of the six vignettes respondents estimated their hypothetical risk of breast cancer recurrence (which we refer to as “perceived risk”) from 0 to 100% and indicated their hypothetical treatment preference (chemotherapy, hormone therapy, both or neither). We created two treatment preference variables to reflect whether participants preferred chemotherapy treatment (yes or no) and preferred hormone treatment (yes or no).
Attitudes toward tests
Two items assessed whether women felt their actual standard and genomic test results could be trusted and were accurate. The 5-point response scale ranged from “strongly disagree” to “strongly agree”, with higher scores reflecting higher trust.
Participant demographic and medical characteristics
The survey assessed demographic characteristics including age, race, income, time since cancer diagnosis, and treatments received. One item assessed whether patients’ recalled that their actual standard and genomic tests produced similar results (“yes”, “no” or “don’t know”). An item assessed how often respondents needed help with written health-related materials; we refer to this as literacy though we acknowledge that this single item measure may also tap other constructs. Response options ranged from “never” to “always.” We assessed numeracy with a 3-item scale (range 0–3 correct answers)(Schwartz et al., 1997).
We reviewed medical records of participants who signed HIPAA authorization forms (87%) to confirm information on cancer treatments and to document actual recurrence risk using two methods. The first method used women’s actual Oncotype DX scores that yielded a recurrence score corresponding to a 10-year distant recurrence risk estimate (0–100%). Recurrence risks were “low” if estimates were about 11% or less, “intermediate” for estimates between 12% and 21%, and “high” for estimates greater than 21% as they appear in Oncotype DX reports. We also calculated women’s 10-year risk for breast cancer recurrence using Adjuvant! Online using standard clinical information (e.g., grade, tumor size) only.
Data analyses
The main outcome measures were perceived risk and treatment preferences. We used generalized estimating equations, which accounted for repeated measurements, to examine whether hypothetical standard test results, genomic test results and their interaction predicted these two outcomes. We also examined whether perceived risk mediated the association of hypothetical test results to chemotherapy treatment preferences (Baron et al., 1986). GEE analyses yielded test statistics that follow a Z distribution. The Z statistic can be interpreted as the chance difference in standard error units between the observed effect parameter and the hypothesized null value if the null were true (Stokes et al., 2000); Z statistics smaller than −1.96 and larger than 1.96 are considered statistically significant in our study. As preliminary analyses showed that the order in which patients received hypothetical genomic test results (e.g., whether high risk results came first) influenced perceived risk of recurrence (p=.003), analyses controlled for vignette order. We used post-hoc, paired t-tests and McNemar’s test to compare responses when hypothetical standard and genomic test results were discordant. We conducted analyses in SAS v 9.2 (SAS Institute Inc., Cary, NC, USA) using two-tailed tests and a critical alpha of .05.
RESULTS
Of 104 women we invited to participate, 78 completed surveys (response rate = 75%). We excluded data from one participant who had incomplete survey data on treatment preferences, resulting in a final sample of 77. Women’s mean age was 58 years (range 38–83) (Table 1). Most were White (81%) and had health insurance (95%). Most women had high literacy (81%), but fewer (31%) answered all numeracy items correctly. The average time since cancer diagnosis was 17 months (range 4–54) with the majority of women (78%) having been diagnosed within two years of the study. Most women had received hormone therapy treatment (92%), and one-third (33%) had received chemotherapy.
Table 1.
Demographic and medical characteristics
| %
|
|
|---|---|
| Age, mean | 58 |
| White race/ethnicity | 81 |
| Married or living as married | 74 |
| College degree or more | 55 |
| Annual household income, > $60,000 | 60 |
| Insured | 95 |
| High literacy (never/rarely needs help) | 81 |
| High numeracy (all 3 answers correct) | 31 |
| Time since diagnosis, mean (months) | 17 |
| Treatment received | |
| Hormone therapy | 92 |
| Radiation | 61 |
| Chemotherapy | 33 |
| Oncotype DX risk category* | |
| Low (<12%) | 51 |
| Intermediate (12–21%) | 37 |
| High (>21%) | 12 |
| Conflicting test results | |
| Unsure/do not remember | 54 |
| Results were the same | 29 |
| Results were discordant | 17 |
Notes. n=77, except variables with missing data: age (n=75), insurance status (n=76), Oncotype risk category (n=67) due to women who did not give permission to review their medical records, and conflicting test results (n=76).
