Abstract
In spite of long-standing evidence showing that the hypothalamus is instrumental in generating behaviors associated with positive and negative emotions, little is known about the role of the hypothalamus in normal human emotional processing. Recent findings have suggested that the hypothalamus plays a role beyond mere control of HPA-axis function; this is also supported by the existence of rich anatomical connections between the hypothalamus and the amygdala, a region known for its important role in emotional processing. However, evidence of emotion-induced hypothalamic activity from neuroimaging studies has been inconsistent, possibly due to methodological limitations (e.g., low spatial resolution). Taking advantage of recent improvements in fMRI technology we set out to explore a possible valence-dependent modulation of hypothalamic activity. Using second order parametric analysis of high-resolution BOLD fMRI, we assessed hypothalamic activation patterns during passive viewing of visual stimuli of varying valence, and compared the results with the activity pattern in the amygdalae, i.e. nuclei with known valence-dependent activity profiles. We show that both hypothalamic and amygdalar activation is modulated by the second-order stimulus valence term, i.e., there is increased neural activity following the processing of both positive and negative stimuli. Our results suggest that the hypothalamus may serve a role in generating emotions broader than generally assumed.
Keywords: fMRI, Hypothalamus, Amygdala, Emotion
Introduction
Based on recent meta-analyses (Kober et al., 2008; Phan et al., 2002) it has been suggested that the function of the hypothalamus in human emotion processing goes beyond the previously postulated role as a simple organizing center integrating various somatic and autonomic responses (Hess, 1954; Ranson, 1934). In the light of recent research focused on revealing the neural underpinnings of emotional processing, a fuller account of hypothalamic involvement in emotional processing is thus warranted (Davidson, 2001).
Similar to the amygdala the hypothalamus could function as valence detector (Habel et al., 2007), as studies using event-related functional magnetic resonance imaging (fMRI) have demonstrated that emotion processing elicited by humorous stimuli causes hypothalamic activity patterns which are comparable to those of the amygdalae (Mobbs et al., 2003; Reiss et al., 2008; Schwartz et al., 2008; Watson et al., 2007; Wild et al., 2003). Also, rich neural connections between amygdala and hypothalamus, via the amygda-lofugal pathway, strongly suggest hypothalamic involvement in emotion processing. The central, basal, accessory basal and lateral amygdaloid nuclei all project to the hypothalamus which in turn projects to the central and medial nuclei of the amygdala (Herman and Cullinan, 1997; Hikosaka et al., 2008; Price, 2003) as well as to the dopaminergic ventral tegmental area (Hikosaka et al., 2008). Clinical evidence indicates that the loss of hypocretin neurons which are solely contained within the hypothalamus, results in the sleep disorder narcolepsy (Thannickal et al., 2000). Cataplectic attacks encompassing complete loss of muscle tone for periods ranging from seconds to several minutes are the cardinal symptom of narcolepsy and occur predominantly following strong and sudden emotional arousal, further suggesting hypothalamic involvement in emotional processing (Guilleminolt and Fromherz, 2005; Siegel and Boehmer, 2006).
The specific role of the hypothalamus during the processing of emotional stimuli is still unclear as fMRI results are less consistent than in the amygdala (Kober et al., 2008; Phan et al., 2002; Taylor et al., 2004). Significant hypothalamic activation has been reported bilaterally (Reiss et al., 2008; Watson et al., 2007) and unilaterally in response to funny, but not neutral, stimuli (Mobbs et al., 2003; Schwartz et al.,2008). Left hypothalamic activity has, also, been reported to increase when anticipating unpleasant stimuli (Herwig et al., 2007). In contrast, at least two previous studies have failed to find hypothalamic activation in response to emotionally salient (i.e., funny) stimuli (Goel and Dolan, 2001; Moran et al., 2004). It seems that most of these discrepancies in hypothalamic activation patterns reported across studies are related to the methodological challenges in performing fMRI in certain brain areas (Merboldt et al., 2001, Robinson et al., 2009). Due to limited spatial resolution and signal-dropouts in ventral brain areas caused by intra-voxel dephasing effects around nasal cavities, detecting significant BOLD-changes in small and heterogeneous structures like the hypothalamus is not a trivial task. Based on recent technical and methodological advancements (Habel et al., 2007; Moser et al., 2007; Robinson et al., 2004, 2008) we aimed in this study to improve the detection of significant BOLD-changes in the hypothalamus.
