Abstract
Adult male and female rat hepatocytes were individually transplanted into the spleens of adult male and female rats. The recipients were euthanized at either 8, 16, 30 or 45 weeks following transplantation at which time hepatic and splenic levels of liver-specific rat albumin mRNA as well as sex-dependent transcript levels of CYP2C11, -2C12, -2C7, -2A1 and -3A2, comprising >60% of the total concentration of hepatic constituent cytochrome P450, were determined. Whereas the pre-infused hepatocytes expressed their expected cytochrome P450 sexual dimorphisms (female-specific CYP2C12, male-specific CYP3A2 and female-predominant CYP2A1), their post transplantational competence now reflected the sexual dimorphisms of the recipient (as observed in the host’s liver) supporting the concept that the sex-dependent growth hormone circulating profiles are the determinants regulating the expression levels of hepatic cytochromes P450. Also expressed at normal concentrations in the pre-infused hepatocytes, male-specific CYP2C11 and female-predominant CYP2C7 were inexplicably undetectable in the spleens of both recipient males and females, irrespective of the sex of the donor hepatocytes, almost one year after transplantation.
Keywords: growth hormone, sex-dependent secretion, growth hormone, regulation of cytochrome P450s, sexual dimorphism, cytochrome P450 expression
Orthotopic liver transplantation is often the only therapeutic response for the treatment of several liver diseases, eg., hepatic cirrhosis, fulminant hepatitis and several lethal hereditary enzyme deficiencies. While the procedure is now routine, it is not without its drawbacks, including post-transplantation complications as well as a shortage of donors. Accordingly, alternative procedures have been investigated to support liver function. Among these is the transplantation of isolated hepatocytes to various systemic sites. Numerous sites have been examined, including fat pads, muscle, subcutaneous tissue, peritoneum, lungs, kidney, liver and spleen (1–5). In spite of the disadvantages of the intrasplenic site, such as the need for syngeneic hepatocytes which would otherwise require immunosuppressive therapy and its size limitation which allows the spleen to accommodate hepatic tissue amounting to only ~3–4% of normal liver weight, the spleen has been shown to be a highly effective site, at least in experimental animals (6). The efficacy of intrasplenic hepatocyte transplants in rats with fulminate hepatitis induced by D-galactosamine (7), carbon tetrachloride (8) and portacaval anastomosis plus 70% hepatectomy (9) was demonstrated by a 20–80%, 100% and 10–60%, respectively, increase in survival. Moreover, intrasplenic hepatocyte transplants were able to supply catalase in a strain of acatalasemic mice (10), reduce both serum bilirubin in the Gunn rat (11, 12) and cholesterol plasma levels in the Watanabe rabbit (13, 14).
The liver contains many vital enzyme systems of which the cytochrome P450 (CYP) system is one. The mammalian CYP superfamily is involved in the synthesis and/or metabolism of prostaglandins, eicosanoids, cholesterol, bile acids, steroid and thyroid hormones, vitamins A and D, biogenic amines, neuroamines and fatty acids. In addition, the enzymes are the body’s first line of defense in metabolizing pharmaceuticals, foreign chemicals and pollutants. Whereas humans, rats and mice each express about 50 to 70 different CYPs in various organs, it is the hepatic isoforms which comprise the bulk of the CYP enzymes (15, 16). Moreover, hepatic CYPs are generally expressed at sexually dimorphic levels, although to different extremes depending on the species (17–19). In spite of the importance of the CYP enzymes system, there remains a paucity of studies examining its expression in isolated hepatocyte transplants, and in particular, intrasplenic transplants. A few reports have investigated the long term (~ 1 year) expression of CYPs in intrasplenic hepatocyte transplants derived from mixed-sex fetal liver. In these studies, western blotting, immunohistochemistry and CYP-dependent monooxygenase activities have indicated the persistent expression of several fetal CYP isoforms, a few minor adult isoforms and the responsiveness of inducible CYPs to induction by dexamethasone, phenobarbital and/or β-naphthoflavone (20–22). In addition, isolated adult hepatocytes transplanted into the spleens of syngeneic Fischer 344 rats survived for at least 15 months and maintained a responsiveness of their inducible CYP isoform (measured at the mRNA level) to phenobarbital induction (23). To date, however, all of the CYP isoforms measured in the intrasplenic hepatocyte transplants account for only a very small percent of the total adult constituent pool. In contrast, the expression of CYP2A1, -2A2, -2C6, -2C7, -2C11, -2C12, -2C13, -2E1 and -3A2, accounting for more than 90% of the total hepatic CYP in adult male or female rats (24), has not been measured in intrasplenic hepatocyte transplants. In the present study, we have investigated the sexually dimorphic regulations of 5 constituent hepatic CYPs, comprising >60% of the total CYP content in male and female rat liver (24) in intrasplenic hepatocytes derived from adult male and female rats and transplanted into adult male and female rats.
EXPERIMENTAL PROCEDURES
Animals
Animals were housed in the University of Pennsylvania Laboratory Animal Resources facility, under the supervision of certified laboratory animal medicine veterinarians and were treated according to a research protocol approved by the university’s Institutional Animal Care and Use Committee. All Fischer 344 inbred rats (males and females, recipients and donors) were nine weeks of age at the time of intrasplenic hepatocyte transplantation. Male and female recipients were implanted with viable hepatocytes from either same or opposite sex rats and euthanized at either 8, 16, 30 or 45 weeks post transplantation. Each donor rat supplied sufficient cells to implant 4 rats of the same or opposite sex representing the 4 euthanasia periods. That is, hepatocytes from the same male donor were infused into the spleens of 4 different recipient males; one of each to be euthanized at 8, 16, 30 and 45 weeks post transplantation. Cells from another male were infused into 4 females; each euthanized at one of the 4 time periods. Hepatocytes harvested from female donors were similarly distributed to male and female recipients. Each treatment group contained 8 rats to assure that at least 6 rats (the number used in the study) contained viable intrasplenic hepatocytes for analysis. Spleens of sham rats (i.e. controls) were infused with hepatocyte buffer containing no cells. At the appointed times, the rats were decapitated, the livers and spleens were quickly removed and minced, and portions reserved for mRNA determinations were stored in RNA-Later (Invitrogen, Carlsbad, CA). In addition, a few random tissue samples were stored in 10% buffered formalin for subsequent histological preparation. The effectiveness of the procedure was verified at necropsy by the presence of obvious nodules in otherwise normal appearing spleen, by random histological preparations demonstrating the presence of hepatocytes in the splenic pulp and by the expression of rat albumin mRNA in the treated spleens. All of these markers were negative in the spleens from sham rats.