Actual Test Results
During previous clinical care, women received Oncotype DX test results that indicated a mean recurrence risk of 12% (range 4% to 32%). Mean risk estimates based on standard clinical parameters that we integrated using Adjuvant! Online were 24% (range 15% to 54%). This difference was statistically significant (p=<.001). About 82% (55/67) of genomic test results were lower by at least 5 percentage points than estimates based on standard test results. About 17% of women recalled that their standard and genomic tests yielded different recurrence risk estimates; 29% recalled that their test results produced similar results; while 54% of women were unsure or could not recall if their results were discordant.
Women trusted their genomic test results more than standard test results (mean scores 3.96, SD=.70 and 3.71, SD=.81 respectively, p=.03). Women also thought genomic test results were more accurate than standard test results (mean scores 3.89, SD=.73 and 3.70, SD=.83 respectively, p=.04). Trust and accuracy were highly correlated for both genomic (r=.86, p<.001) and standard tests (r=.74, p<.001).
Hypothetical Test Results
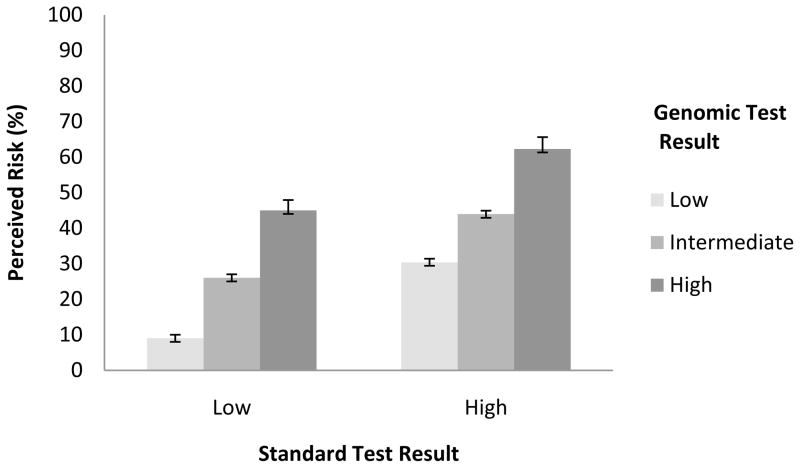
Hypothetical standard and genomic tests that reported results with higher risk magnitudes led women to state higher perceived risks of cancer recurrence (Figure 1) (z = 11.58 and 14.71 respectively, both p <.001). However, the association of genomic test results with perceived risk did not vary by standard test results (p =.11 for interaction)
Figure 1. Perceived Recurrence Risk.
Bars depict standard errors
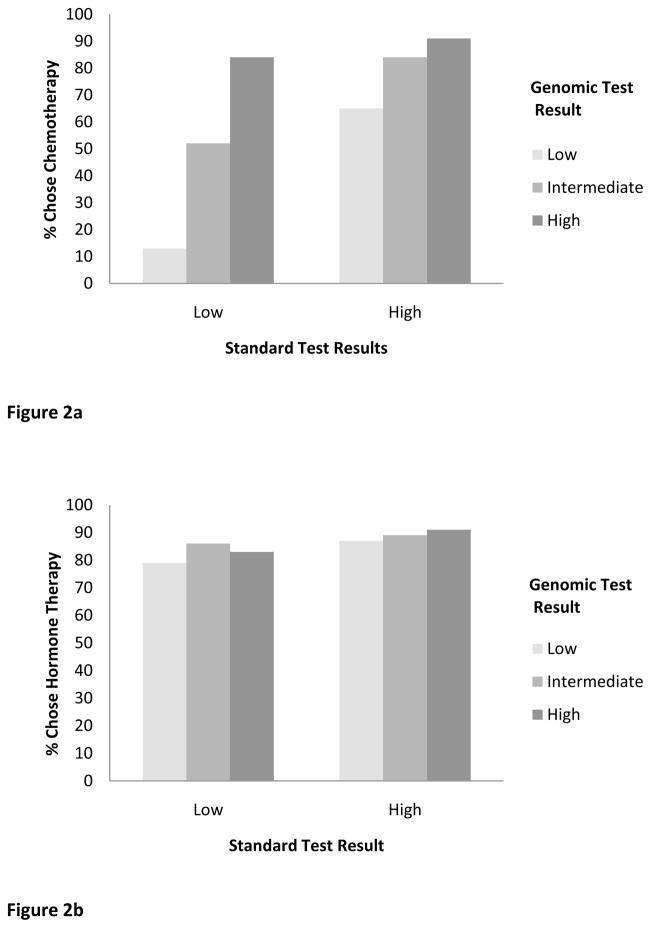
Hypothetical standard and genomic test results indicating higher risk increased preference for chemotherapy treatment (z = 6.10 and 6.65 respectively, both p <.001) (Figure 2a). The effect of genomic test results on chemotherapy preferences varied by standard test results (p <.001 for interaction). Post-hoc tests revealed that, when standard test results indicated low risk, results of genomic tests had a strong effect on chemotherapy preferences (z = 8.00, p <.001) (represented by the slope of bars 1–3, Figure 2a). When standard test results indicated high risk, interest in chemotherapy was generally high, and genomic test results had a smaller effect on chemotherapy preferences (z = 4.42, p <.001) (represented by the slope of bars 4–6, Figure 2a).