As previous studies have exclusively focused on hypothalamic activation due to positive (i.e., humor) emotion processing, our aim is to shed further light on the role of the hypothalamus in processing both positive and negative emotions. We employed high-resolution BOLD fMRI, specifically optimized for ventral brain areas to determine valence-dependent modulation of hypothalamic activation in healthy individuals during passive viewing of visual stimuli. A key issue in understanding hypothalamic involvement in emotional processing, beyond the role suggested by the neuroanatomy, is to specifically characterize functional responses of the hypothalamus during normal emotional processing with regard to changes in amygdalar activation. In this study, hypothalamic results were therefore compared with activity patterns in the amygdala, which represents a brain structure with known valence-dependent activity profile. We hypothesize that there is increased neural activity at the level of the hypothalamus following the processing of both positive and negative stimuli.
Methods
Still pictures were used to manipulate the emotional state on the funny-to-sad dimension in 20 healthy subjects (16 males and 4 females; mean age 26±2.9 years) during an fMRI session. All experiments were performed at the MR Center of Excellence, Medical University of Vienna, Vienna, Austria, in accordance with the 1975 Helsinki declaration and local ethics regulations.
Stimuli and paradigm
Over 200 color and black–white still pictures depicting humans, animals, objects or landscapes were pre-selected from commercial databases (e.g., iStockphoto, Getty Images). The pictures did not contain any written material or cartoon drawings and depicted a wide range of scenes such as: car crashes, empty office buildings, electrical appliances, humans and animals in comic situations, and trees. These pictures were rated by 50 naive participants (22 males and 28 females; mean age 29±3.1) under controlled conditions where participants received standardized instructions. Participants viewed the stimuli in randomized order on a computer screen for a maximum of 8 s each, whereby participants could press a button to progress to the next picture once it had been rated. Each picture was rated using a 7-point Likert scale, on the funny-to-sad dimension, where 1 indicated funniest, 4 neutral and 7 saddest, respectively. Using these ratings, the twenty stimuli within each category which showed the highest inter-rater agreement (funny: 2.4±1.01; neutral: 3.9±0.35; sad: 6.1±1.04; values are mean±standard deviation) were selected for the experiment. During the fMRI session each stimulus was shown once for 4 s, and stimulus presentation order was randomized with inter-stimulus intervals ranging from 8 s to 12 s. A white cross on black background served as a baseline and was shown between each stimulus. Study participants were asked to watch the stimuli without being instructed to fixate a specific part of the image.
Data acquisition
Functional imaging was performed on a Tim Trio Scanner (Siemens Medical, Erlangen, Germany) using a 12-channel head coil and employing high-resolution gradient-recalled EPI, following the sequence parameter sets recently presented by our group (Robinson et al., 2004, 2008, 2009). Twenty axial slices centered at the hypothalamus and aligned along ac-pc were acquired with a slice thickness of 1.9 mm, a slice gap of 0.9 mm and matrix size of 128×128 (in-plane voxel size 1.7×1.7 mm2). This high spatial resolution acquisition scheme significantly reduces signal drop-out in the amygdala-hypothalamus region (Robinson et al., 2004, 2008). Parallel imaging with a GRAPPA factor of two was used to enable both a TE of 40 ms and a TR of 2000 ms at this high spatial resolution. Paradigm duration was approximately 15 min, during which 430 functional volumes were acquired.
Data analysis
Functional MRI time series data were analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk) after standard preprocessing procedures including motion- and slice-timing correction, spatial normalization to MNI space using the SPM5 EPI-template and spatial smoothing (Gaussian kernel with 7 mm FWHM) was applied.