Hepatocyte Isolation and Transplantation
Isolation of rat hepatocytes was performed with minor modifications (25) by in situ perfusion of collagenase through the portal vein of anesthetized rats (26). In brief, initial perfusion with a calcium-free buffer was followed by a solution of collagenase (0.05% w/v). The “softened” liver was excised, and the hepatocytes were separated from connective tissue by filtering through 100 μm macroporous filters (Spectrum Co., Laguna Hills, CA) and from nonparenchymal cells by repeated low speed centrifugation in wash medium: high glucose DMEM (4.5 g/l) containing streptomycin (100 μg/ml), penicillin (100 U/ml), gentamycin (50 μg/ml), fungizone (0.25 μg/ml) and Hepes (15 mM). The viability of the final cell suspension of hepatocytes was typically between 50 and 60% (trypan blue)1. An aliquot of isolated hepatocytes from every donor rat was stored for subsequent mRNA analysis of albumin and CYP isoforms.
Hepatocyte transplantation was carried out on laparotomized rats under inhalation anesthesia with isofluorine. The exposed spleen was kept moist throughout the procedure. A hepatocyte suspension containing 4 to 5 × 106 live cells in 0.3 ml of DMEM was very slowly injected into the splenic pulp through a 25-gauge needle. Hemostasis was controlled by compression of the injection site with a sterile cotton tip. Leakage from the site of injection and run off into the portal vein were minimized by temporary occlusion of the vascular system at the splenic hilum. The entire procedure took no more than 10 minutes.
RNA Analysis
Total hepatic and splenic RNA was isolated and prepared as previously described (27, 28). Basically, 10 μg (liver) or 30 μg (spleen) of RNA was electrophoresed under formaldehyde denaturing conditions on 1% agarose and transferred to Nytran nylon membranes (Schleicher & Scheull, Keene, NH). The northern blots were probed and occasionally reprobed with 32P-labeled oligonucleotides by using hybridization and high-stringency washing conditions. The nucleotide sequence of oligonucleotide probes for CYP2A1, -2C7, -2C11, -2C12 (30), -3A2 (31), and rat albumin (32) have been reported. The consistency of RNA loadings between samples was confirmed by ethidium bromide staining of 18S and 28S rRNAs and was verified with an 18S oligonucleotide probe (33). The hybridized mRNA signals were quantified by scanning the autoradiographs by using an Alpha Innotech FluorChem 8800 and normalized to the 18S rRNA signals in each lane.
Histology
Light microscopy was performed on hematoxylin and eosin-stained sections of 6 spleens 45 weeks after hepatocyte transplantation.
Statistics
All data were subjected to two way analysis of variance (alpha=0.05) demonstrating statistically significant interactions between groups, and differences were determined with t statistics and the Bonferroni procedure for multiple comparisons.
RESULTS
Morphological Appearance of Intrasplenic Hepatocytes
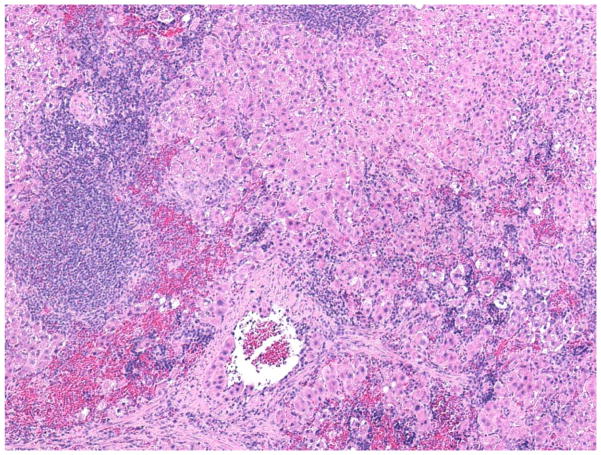
The presented histological section (Fig. 1) is that of a spleen from a male rat 45 weeks after the infusion of donor female hepatocytes. The sex of the donor hepatocytes as well as the sex of the recipient spleens had no observable effect on the morphology of the intrasplenic hepatocytes 45 weeks post transplantation. Our limited number of samples showed hepatocytes restricted to the red pulp and often presenting as disoriented and abbreviated cord-like structures, inferior to cords observed in normal liver organized about a central vein. In addition, some areas contained unstructured masses of cells appearing as clumps. Histological sections of liver and control spleens, i.e., infused with hepatocyte buffer alone, were unremarkable and have not been presented.
Fig. 1.
Morphological appearance of intrasplenically transplanted hepatocytes. Light microscopic appearance of intrasplenic hepatocytes 45 weeks after transplantation (×200). Spleens were fixed in 10% buffered formalin and stained with hematoxylinand eosin. Large masses of hepatocytes within the red pulp occupy -70% of the figure (from left to right).
Isolated Hepatocyte CYP mRNAs
In order to determine whether the hepatocytes expressed normal transcript levels at the time of transplantation, an aliquot of isolated cells from every donor rat was stored for analyses at the time the remaining hepatocytes were infused into recipient spleens. We found that the isolated hepatocytes expressed the expected sexually dimorphic levels and concentrations of, CYP2A1, -2C7, -2C11, -2C12 and -3A2 mRNAs as observed in the same sex livers of recipients (see subsequent tables), and accordingly the mRNA values for the pre-infused hepatocytes have not been presented.
Albumin
There were no statistical differences in the concentrations of albumin mRNA in the livers of any treatment group. That is, irrespective of sex, the presence of splenic hepatocytes, or the number of weeks following transplantation, the livers of rats in all groups contained similar levels of the transcript (Fig. 2).
Fig. 2.