Figure 2.
Figure 2a. Chemotherapy Treatment Preference.
Figure 2b. Hormone Therapy Treatment Preference.
Hypothetical standard test results reflecting higher risk increased preference for hormone therapy treatment (z = 2.33, p =.02) (Figure 2b). However, genomic test results did not affect preference for hormone therapy (z = 1.11, p =.27) nor was the interaction between standard and genomic test results statistically significant (p=.64).
Mediation
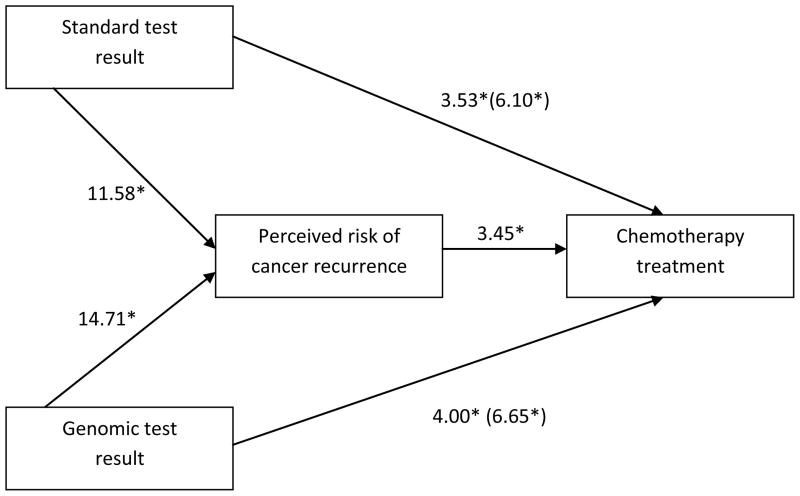
We conducted mediation analyses to assess whether perceived risk was the mechanism through which hypothetical test results influenced hypothetical preferences for chemotherapy treatment. As previously reported, standard and genomic test results indicating higher recurrence risk increased perceived risk of recurrence and preference for chemotherapy. Higher perceived risk was associated with a higher likelihood of opting for chemotherapy treatment, controlling for test results (p<.001). Effects of standard and genomic test results on chemotherapy preferences were attenuated when controlling for perceived risk (z values dropped from 6.10 to 3.53 and 6.65 to 4.00 respectively), suggesting partial mediation (Figure 3). Thus, genomic and standard test results indicating higher recurrence risk lead to greater perceived risk, which in turn, lead to an increased likelihood of opting for chemotherapy treatment. We did not conduct mediation analyses for interest in hormone treatment due to lack of association with genomic test results.
Figure 3. Perceived risk as mediator.
Values are z statistics from generalized estimable models.
*p<.001
Moderation
Effects of hypothetical standard and genomic test results on hypothetical treatment preferences were not modified by literacy, numeracy, trust, perceived accuracy, whether patients received actual chemotherapy treatment, or patients’ actual recurrence risk based on Oncotype DX and Adjuvant! Online scores (all p>.05). As half (54%) of patients could not recall whether their actual results were similar or discordant, we did not examine this variable as a potential moderator.
Discordant Test Results
A critical question concerned whether women gave more weight to standard or genomic tests when hypothetical results were discordant. To address this question, we compared risk perceptions and chemotherapy preferences in the two “mismatched” results conditions (i.e., a low risk result from the standard test coupled with a high risk result from the genomic test and vice versa). Mean perceived recurrence risk was 45% when standard test results were low but genomic test results were high, higher than the mean of 30% when standard tests were high but genomic tests were low (p <.001, represented by bars 3 and 4 respectively, Figure 1). Interest in chemotherapy also differed when discordant genomic rather than standard tests showed high risk (84% vs. 65%, p <.001; bars 3 and 4 of Figure 2a).