To account for individual differences in the appraisal of the presented stimuli, participants performed stimulus rating immediately after the fMRI session using the same 7-point Likert scale as described above. Based on these subject-specific stimulus ratings, a high-order parametric modulation model was constructed. The model incorporated a regressor modeling the occurrence of all stimuli (0th order) and five regressors as 1st to 5th order parametric modulators of the stimulus-specific hemodynamic response (Buchel et al., 1998). In addition, realignment parameters were added as nuisance regressors to model for residual motion effects. For second-level analysis, a series of six one-sample t-tests for all modulation orders was performed and thresholded at p<0.05 (FDR-corrected), as implemented in SPM5. Bonferroni correction was applied to account for repeated testing.
Results
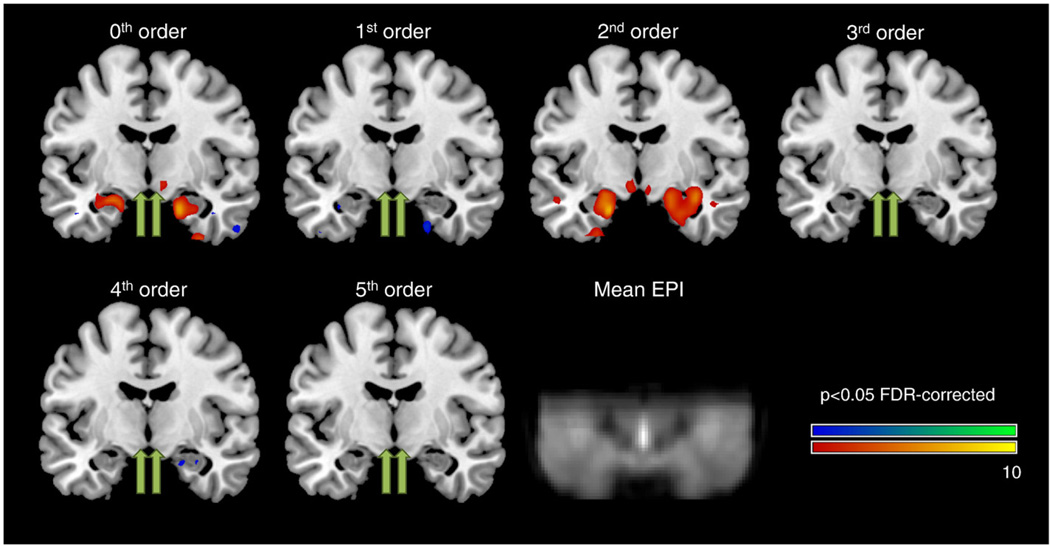
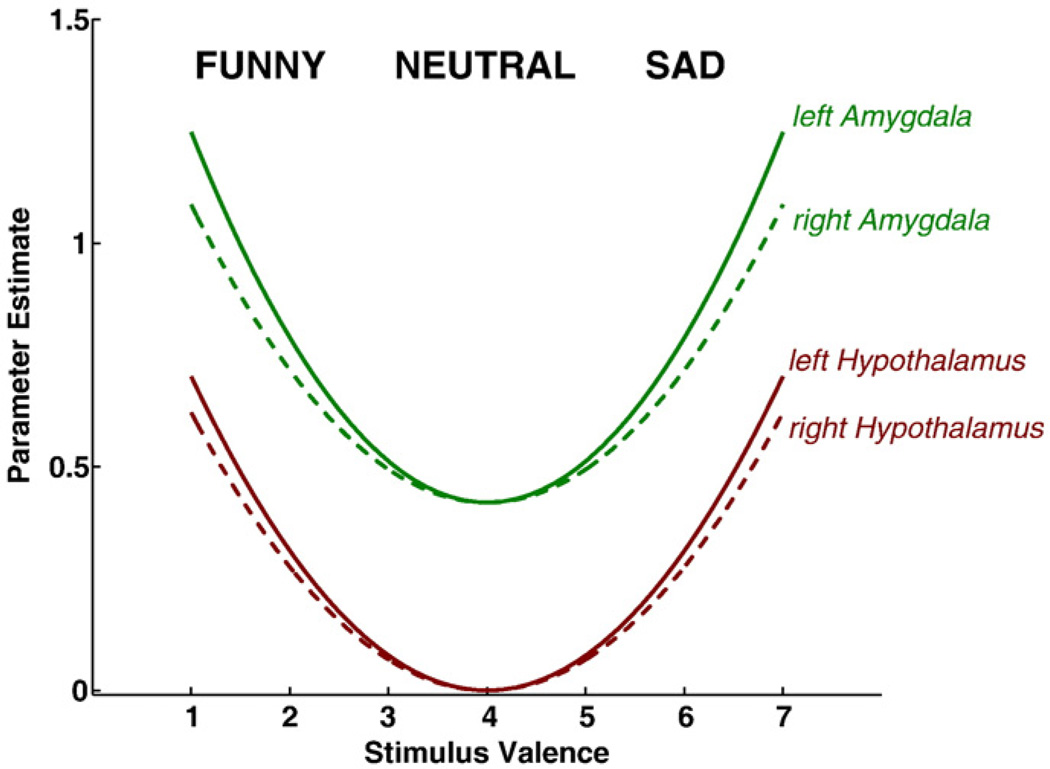
Fig. 1 shows the group results of modulatory effects for 0th to 5th order (coronal plane at MNIy=−8 mm). In accordance with our hypothesis, areas with significant second-order modulation across the brain were extracted and are given in Table 1. Maximum second-order modulation in left and right hypothalamus was found at MNI-coordinates −6, −8, −8 (T=4.59; df=21; p<0.02 FDR) and 6, −8, −12 (T=3.94; p<0.04 FDR), respectively. For comparison, both amygdalae also showed significant modulation effects: left amygdala −18, −6, −24 (T=9.60; p<0.001 FDR), right amygdala 28, −2, −26 (T=8.58; p<0.001 FDR), respectively. Other areas with significant activation modulation were found in bilateral fusiform gyrus, bilateral thalamus and bilateral inferior frontal gyrus (see Table 1). Table 2 shows mean parameter estimates and t-values of 0th to 5th order modulation for bilateral hypothalamus and amygdala, respectively. Significant modulations