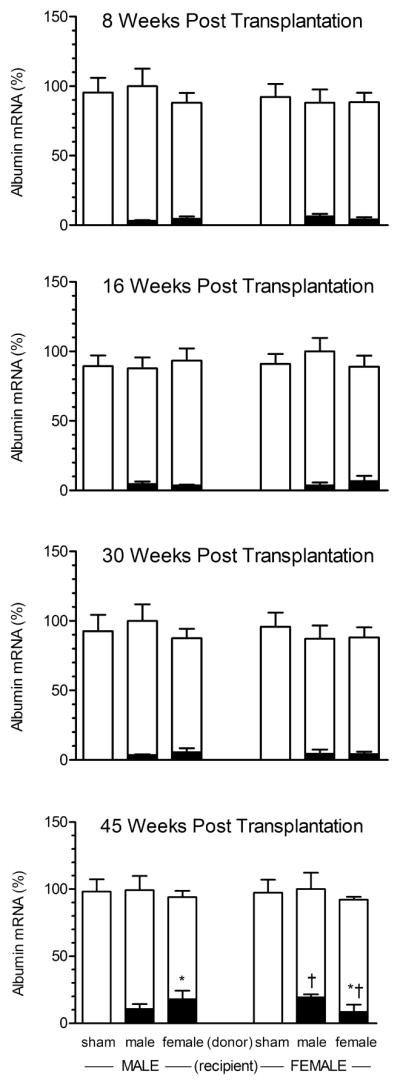
Rat albumin mRNA levels in liver and spleen of recipient male and female rats bearing intrasplenic hepatocyte transplants from same age male or female donor rats. Spleens from sham rats were infused with hepatocyte media containing no cells. Recipient rats were euthanized at either 8, 16, 30 or 45 weeks post transplantation. Each data point is a mean ± SD from 6 rats. The open bars represent hepatic albumin mRNA expressed as a percentage of the mean value of the group with highest concentration (designated 100%) of the transcript. The black bars represent the concentration of splenic albumin mRNA expressed as a percentage of their autologous liver albumin mRNA. *, P<0.05 compares the effects of donor sex in same sex recipient; †, P<0.05 compares the effects of recipient sex containing hepatocytes from same sex donors.
Not unexpectedly, having been infused with only hepatocyte media, the spleens of both male and female sham rats contained no measurable rat albumin mRNA. In general, splenic hepatocytes in male and female recipients, regardless of donor sex, contained statistically similar concentrations of rat albumin mRNA; albeit at only ~4 to 6% of the concentrations in their autologous livers. Only at 45 post transplantation weeks, however, did we observe a sex-dependent effect in which albumin content was statistically greater in the spleens of recipients that received hepatocyte implants from opposite sex donors (Fig. 2). That is, at 45 weeks post transplantation female-derived hepatocytes expressed higher levels of the transcript than male-derived cells when implanted in the spleens of male recipients. Correlatively, hepatocytes obtained from males contained greater concentrations of albumin mRNA than those from females when maintained in the spleens of females.
CYP in Sham-treated Rats
Although we analyzed the livers and spleens of the sham-treated rats for all the measured CYP transcripts at the four post transplantation ages, our finding have not been presented. Since the sham spleens contained neither hepatocytes nor albumin, expectedly we observed no detectable concentrations of CYP2A1, -2C7, -2C11, -2C12 and -3A2 mRNAs. The livers of the sham-treated rats expressed the same sexually dimorphic levels of CYP transcripts as we report for the livers of the recipients thus generating no new findings.
CYP2C12
The liver findings clearly agree with the dozens of earlier reports, many of them our own (17, 19), demonstrating female-specific expression of hepatic CYP2C12. That is, male liver, regardless of age or splenic implant, had not detectable levels of CYP2C12 mRNA (Table 1). On the other hand, female liver expressed robust levels of the enzyme transcript [~40% of its total hepatic CYP (15,16)] that were uneffected by either the sex of the splenic transplant or the age of the rat.
Table 1.
Sexually Dimorphic Expression of CYP2C12 mRNA in Liver and Spleen-containing Hepatocyte Transplants.
| Weeks Post | |||||
|---|---|---|---|---|---|
| Transplantation
|
Recipient
|
Donor
|
CYP2C12/Albumin
|
% Liver CYP2C12
|
|
| 8 | Male | Male | Liver | ND | |
| Spleen | ND | _____ | |||
| Female | Liver | ND | _____ | ||
| Spleen | ND | _____ | |||
| Female | Male | Liver | 0.48±0.09 | ||
| Spleen | ND | _____ | |||
| Female | Liver | 0.51±0.04 | |||
| Spleen | ND | _____ | |||
|
| |||||
| 16 | Male | Male | Liver | ND | |
| Spleen | ND | _____ | |||
| Female | Liver | ND | |||
| Spleen | 0.41±0.09a | 2.2±0.4b | |||
| Female | Male | Liver | 0.63±0.10 | ||
| Spleen | 0.87±0.27 | 3.6±1.2 | |||
| Female | Liver | 0.78±0.11 | |||
| Spleen | 0.89±0.24† | 4.3±1.3† | |||
|
| |||||
| 30 | Male | Male | Liver | ND | |
| Spleen | ND | _____ | |||
| Female | Liver | ND | |||
| Spleen | 0.13±0.04a | 1.0±0.1b | |||
| Female | Male | Liver | 0.64±0.11 | ||
| Spleen | 0.61±0.12 | 4.3±1.1 | |||
| Female | Liver | 0.59±0.07 | |||
| Spleen | 0.56±0.09† | 4.1±1.0† | |||
|
| |||||
| 45 | Male | Male | Liver | ND | |
| Spleen | ND | _____ | |||
| Female | Liver | ND | |||
| Spleen | 0.07±0.02a | 0.9±0.2b | |||
| Female | Male | Liver | 0.73±0.11 | ||
| Spleen | 0.61±0.12 | 12.6±3.8 | |||
| Female | Liver | 0.76±0.17 | |||
| Spleen | 0.63±0.13† | 6.1±0.7†# | |||
Donor hepatocytes isolated from adult male or female rats were infused into the spleens of same age male and female recipient rats. The recipients were euthanized at 8, 16, 30 and 45 weeks after transplantation when CYP2C12 mRNA levels were determined in both organs. Values are presented as CYP2C12 mRNA per rat albumin mRNA (liver and spleen) and as % of autologous liver CYP2C12 mRNA/mg (spleen). Each value is a mean ±SD with n=6 unless otherwise indicated. ND, not detectable,
, P<0.05 compares spleen with liver of the same rat.
, P<0.05 compares same sex donors in opposite sex recipients of the same age.
, P<0.05 compares opposite sex donors in same sex recipients of the same age.
, n=3,
, % of female liver.