DISCUSSION
Genomic testing for breast cancer recurrence risk is becoming an increasingly important component of cancer care in the age of personalized medicine. Test results can inform women and their doctors of the likelihood of cancer recurrence, information that they can use to make decisions about inclusion of chemotherapy treatment in addition to hormone therapy. As we predicted, hypothetical genomic tests had the intended effect of influencing women’s perceptions of recurrence risk, which in turn, informed hypothetical decisions to undergo or forgo chemotherapy treatment. These findings corroborate our previous finding that most women understand that genomic test results provide information about the potential for recurrence and that results can help inform treatment decisions (Brewer et al., 2009; Richman et al., 2011).
Women’s receptivity to this risk information replicates our previous work showing that early stage breast cancer patients’ recurrence risk beliefs corresponded closely to their actual Oncotype DX results (Tzeng et al., 2010). These findings differ markedly from previous work on communicating with women about BRCA 1/2 mutations that found that women resisted information on breast cancer risk (Gurmankin et al., 2005). It may be that women think differently about information relevant to treatment decisions (e.g., Oncotype DX) than they do about risk for future disease (e.g., BRCA 1/2), a topic that merits additional exploration in the same study.
As expected, hypothetical genomic test results had no influence on preference for hormone therapy. The purpose of genomic tests is to inform decisions as they relate to chemotherapy treatment, and thus results should not influence decisions to take hormone therapy. However, differences in standard test results did inform preferences for hormone therapy. While this pattern of findings suggests that women understand the specificity of meaning of high genomic recurrence risk for treatment, the uniformly high interest in hormone therapy may have obscured any differences.
About half of women in our study could not recall or were unsure whether their actual standard and genomic test results received during clinical care were discordant. Women’s limited ability to recall discordance of test results could be due to time since cancer diagnosis which was 17 months on average, making details regarding similarity between standard and genomic test results less salient. Low recall of whether actual results were discordant might also indicate that some women did not understand what they were told about their recurrence risk based on either test. Our previous research showed that although many early stage breast cancer patients said they understood discussions about their genomic test results, a third reported not fully understanding these discussions (Tzeng et al., 2010). Furthermore, while recalled recurrence risk was highly concordant with the results from Oncotype DX, a third could not recall their recurrence risk result (Tzeng et al., 2010).
Women in our study placed more weight on hypothetical genomic over standard test results when the results conflicted. Women also perceived genomic tests as more accurate and trustworthy compared to standard tests. These findings imply that one of the main promises of genomic testing for patient decisions–helping some women to choose to forgo chemotherapy and its potential side effects–is being realized and that women place high trust in their test results. Women in our study were more willing to incorporate information from genomic tests when standard test results indicate low rather than high recurrence risk. Women still placed emphasis on standard clinic markers for recurrence (i.e., tumor size etc.) and were less willing to forgo chemotherapy when these test results suggest that they are at a higher risk of recurrence.
Strengths of the study are the inclusion of patients who received the Oncotype DX test–the population for whom the findings are clinically relevant–and a high response rate. A limitation is that participants were mostly Caucasian, well-educated and insured women who received medical care from the same institution. The generalizability of our findings to more diverse populations remains to be established. Future research on women’s reactions to genomic testing should include socio-economically diverse samples from multiple clinics. Our findings did not vary by factors such as demographic characteristics and numeracy. However, given our modest sample size, we view these moderation analyses as exploratory. We also acknowledge that results from our hypothetical information may differ from real life treatment decisions that happen with support and advice from a clinical care team. As growing numbers of women undergo genomic testing, it will be important to replicate findings from our survey with real treatment decisions.
Nonetheless, these findings suggest that many women understand the benefit of genomic testing and properly use their results to inform treatment decisions. While many women will be reluctant to forgo chemotherapy when standard test results indicate they are at risk of recurrence, a sizable proportion reported they would be willing to do without this treatment based on results of their genomic tests.
Acknowledgments
We are grateful to the physicians and nurses of the University of North Carolina Breast Center for their assistance with this study. Most importantly, we thank the women who participated in this study. We thank Alice Richman and Janice Tzeng for their work on the study. The study received generous financial support from the American Cancer Society (MSRG-06-259-01-CPPB). Jessica T. DeFrank was funded by the UNC Cancer Care and Quality Training Program (NCI R25 Grant, CA116339).
Footnotes
The authors have no financial disclosures or conflicts of interest to declare.