are printed in bold. It can be seen that both amygdale and hypothalamus show significant 2nd order modulation while bilateral amygdala also showed significant 0th order modulation. Note that several other modulation terms show trends towards significance. From these results mean modulation curves over stimulus valence for all four regions were calculated based on all significant modulation terms, i.e. 0th and 2nd order for amygdala and 2nd order for hypothalamus, and are shown in Fig. 2. Note the similarity in modulation of left and right activation, both for amygdala and hypothalamus.
Fig. 1.
Coronal plane (MNIy=−8 mm) overlaid with areas of significant parametric modulations for 0th to 5th order (p<0.05 FDR-corrected). In accordance with the hypothesis, significant second-order modulation was found in left and right hypothalamus (yellow arrows) as well as bilateral amygdala. For comparison, hypothalamic second-order modulation maxima are also indicated for all other modulation orders (green arrows). For details please refer to Table 1. In addition, the group-averaged EPI is also shown to indicate brain coverage and to demonstrate the high quality of fMRI data in hypothalamus/amygdala regions.
Table 1.
Areas with significant second-order activation modulation (p<0.05, FDR-corrected). Coordinates are given in the left-posterior-inferior (LPI) system.
| Talairach-Tournoux atlas | Focus point (LPI) | T-value |
|---|---|---|
| Left amygdala | −18, −6, −24 | 9.60 |
| Right amygdala | 28, −2, −26 | 8.58 |
| Left fusiform gyrus | −42, −54, −22 | 8.50 |
| Right fusiform gyrus | 42, −52, −22 | 6.85 |
| Right inferior frontal gyrus | 34, 28, −20 | 6.46 |
| Left inferior frontal gyrus | −34, 22, −22 | 5.36 |
| Left thalamus | −10, −24, −6 | 5.34 |
| Right thalamus | 10, −24, −2 | 4.74 |
| Left hypothalamus | −6, −8, −8 | 4.59 |
| Right hypothalamus | 6, −8, −12 | 3.94 |
Table 2.