Eight weeks after transplantation, in spite of the presence of albumin mRNA, the female-derived splenic hepatocytes appear to have lost their ability to express CYP2C12 mRNA whereas the male-derived splenic hepatocytes had not obtained that function (Table 1). However, following 16, 30 and 45 weeks of transplantation, the splenic hepatocytes in recipient females, irrespective of donor sex, had the same concentration (per hepatocyte) of the transcript as their autologous livers. Since the actual number of hepatocytes capable of residing in the spleen are profoundly lower than the number of hepatocytes in the liver, it is not surprising that the spleens expressed only a fraction of the total CYP2C12 mRNA of that measured in the livers. As expected, male-derived splenic hepatocytes in recipient males expressed no detectable levels of CYP2C12 mRNA at all time points measured. The fact, however, that the female-derived splenic hepatocytes expressed CYP2C12 mRNA in male recipients, albeit at much lower and declining levels than female liver, and in only half the recipients, suggests that expression of the female-specific enzyme is to some degree independent of suppressive factors in the male host.
CYP2A1
Hepatic CYP2A1 is a known female-predominant hepatic isoform whose ~3-fold higher expression in females (Table 2) is clearly in agreement with previous reports (17, 19). Neither the age of the rats nor the presence of splenic hepatocytes, male or female-derived, had any effect on the characteristic sexually dimorphic expression of liver CYP2A1 mRNA.
Table 2.
Sexually Dimorphic Expression of CYP2A1 mRNA in Liver and Spleen-containing Hepatocyte Transplants.
| Weeks Post | |||||
|---|---|---|---|---|---|
| Transplantation
|
Recipient
|
Donor
|
CYP2CA1/Albumin
|
% Liver CYP2CA1
|
|
| 8 | Male | Male | Liver | 0.25±0.04 | |
| Spleen | ND | _____ | |||
| Female | Liver | 0.24±0.04 | _____ | ||
| Spleen | ND | _____ | |||
| Female | Male | Liver | 0.81±0.06† | ||
| Spleen | ND | _____ | |||
| Female | Liver | 0.82±0.09† | |||
| Spleen | ND | _____ | |||
|
| |||||
| 16 | Male | Male | Liver | 0.21±0.04 | |
| Spleen | ND | _____ | |||
| Female | Liver | 0.24±0.07 | |||
| Spleen | ND | _____ | |||
| Female | Male | Liver | 0.69±0.08† | ||
| Spleen | 0.77±0.19a | 4.6±0.8 | |||
| Female | Liver | 0.68±0.04† | |||
| Spleen | 0.76±0.08a | 5.9±1.2 | |||
|
| |||||
| 30 | Male | Male | Liver | 0.17±0.03 | |
| Spleen | 0.22±0.05a | 5.4±0.9 | |||
| Female | Liver | 0.18±0.03 | |||
| Spleen | 0.20±0.05a | 5.7±0.9 | |||
| Female | Male | Liver | 0.61±0.10† | ||
| Spleen | 0.68±0.11a† | 6.0±1.1 | |||
| Female | Liver | 0.58±0.03† | |||
| Spleen | 0.60±0.09† | 5.0±0.8 | |||
|
| |||||
| 45 | Male | Male | Liver | 0.27±0.05 | |
| Spleen | 0.26±0.06 | 9.1±2.3 | |||
| Female | Liver | 0.27±0.05 | |||
| Spleen | 0.34±0.06 | 19.3±3.7# | |||
| Female | Male | Liver | 0.73±0.12† | ||
| Spleen | 0.71±0.06† | 18.6±2.6† | |||
| Female | Liver | 0.73±0.06† | |||
| Spleen | 0.75±0.14† | 9.3±1.8†# | |||
Donor hepatocytes isolated from adult male or female rats were infused into the spleens of same age male and female recipient rats. The recipients were euthanized at 8, 16, 30 and 45 weeks after transplantation when CYP2A1 mRNA levels were determined in both organs. Values are presented as CYP2A1 mRNA per rat albumin mRNA (liver and spleen) and as % of autologous liver CYP2A1 mRNA/mg (spleen). Each value is a mean ±SD with n=6 unless otherwise indicated. ND, not detectable,
, P<0.05 compares spleen with liver of the same rat.
P<0.05 compares same sex donors in opposite sex recipients of the same age.
P<0.05 compares opposite sex donors in same sex recipients of the same age.
, n=3.
In spite of the expression of albumin mRNA in all of the hepatocyte transplants at 8 weeks post transplantation, none of the cells, regardless of recipient or donor sex, contained detectable concentrations of CYP2A1 mRNA. At 16 post transplantation weeks, half of the female recipients with splenic hepatocytes from either sex, expressed high autologous liver-like concentrations of CYP2A1 mRNA. The remaining 50% of the female recipients exhibited no measurable splenic CYP2A1. In contrast, CYP2A1 levels in splenic hepatocytes, male- and female-derived remained undetectable in all the male recipients at 16 post transplantation weeks (Table 2). At 30 and 45 weeks post transplantation, donor hepatocytes in female, and now male recipients, expressed CYP2A1 mRNA. The transcript levels, however, reflected the sex of the host and not that of the donor. That is, isoform levels in male-derived splenic hepatocytes in female recipients were now elevated to female-like hepatic concentrations whereas CYP2A1 mRNA levels in female-derived splenic hepatocytes in male recipients were suppressed to male-like hepatic concentrations. Total splenic CYP2A1 mRNA levels (i.e., percent of autologous liver CYP2A1 mRNA/mg) generally reflected albumin mRNA levels (Table 2).
CYP2C7
As previously reported (17, 19), we found that CYP2C7 mRNA is another female-predominant hepatic isoform whose expression was ~ 3-fold greater in female liver than male liver (data not presented). Neither the age of the rats nor the presence of splenic hepatocytes altered the sex-dependent hepatic ratio of the transcript. Regardless of the sex of either the recipients or donors, all of the transplanted splenic hepatocytes lost the ability to express measurable levels of CYP2C7 mRNA at any of the determined time points. (One or 2 spleens with female-derived hepatocytes in female recipients contained trace amounts of the transcript at 16 and 30 weeks post transplantation which was no longer observed 15 weeks later.)