References
- Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. The Lancet Oncology. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Cuite CL, Herrington JE, Weinstein ND. Risk compensation and vaccination: Can getting vaccinated cause people to engage in risky behaviors? Annals of Behavioral Medicine. 2007;34:95–99. doi: 10.1007/BF02879925. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Edwards AS, O’Neill SC, Tzeng JP, Carey LA, Rimer BK. When genomic and standard test results diverge: Implications for breast cancer patients’ preference for chemotherapy. Breast Cancer Research and Treatment. 2009;117:25–29. doi: 10.1007/s10549-008-0175-2. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. Journal of Clinical Oncology. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- Gianni L, Zambetti M, Clark K, Baker J, Cronin M, Wu J, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. Journal of Clinical Oncology. 2005;23:7265–7277. doi: 10.1200/JCO.2005.02.0818. [DOI] [PubMed] [Google Scholar]
- Gurmankin AD, Domchek S, Stopfer J, Fels C, Armstrong K. Patients’ resistance to risk information in genetic counseling for BRCA1/2. Archives of Internal Medicine. 2005;165:523–529. doi: 10.1001/archinte.165.5.523. [DOI] [PubMed] [Google Scholar]
- Guttmacher AE, Collins FS, Guttmacher AE, Collins FS. Genomic medicine—a primer. New England Journal of Medicine. 2002;347:1512–1520. doi: 10.1056/NEJMra012240. [DOI] [PubMed] [Google Scholar]
- Habel LA, Shak S, Jacobs MK, Capra A, Alexander C, Pho M, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Research. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. Journal of Clinical Oncology. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- Lerman C, Hughes C, Croyle RT, Main D, Durham C, Snyder C, et al. Prophylactic surgery decisions and surveillance practices one year following BRCA1/2 testing. Preventive Medicine. 2000;31:75–80. doi: 10.1006/pmed.2000.0684. [DOI] [PubMed] [Google Scholar]
- Lo SS, Mumby PB, Norton J, Rychlik K, Smerage J, Kash J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. Journal of Clinical Oncology. 2010;28:1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- Morris SR, Carey LA. Gene expression profiling in breast cancer. Current Opinion in Oncology. 2007;19:547–551. doi: 10.1097/CCO.0b013e3282f0ada3. [DOI] [PubMed] [Google Scholar]
- O’Neill SC, Brewer NT, Lillie SE, Morrill EF, Dees EC, Carey LA, et al. Women’s interest in gene expression analysis for breast cancer recurrence risk. Journal of Clinical Oncology. 2007;25:4628–4634. doi: 10.1200/JCO.2006.09.6255. [DOI] [PubMed] [Google Scholar]
- Oratz R, Kim B, Chao C, Skrzypczak S, Ory C, Bugarini R, et al. Physician survey of the effect of the 21-gene recurrence score assay results on treatment recommendations for patients with lymph node-positive, estrogen receptor-positive breast cancer. Journal of Oncology Practice. 2011;7:94–99. doi: 10.1200/JOP.2010.000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oratz R, Paul D, Cohn AL, Sedlacek SM. Impact of a commercial reference laboratory test recurrence score on decision making in early-stage breast cancer. Journal of Oncology Practice. 2007;3:182–186. doi: 10.1200/JOP.0742001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. New England Journal of Medicine. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor positive breast cancer. Journal of Clinical Oncology. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- Paik S. Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. The Oncologist. 2007;12:631–635. doi: 10.1634/theoncologist.12-6-631. [DOI] [PubMed] [Google Scholar]
- Richman AR, Tzeng JP, Carey LA, Retèl VP, Brewer NT. Knowledge of genomic testing among early-stage breast cancer patients. Psycho-Oncology. 2011;20:28–35. doi: 10.1002/pon.1699. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Annals of Internal Medicine. 1997;127:966–972. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. Journal of Clinical Oncology. 2008;26:721–728. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- Stokes ME, Davis CS, Koch GG. Categorical Data Analysis using the SAS® System. 2. Cary, NC: SAS Institute, Inc; 2000. [Google Scholar]
- Tzeng JP, Mayer D, Richman AR, Lipkus I, Han PK, Valle CG, et al. Women’s experiences with genomic testing for breast cancer recurrence risk. Cancer. 2010;116:1992–2000. doi: 10.1002/cncr.24990. [DOI] [PubMed] [Google Scholar]
- Van De Vijver MJ, He YD, Van’t Veer LJ, Dai H, Hart AAM, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. New England Journal of Medicine. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]