Mean parameter for 0th to 5th order modulation. Numbers in brackets are the corresponding t-values. Statistically significant modulations are printed in bold (p<0.05, FDR-corrected). Coordinates are given in the left-posterior-inferior (LPI) system.
| Talairach–Tournoux atlas | Focus point (LPI) | 0th order | 1st order | 2nd order | 3rd order | 4th order | 5th order |
|---|---|---|---|---|---|---|---|
| Left amygdala | −18, −6, −24 | 0.42 (4.50) | −0.012 (−0.52) | 0.092 (9.60) | −0.036 (−2.25) | −0.023 (−2.20) | −0.005 (−0.46) |
| Right amygdala | 28, −2, −26 | 0.42 (5.28) | −0.034 (−1.59) | 0.074 (8.58) | −0.016 (−2.72) | −0.014 (−2.95) | −0.0002 (−0.04) |
| Left hypothalamus | −6, −8, −8 | 0.21 (2.21) | −0.012 (−0.56) | 0.078 (4.59) | −0.009 (−0.85) | 0.012 (1.33) | 0.010 (1.28) |
| Right hypothalamus | 6, −8, −12 | 0.32 (3.32) | 0.0056 (0.16) | 0.069 (3.94) | 0.004 (0.34) | −0.003 (−0.43) | −0.002 (−0.27) |
Fig. 2.
Mean modulation amplitude curves for amygdala (green) and hypothalamus (red). Solid lines refer to left hemispheric regions, dashed lines indicate right hemispheric regions.
Discussion
In this study the hypothalamus was found to exhibit significant second-order modulation of activation indicating increased BOLD activation when subjects were viewing funny and sad stimuli in comparison to neutral stimuli. A similar pattern was found in the amygdala. However, no significant hypothalamic activation was found for processing neutral stimuli, while both amygdalae were significantly activated by neutral stimuli.
Our findings regarding the processing of funny pictures are in agreement with recent reports on hypothalamic activity during humor processing (Reiss et al., 2008; Schwartz et al., 2008). Interestingly, the measured response appears at an anatomical location corresponding to that of hypocretin neurons (Thannickal et al., 2000). Assuming that the response is indeed mediated by hypocretin neurons it is consistent with a recent hypothesis suggesting that, under normal conditions, hypocretin neurons of the hypothalamus are activated following positive emotional arousal, such as laughter or joking. In doing so, they may serve to counterbalance muscle inhibition that is a by-product of limbic system activation (Siegel and Boehmer, 2006). In the absence of the influence of hypocretin neurons, as in the case of narcolepsy, the same emotional activation can lead to cataplexy (Siegel and Boehmer, 2006). The two published studies, where BOLD patterns were measured in narcoleptic patients following humor processing, have yielded differing results: either increased hypothalamic activation when compared to controls (Reiss et al., 2008) or vice versa (Schwartz et al., 2008). Only the latter result is consistent with the hypothesis of Siegel and Boehmer. This issue of hypothalamic activity in response to stimuli with positive valence in narcoleptics remains to be resolved.
Here, we have further demonstrated increased hypothalamic activity in response to viewing very sad stimuli in healthy individuals, suggesting that hypocretin cells may be activated during both positive and negative emotional processing. However, even though laughter may be the most common trigger of cataplexy in narcoleptics, there have been reports on negative emotions triggering cataplectic attacks (Guilleminolt and Fromherz, 2005). Thus, it seems possible that hypothalamic activation serves to counteract muscle weakness following both positive and negative emotional arousal in healthy individuals. Alternatively, hypocretin neurons may be activated while viewing very funny stimuli whereas other cell groups (e.g., melanin concentrating hormone neurons) that are intermixed with hypocretin neurons, are activated while watching very sad stimuli; these intermixed neuronal populations have been shown to exhibit antagonistic discharge profiles in response to extracellular glucose levels and noradrenaline stimulation in animal studies (Bayer et al., 2005; Burdakov and Alexopoulos, 2005). It is, therefore, tempting to speculate that these neuronal groups exhibit similarly antagonistic activity profiles to emotional processing in humans.