CYP2C11
CYP2C11 is a male-specific hepatic isoform that comprises >50% of the total liver cytochrome P450 in adult male rats (17, 19). In agreement, while we found robust levels of the transcript in the livers of males at all ages, we were unable to detect CYP2C11 mRNA in female livers at any of the ages analyzed (data not presented). Although the pre-infused male hepatocytes expressed CYP2C11 mRNA at concentrations no different than that observed in the livers of recipient male rats, none of cells expressed measurable levels of the transcript when transplanted into the spleens of recipient males as well as females after 8, 16, 30 and even 45 weeks. Similarly, the isolated female hepatocytes, containing no detectable CYP2C11 mRNA at the time of infusion, remained so when transplanted into the inducible environment of the male rat.
CYP3A2
Like CYP2C11, CYP3A2 is a male-specific hepatic isoform whose expression is suppressed in female rats (17, 19). In agreement, we observed that expression of CYP3A2 mRNA was limited to male liver and that the concentration of the transcript was independent of the male’s age and the presence of splenic hepatocytes (Table 3). Unlike CYP2C11 mRNA, CYP3A2 mRNA was expressed, sex-dependently, in splenic hepatocytes. Although none of the female recipients, regardless of donor sex, expressed any detectable splenic levels of the transcript, hepatocytes of either sex, transplanted into the spleens of males, did contain CYP3A2 mRNA 30 and 45 weeks after infusion. However, the concentrations of the isoform in splenic hepatocytes were only ~30% of that observed in the recipient males’ livers (Table 3). Consequently, the amount of CYP3A2 mRNA in a mg of implanted spleen was only ~2.5% of that found in a mg of autologous liver.
Table 3.
Sexually Dimorphic Expression of CYP3A2 mRNA in Liver and Spleen-containing Hepatocyte Transplants.
| Weeks Post | |||||
|---|---|---|---|---|---|
| Transplantation
|
Recipient
|
Donor
|
CYP3A2/Albumin
|
% Liver CYP3A2
|
|
| 8 | Male | Male | Liver | 0.49±0.04 | |
| Spleen | ND | _____ | |||
| Female | Liver | 0.46±0.07 | |||
| Spleen | ND | _____ | |||
| Female | Male | Liver | ND | ||
| Spleen | ND | _____ | |||
| Female | Liver | ND | |||
| Spleen | ND | _____ | |||
|
| |||||
| 16 | Male | Male | Liver | 0.55±0.08 | |
| Spleen | ND | _____ | |||
| Female | Liver | 0.57±0.08 | |||
| Spleen | ND | _____ | |||
| Female | Male | Liver | ND | ||
| Spleen | ND | _____ | |||
| Female | Liver | ND | |||
| Spleen | ND | _____ | |||
|
| |||||
| 30 | Male | Male | Liver | 0.47±0.05 | |
| Spleen | 0.16±0.03* | 2.8±0.7 | |||
| Female | Liver | 0.45±0.06 | |||
| Spleen | 0.13±0.02* | 2.4±0.7 | |||
| Female | Male | Liver | ND | ||
| Spleen | ND | _____ | |||
| Female | Liver | ND | |||
| Spleen | ND | _____ | |||
|
| |||||
| 45 | Male | Male | Liver | 0.52±0.07 | |
| Spleen | 0.15±0.03* | 2.4±0.05 | |||
| Female | Liver | 0.55±0.08 | |||
| Spleen | 0.17±0.03* | 2.5±0.07 | |||
| Female | Male | Liver | ND | ||
| Spleen | ND | _____ | |||
| Female | Liver | ND | |||
| Spleen | ND | _____ | |||
Donor hepatocytes isolated from adult male or female rats were infused into the spleens of same age male and female recipient rats. The recipients were euthanized at 8, 16, 30 and 45 weeks after transplantation when CYP3A2 mRNA levels were determined in both organs. Values are presented as CYP3A2 mRNA per rat albumin mRNA (liver and spleen) and as % of autologous liver CYP3A2 mRNA/mg (spleen). Each value is a mean ±SD with n=6 unless otherwise indicated. ND, not detectable,
, P<0.05 compares spleen with liver of the same rat.
P<0.05 compares same sex donors in opposite sex recipients of the same age.
P<0.05 compares opposite sex donors in same sex recipients of the same age.
DISCUSSION
Whereas the pre-infused hepatocytes expressed their expected CYP sexual dimorphisms, their post transplantational competence, depending on the isoform, was either profoundly delayed, if not permanently lost and now reflected the sexual dimorphisms of the recipient (as observed in the host’s liver) rather than their pre-infused dimorphisms. This finding that CYP expression in the transplanted hepatocytes take on the sexual characteristics of the recipient’s liver supports previous observations that the sex-dependent growth hormone (GH) secretory profiles to which the hepatocytes are exposed (in the present case that of the host) determines which sexually dimorphic CYP isoforms are expressed. Presently, all species examined, including rats, mice, chickens (17) and humans (34, 35) exhibit sex differences in GH secretory profiles. In the case of the rat, males secrete GH in episodic bursts (~200–300 ng/ml of plasma) about every 3.5 to 4 hr. Between the peaks GH is absent. In females, the hormone pulses are more frequent and irregular and are of lower magnitude than those in males, whereas substantial concentrations of GH are always secreted between peaks. While the sexually dimorphic GH profiles are not exactly the same in all species, there are several commonalities such as the higher pulse amplitudes in the males and greater pulse frequencies with corresponding briefer interpeak periods in the females. In fact, it is now clear that sex differences in the circulating GH profiles, and not GH concentrations, per se, are responsible for sexual dimorphisms ranging from body growth, muscle mass, bone density and fat metabolism as well as the expression of hepatic isoforms of CYP in all species examined (cf. 28).