Due to the increased coil sensitivity (12-channel vs. CP) and accelerated data acquisition (GRAPPA factor of 2) clear improvement in resolution is seen at the level of the amygdale, as compared to previous studies (Habel et al., 2007; Morawetz et al., 2008; Moser et al., 2007; Robinson et al., 2004). Moreover, the optimized EPI protocol could be of value when studying diseases with hypothalamic underpinnings, e.g., sleep and circadian disorders, emotional and eating disorders as currently significant changes in hypothalamus activation are detectable in grouped data only. In order to enable single-subject hypothalamic activation assessment, sensitivity must still be increased. This could be achieved via array-coils with more elements, e.g., 32 channels (Wiggins et al., 2006), at 3 T with an expected SNR gain of 50% in image SNR or even at 7 T. Whether physiological noise will be limiting the latter approach (Triantafyllou et al., 2005) or may be overcome by appropriate noise correction strategies remains to be shown.
In conclusion, extensive connections of the hypothalamus to amygdala, reward centers and structures implicated in humor processing (Herman and Cullinan, 1997; Hikosaka et al., 2008; Wild et al., 2003), along with long-standing evidence of the severe effects of hypothalamic lesions on emotional behavior, strongly suggest a hypothalamic role in the integration of various autonomic and somatic responses (Hess, 1954; Ranson, 1934). Here we have demonstrated valence-dependent modulation of hypothalamic and amygdalar activation following passive viewing of funny as well as sad stimuli indicating that the hypothalamus may serve a role in generating emotions broader than generally assumed.
Acknowledgments
Financially supported by Icelandic Centre for Research grant no. 070431022 (to KÆK) and Reykjavik University Development fund no. 14630 (to KÆK). We are grateful to M. Fürsatz (Vienna) for his support with the initial experiments, as well as H. Arnadottir and I. Johannesdottir (Reykjavik) for collecting and validating the stimulus material.
References
- Bayer I, Eggerman E, Serafin M, Grivel J, Machard D, Muhlethaler M, Jones BE. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–811. doi: 10.1016/j.neuroscience.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. NeuroImage. 1998;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Alexopoulos H. Metabolic state signalling through central hypocretin/orexin neurons. J. Cell Mol. Med. 2005;9:795–803. doi: 10.1111/j.1582-4934.2005.tb00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Toward a biology of personality and emotion. Ann. N. Y. Acad. Sci. 2001;935:191–207. doi: 10.1111/j.1749-6632.2001.tb03481.x. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. The functional anatomy of humor: segregating cognitive and affective components. Nat. Neurosci. 2001;4:237–238. doi: 10.1038/85076. [DOI] [PubMed] [Google Scholar]
- Guilleminolt C, Fromherz S. Narcolepsy: Diagnosis and management. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsivier; 2005. [Google Scholar]
- Habel U, Windischberger C, Derntl B, Robinson S, Kryspin-Exner I, Gur RC, Moser E. Amygdala activation and facial expressions: explicit emotion discrimination versus implicit emotion processing. Neuropsychologia. 2007;45:2369–2377. doi: 10.1016/j.neuropsychologia.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herwig U, Abler B, Walter H, Erk S. Expecting unpleasant stimuli—an fMRI study. Psychiatry Res. 2007;154:1–12. doi: 10.1016/j.pscychresns.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Hess WR. Diencephalon; Autonomic and Extrapyramidal functions. New York: Grune and Stratton; 1954. [Google Scholar]
- Hikosaka O, Bromberg-Martin E, Hong S, Matsumoto M. New insights on the subcortical representation of reward. Curr. Opin. Neurobiol. 2008;18:203–208. doi: 10.1016/j.conb.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a metaanalysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merboldt KD, Fransson P, Bruhn H, Frahm J. Functional MRI of the human amygdala. NeuroImage. 2001;14:253–257. doi: 10.1006/nimg.2001.0802. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Greicius MD, Abdel-Azim E, Menon V, Reiss AL. Humor modulates the mesolimbic reward centers. Neuron. 2003;40:1041–1048. doi: 10.1016/s0896-6273(03)00751-7. [DOI] [PubMed] [Google Scholar]
- Moran JM, Wig GS, Adams RB, Jr, Janata P, Kelley WM. Neural correlates of humor detection and appreciation. NeuroImage. 2004;21:1055–1060. doi: 10.1016/j.neuroimage.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Morawetz C, Holz P, Lange C, Baudewig J, Weniger G, Irle E, Dechent P. Improved functional mapping of the human amygdala using a standard functional magnetic resonance imaging sequence with simple modifications. Magn. Reson. Imaging. 2008;26:45–53. doi: 10.1016/j.mri.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Moser E, Derntl B, Robinson S, Fink B, Gur RC, Grammer K. Amygdala activation at 3 T in response to human and avatar facial expressions of emotions. J. Neurosci. Methods. 2007;161:126–133. doi: 10.1016/j.jneumeth.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Ann. N. Y. Acad. Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Ranson SW. The hypothalamus: its significance for visceral innervation and emotional expression. Transactions Coll. Physicians Philadelphia. 1934;2:222–242. [Google Scholar]
- Reiss AL, Hoeft F, Tenforde AS, Chen W, Mobbs D, Mignot EJ. Anomalous hypothalamic responses to humor in cataplexy. PLoS ONE. 2008;e2225:3. doi: 10.1371/journal.pone.0002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Windischberger C, Rauscher A, Moser E. Optimized 3 T EPI of the amygdalae. NeuroImage. 2004;22:203–210. doi: 10.1016/j.neuroimage.2003.12.048. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Pripfl J, Bauer H, Moser E. The impact of EPI voxel size on SNR and BOLD sensitivity in the anterior medio-temporal lobe: a comparative group study of deactivation of the Default Mode. Magn. Mater. Phys. 2008;21:279–290. doi: 10.1007/s10334-008-0128-0. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Moser E, Peper M. Functional magnetic resonance imaging of emotion. In: Filippi M, editor. “FMRI techniques and protocols”: Series: Neuromethods. vol. 41. Totowa, NJ: Humana Press; 2009. pp. 411–456. [Google Scholar]
- Schwartz S, Ponz A, Poryazova R, Werth E, Boesiger P, Khatami R, Bassetti CL. Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain. 2008;131:514–522. doi: 10.1093/brain/awm292. [DOI] [PubMed] [Google Scholar]
- Siegel JM, Boehmer LN. Narcolepsy and the hypocretin system—where motion meets emotion. Nat. Clin. Pract. Neurol. 2006;2:548–556. doi: 10.1038/ncpneuro0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ. A functional neuroimaging study of motivation and executive function. NeuroImage. 2004;21:1045–1054. doi: 10.1016/j.neuroimage.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllou C, Hoge RD, Krueger G, Wiggins CJ, Potthast A, Wiggins GC, Wald LL. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. NeuroImage. 2005;26:243–250. doi: 10.1016/j.neuroimage.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Watson KK, Matthews BJ, Allman JM. Brain activation during sight gags and language-dependent humor. Cereb. Cortex. 2007;17:314–324. doi: 10.1093/cercor/bhj149. [DOI] [PubMed] [Google Scholar]
- Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn. Reson. Med. 2006;56:216–223. doi: 10.1002/mrm.20925. [DOI] [PubMed] [Google Scholar]
- Wild B, Rodden FA, Grodd W, Ruch W. Neural correlates of laughter and humour. Brain. 2003;126:2121–2138. doi: 10.1093/brain/awg226. [DOI] [PubMed] [Google Scholar]