Of the near dozen sex-dependent isoforms of CYP in the rat liver, comprising the bulk of constituent activity, some are only expressed in either males or females (sex-specific) whereas others are expressed in both sexes, but at considerably different concentrations (sex-predominant). In this regard, we have discovered numerous inherent “regulatory signals” in the sexually dimorphic GH profiles that are individually responsible for either inducing or suppressing each of the sex-dependent CYP isoforms. For example, CYP2C12, the major female-specific rat isoform, requires a continuous (no GH-devoid interpulse of any length) profile for expression (36–39). Accordingly, male hepatocytes, before isolation, were exposed to the episodic GH profile and thus were unable to express CYP2C12. Transplantation of the male-derived hepatocytes into the spleens of females exposed them to the continuous GH profile now inducing CYP2C12 expression. As would be expected, the elevated expression levels of CYP2C12 observed in pre-infused female hepatocytes were completely suppressed when the cells were transplanted into male spleens. In contrast, male-like levels of hepatic CYP3A2 are dependent upon the masculine episodic GH profile, the feminine continuous GH profile is completely suppressive (36–39). Accordingly, pre-infused female hepatocytes expressed no measurable CYP3A2 mRNA. Following transplantation into the spleens of male rats, the hepatocytes were now exposed to the masculine circulating GH profile which subsequently induced expression of the isoform in the female hepatocytes. In agreement with the proposed regulatory mechanism, the elevated expression levels of CYP3A2 observed in pre-infused male hepatocytes were completely suppressed when the cells were transplanted into the spleens of female rats. Unlike CYP2C12 and CYP3A2, CYP2A1 is not a sex-specific isoform, but rather a sex-predominant isoform. Whereas the masculine GH secretory profile can induce some CYP2A1 expression, the isoform is more responsive to the feminine continuous profile resulting in a male to female sex ratio of 1 to 3 (36–39). Accordingly, pre-transplanted male hepatocytes expressed the lower male-like levels of CYP2A1. Following transplantation into the spleens of females, concentrations of the isoform increased 3-fold to the characteristic female-like levels. Moreover, the elevated pre-infusion expression levels of CYP2A1 in female hepatocytes were reduced to male-like concentrations when the cells were transplanted into the spleens of males.
While the CYP2C12, -3A2 and -2A1 findings support the reported mechanism that circulating GH profiles are the regulators of sex differences in expression levels of hepatic CYP isoforms, we have otherwise noted two confounding results. First, while the transplanted hepatocytes expressed albumin mRNA at all measured time points, expression of the three detectable isoforms were delaying until 16 to 30 weeks post transplantation. Second, and perhaps more significant, two of the isoforms, the major male-specific CYP2C11 and the female-predominant CYP2C7, remained statistically undetectable almost a year after transplantation. It is possible had CYP transcripts been measured by real time quantitative PCR, as we have reported in previous studies (40), we may have observed an earlier appearance of CYP2C12, -3A2 and -2A1 and possible nominal, but physiologically inconsequential concentrations of CYP2C11 and CYP2C7.
The fact that the intrasplenic hepatocytes did not express CYP2C12, -3A2 and - 2A1 for 4 to 7 months after transplantation and exhibited a permanent loss of CYP2C11 and CYP2C7, in spite of normal expression levels of all the isoforms at the time of infusion, suggest an initial, prolonged and sometimes permanent period of CYP repression followed by a subsequent re-expression of some isoforms. These findings of an initial suppression of isoform expression somewhat parallels in vitro findings of hepatocytes in cell culture (41, 42). We have reported (27, 43) that primary hepatocytes in culture initially experience a dramatic decline in the expression levels of their near dozen sex-dependent constituent CYP isoforms, some isoforms never recovering; as also seen in the intrasplenic hepatocytes. Although careful replication of the species and gender-dependent GH profiles to the culture media can restore physiologic concentrations of many of the constituent isoforms, some remain permanently repressed (27, 43), again, similar to the present findings with the intrasplenic transplanted hepatocytes.
It may be concluded that while CYP enzymes are both an ancient (~ 1.5 billion years old) and an enormous superfamily (comprising many hundreds of isoforms) expressed in near every, if not all phyla and within almost every cell (15, 16, 44), they simply do not function well ex-situ. In this regard, the hepatic acinus, the structural and functional unit of hepatic parenchyma, is composed of hepatocytes differing in morphological, biochemical and functional abilities, depending upon the location of the cells in relation to the inflow or outflow of portal blood, i.e., periportal or perivenous (45, 46). It is now well known that most of the sex-dependent rat hepatic CYP isoforms which comprise the bulk of constituent activity, exhibit a particularly prominent zonation within the acinus which then determines both their constituent and inducible levels (47, 48). These isoforms include CYP2A1, -3A1, -3A2, -2C11, -2C12 and -2E1 (47, 48). While the intrasplenic hepatocytes exhibit some cord-like structures, their general architecture does not resemble that of a normal hepatic acinus and might explain the inability of the transplanted liver cells to express CYP2C11 and CYP2C7. However, primary hepatocytes in cell culture whose architecture in no way resembles an hepatic acinus, are capable of expressing physiologic-like concentrations of CYP2C11 but no detectable CYP3A2 (43). Clearly the loss of architectural integrity of the transplanted intrasplenic hepatocytes as well as primary hepatocytes in cultures is not sufficient to explain the inability of ex-situ hepatocytes to express their normal complement of constituent CYP isoforms. Normal expression of some of the constituent isoforms outside the confines of the normal acinus structure, apparently require yet to be identified factors.2 Although the likely gender-dependent expression of CYP isoforms of human intrasplenic hepatocyte transplants is speculative, it is noteworthy to mention that the major human CYP isoforms are also expressed at sexually dimorphic levels regulated by gender differences in circulating GH profiles (18, 49).
Acknowledgments
Financial Support: This work was supported in part by National Institutes of Health Grant HD-061285 and the Chutzpah Foundation.
Abbreviations
- CYP
cytochrome P450
- GH
growth hormone
Footnotes
When compared to our previous reports (27, 28) the lower percent viability obtained in the present study may be due to our omission of the Percoll gradient known to remove dead as well as small (i.e. immature) hepatocytes; the latter considered important for colonizing the spleen (29).
Differences in the source of arterial blood are unlikely to explain abnormalities in CYP expression of intrasplenic hepatocytes since both the liver and spleen are perfused by branches of the celiac artery.
References
- 1.Fuller BJ. Transplantation of isolated hepatocytes. A review of current ideas. J Hepatology. 1988;7:368–376. doi: 10.1016/s0168-8278(88)80010-2. [DOI] [PubMed] [Google Scholar]
- 2.Balladur P, Honiger J, Calmus Y, Vaubourdolle M, Delelo R, Capeau J, Nordlinger B. Transplantation d’hepatocytes allogeniques sans immunosuppression: survie a long terme. Ann Gastroenterol Hepatol (Paris) 1994;30:265–269. [PubMed] [Google Scholar]
- 3.Selden C, Darby H, Hodgson HJF. Further observations on the survival, proliferation and function of ectopically implanted syngeneic and allogeneic liver cells in rat spleen. Eur J Gastroenterol Hepatol. 1991;3:607–611. [Google Scholar]
- 4.Rivas P, Fabrega AJ, Schwartz D, Digiantis W, Pollak R. Preservation and transplantation of purified canine hepatocytes. Transpl Proc. 1992;24:2833–2836. [PubMed] [Google Scholar]
- 5.Sandbichler P, Then P, Vogel W, Erhart R, Dietze O, Philadelphy H, et al. Hepatocellular transplantation into the lung for temporary support of acute liver failure in the rat. Gastroenterology. 1992;102:605–609. doi: 10.1016/0016-5085(92)90109-c. [DOI] [PubMed] [Google Scholar]
- 6.Dixit V. Transplantation of isolated hepatocytes and their role in extrahepatic life support systems. Scand J Gastroenterol. 1995;30 (Suppl 208):101–110. doi: 10.3109/00365529509107770. [DOI] [PubMed] [Google Scholar]
- 7.Sommer BG, Sutherland DER, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation for treatment of D-galactosamine induced acute liver failure in rats. Transplant Proc. 1979;11:578–584. [PubMed] [Google Scholar]
- 8.Ochenashko OV, Volkova NA, Mazur SP, Somov AY, Fuller BJ, Petrenko AY. Cryopreserved fetal liver cell transplants support the chronic failing liver in rats with CCl4-induced cirrhosis. Cell Transplant. 2006;15:23–33. doi: 10.3727/000000006783982232. [DOI] [PubMed] [Google Scholar]
- 9.Cuervas-Mons V, Cienfuegos JA, Maganto P, Golitsin A, Eroles G, Castillo-Olivares J, et al. Time related efficacy of liver cell isografts in fulminant hepatic failure. Transplantation. 1984;38:23–25. doi: 10.1097/00007890-198407000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland DER, Sommer BG, Hong C, Numata M, Najarian JS. Liver cell transplantation. In: Najarian JS, Delame JP, editors. Hepatic, Biliary and Pancreatic Surgery. Chicago, London: Mosby Year Book Medical Publishers; 1980. pp. 531–551. [Google Scholar]
- 11.Woods RJ, Parbhoo SP. An explanation for the reduction in bilirubin levels in congenitally jaundiced Gunn rats after transplantation of isolated hepatocytes. Eur Surg Res. 1981;13:278–284. doi: 10.1159/000128194. [DOI] [PubMed] [Google Scholar]
- 12.Vroemen JPAM, Buurman WA, Heirwegh KPM, Van der Linden CJ, Kootstra G. Hepatocyte transplantation for enzyme deficiency disease in congenic rats. Transplantation. 1986;42:130–135. doi: 10.1097/00007890-198608000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Wiederkehr JC, Kondos GT, Pollak R. Hepatocyte transplantation for the low- density lipoprotein receptor-deficient state. Transplantation. 1990;50:466–476. doi: 10.1097/00007890-199009000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Tejera ML, Cienfuegos JA, Maganto P, Pardo F, Santamaria L, Codesal J, et al. Reduction of cholesterol levels following liver cell grafting in hyperlipidemic (WHHL) rabbits. Transplant Proc. 1992;24:160–161. [PubMed] [Google Scholar]
- 15.Guengerich FP. Comparisons of catalytic selectivity of cytochrome P450 subfamily enzymes from different species. Chemico-Biol Interact. 1997;106:161–182. doi: 10.1016/s0009-2797(97)00068-9. [DOI] [PubMed] [Google Scholar]
- 16.Nelson DR, Zeldin DC, Hoffman SMG, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenet. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro BH, Agrawal AK, Pampori NA. Gender differences in drug metabolism regulated by growth hormone. Int J Biochem Cell Biol. 1995;27:9–20. doi: 10.1016/1357-2725(94)00056-5. [DOI] [PubMed] [Google Scholar]
- 18.Dhir RN, Dworakowski W, Thangavel C, Shapiro BH. Sexually dimorphic regulation of hepatic isoforms of human cytochrome P450 by growth hormone. J Pharmacol Exp Ther. 2006;316:87–94. doi: 10.1124/jpet.105.093773. [DOI] [PubMed] [Google Scholar]
- 19.Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–228. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato K, Hodgson JB, Lutton JD, Abraham NG. Developmental expression of cytochrome P450s within intrasplenically transplanted fetal hepatocytes from spontaneously hypertensive rats. Cell Transplant. 1994;3:15–21. doi: 10.1177/096368979400300104. [DOI] [PubMed] [Google Scholar]
- 21.Lupp A, Hugenschmidt S, Danz M, Müller D. Influence of recipient gender on cytochrome P450 isoforms expression in intrasplenic fetal liver tissue transplants in rats. Toxicology. 2003;188:171–186. doi: 10.1016/s0300-483x(03)00080-5. [DOI] [PubMed] [Google Scholar]
- 22.Lupp A, Hugenschmidt S, Rost M, Müller D. Influence of recipient gender on intrasplenic fetal liver tissue transplants in rats: cytochrome P450-mediated monooxygenase functions. Toxicology. 2004;197:199–212. doi: 10.1016/j.tox.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Maganto P, Traber PG, Rusnell C, Dobbins WO, Keren D, Gumucio JJ. Long-term maintenance of the adult pattern of liver-specific expression for P-450b, P-450e, albumin and α-fetoprotein genes in intrasplenically transplanted hepatocytes. Hepatology. 1990;11:585–593. doi: 10.1002/hep.1840110410. [DOI] [PubMed] [Google Scholar]
- 24.Ryan DE, Levin W. Age- and gender-related expression of rat liver cytochrome P450. In: Schenkman JB, Greim H, editors. Cytochrome P450 (Handbook of Experimental Pharmacology) New York: Springer-Verlag; 1993. pp. 461–476. [Google Scholar]
- 25.Garcia MC, Thangavel C, Shapiro BH. Epidermal growth factor regulation of female- dependent CYP2A1 and CYP2C12 in primary rat hepatocyte culture. Drug Metab Dispos. 2001;29:111–120. [PubMed] [Google Scholar]
- 26.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 27.Thangavel C, Garcia MC, Shapiro BH. Intrinsic sex differences determine expression of growth hormone-regulated female cytochrome P450s. Mol Cell Endocrinol. 2004;220:31–39. doi: 10.1016/j.mce.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Thangavel C, Shapiro BH. A molecular basis for the sexually dimorphic response to growth hormone. Endocrinology. 2007;148:2894–2903. doi: 10.1210/en.2006-1333. [DOI] [PubMed] [Google Scholar]
- 29.Rajvanshi P, Liu D, Ott M, Gagandeep S, Schilsky ML, Gupta S. Fractionation of rat hepatocyte subpopulations with varying metabolic potential, proliferative capacity, and retroviral gene transfer efficiency. Exp Cell Res. 1998;244:405–419. doi: 10.1006/excr.1998.4223. [DOI] [PubMed] [Google Scholar]
- 30.Waxman DJ. Rat hepatic P450IIA and P450IIC subfamily expression using catalytic, immunochemical and molecular probes. Methods Enzymol. 1991;206:249–267. doi: 10.1016/0076-6879(91)06095-k. [DOI] [PubMed] [Google Scholar]
- 31.Ram PA, Waxman DJ. Hepatic P450 expression in hypothyroid rats: Differential responsiveness of male-specific P450 forms 2a (IIIA2), 2c (IIC11), and RLM2 (IIA2) to thyroid hormone. Mol Endocrinol. 1991;5:13–20. doi: 10.1210/mend-5-1-13. [DOI] [PubMed] [Google Scholar]
- 32.Arosio B, Santambrogio D, Gagliano N, Annoni G. Changes in expression of the albumin, fibronectin and type I procollagen genes in CCl4-induced liver fibrosis: effect of pyridoxol L, 2-pyrrolidon-5 carboxylate. Pharmacol Toxicol. 1993;73:301– 304. doi: 10.1111/j.1600-0773.1993.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 33.Ramsden R, Sommer KM, Omiecinski CJ. Phenobarbital induction and tissue- specific expression of the rat CYP2B2 gene in transgenic mice. J Biol Chem. 1993;286:21722–21726. [PubMed] [Google Scholar]
- 34.Hartman ML, Iranmanesh A, Thorner MO, Veldhuis JD. Evaluation of pulsatile patterns of growth hormone release in humans: a brief review. Am J Human Biol. 1993;5:603–614. doi: 10.1002/ajhb.1310050603. [DOI] [PubMed] [Google Scholar]
- 35.Enstrom BE, Karlsson FA, Wide L. Marked gender differences in ambulatory morning growth hormone values in young adults. Clin Chem. 1998;44:1289–1295. [PubMed] [Google Scholar]
- 36.Pampori NA, Shapiro BH. Feminization of hepatic cytochrome P450s by nominal levels of growth hormone in the feminine plasma profile. Mol Pharmacol. 1996;50:1148–1156. [PubMed] [Google Scholar]
- 37.Pampori NA, Shapiro BH. Gender differences in the responsiveness of the sex- dependent isoforms of hepatic P450 to the feminine plasma growth hormone profile. Endocrinology. 1999;140:1245–1254. doi: 10.1210/endo.140.3.6545. [DOI] [PubMed] [Google Scholar]
- 38.Agrawal AK, Shapiro BH. Differential expression of gender-dependent hepatic isoforms of cytochrome P-450 by pulse signals in the circulating masculine episodic growth hormone profile of the rat. J Pharmacol Exp Ther. 2000;292:228–237. [PubMed] [Google Scholar]
- 39.Agrawal AK, Shapiro BH. Intrinsic signals in the sexually dimorphic circulating growth hormone profiles of the rat. Mol Cell Endocrinol. 2001;173:167–181. doi: 10.1016/s0303-7207(00)00401-9. [DOI] [PubMed] [Google Scholar]
- 40.Thangavel C, Dhir RN, Volgin DV, Shapiro BH. Sex-dependent expression of CYP2C11 in spleen, thymus and bone marrow regulated by growth hormone. Biochem Pharmacol. 207(74):1476–1484. doi: 10.1016/j.bcp.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright MC, Pain AJ. Evidence that the loss of rat liver cytochrome P450 in vitro is not solely associated with the use of collagenase, the loss of cell-cell contacts and/or the absence of an extracellular matrix. Biochem Pharmacol. 1992;43:237–243. doi: 10.1016/0006-2952(92)90283-o. [DOI] [PubMed] [Google Scholar]
- 42.Niwa T, Koide N, Tsuji T, Imaoka S, Ishibashi F, Funae Y, Katagiri M. Cytochrome P450s of isolated rat hepatocytes in spheroid and monolayer cultures. Res Commun Mol Pathol Pharmacol. 1996;91:372–378. [PubMed] [Google Scholar]
- 43.Thangavel C, Dworakowski W, Shapiro BH. Inducibility of male-specific isoforms of cytochrome P450 by sex-dependent growth hormone profiles in hepatocyte cultures from male but not female rats. Drug Metab Dispos. 2006;34:410–419. doi: 10.1124/dmd.105.007716. [DOI] [PubMed] [Google Scholar]
- 44.Nelson DR. Metazoan cytochrome P450 evolution. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;121:15–22. doi: 10.1016/s0742-8413(98)10027-0. [DOI] [PubMed] [Google Scholar]
- 45.Jungermann K. Functional heterogeneity of periportal and perivenous hepatocytes. Enzyme. 1986;35:161–180. doi: 10.1159/000469338. [DOI] [PubMed] [Google Scholar]
- 46.Traber PG, Chianale J, Gumucio JJ. Physiologic significance and regulation of hepatocellular heterogeneity. Gastroenterology. 1988;95:1130–1143. doi: 10.1016/0016-5085(88)90194-1. [DOI] [PubMed] [Google Scholar]
- 47.Buhler R, Lindros KO, Nordling A, Johansson I, Ingelman-Sundberg M. Zonation of cytochrome-P450 isozyme expression and induction in rat liver. Eur J Biochem. 1992;204:407–412. doi: 10.1111/j.1432-1033.1992.tb16650.x. [DOI] [PubMed] [Google Scholar]
- 48.Lindros KO. Zonation of cytochrome P450 expression, drug metabolism and toxicity in liver. Gen Pharmacol. 1997;28:191–196. doi: 10.1016/s0306-3623(96)00183-8. [DOI] [PubMed] [Google Scholar]
- 49.Jaffe CA, Turgeon DK, Lown K, Demott-Friberg R, Watkins APB. Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol. 2002;283:E1008–E1015. doi: 10.1152/ajpendo.00513.2001. [DOI] [PubMed] [Google Scholar]