Abstract
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that can be released or activated in a neuronal activity dependent manner. Although pathologically elevated levels of MMPs may be synaptotoxic, physiologically appropriate levels of MMPs may instead enhance synaptic transmission. MMP inhibitors can block long term potentiation (LTP), and at least one family member can affect an increase in the volume of dendritic spines. While the mechanism by which MMPs affect these changes is not completely understood, one possibility is that the cleavage of specific synaptic cell adhesion molecules plays a role. In the present study, we have examined the ability of neuronal activity to stimulate rapid MMP dependent shedding of the intercellular adhesion molecule-5 (ICAM-5), a synaptic adhesion molecule that is thought to inhibit the maturation and enlargement of dendritic spines. Since such cleavage would likely occur within minutes if it were relevant to a process such as LTP, we focused on post stimulus time points of thirty minutes or less. We show that NMDA can stimulate rapid shedding of ICAM-5 from cortical neurons in dissociated cell cultures and that such shedding is diminished by pretreatment of cultures with inhibitors that target MMP-3 and -9, proteases thought to influence synaptic plasticity. Additional studies suggest that MMP mediated cleavage of ICAM-5 occurs at amino acid 780, so that the major portion of the ectodomain is released. Since reductions in ICAM-5 have been linked to changes in dendritic spine morphology that are associated with LTP, we also examined the possibility that MMP dependent ICAM-5 shedding occurs following high frequency tetanic stimulation of hippocampal slices. Results show that the shedding of ICAM-5 occurs in association with LTP, and that both LTP and the associated ICAM-5 shedding are reduced when slices are pretreated with an MMP inhibitor. Together, these findings suggest that neuronal activity is linked to the shedding of a molecule that may inhibit dendritic spine enlargement and that MMPs can affect this change. While further studies will be necessary to determine the extent to which cleavage of ICAM-5 in particular contributes to MMP dependent LTP, our data support an emerging body of literature suggesting that MMPs are critical mediators of synaptic plasticity.
Keywords: Telencephalin, NMDA, MMPs, dendrite, plasticity, shedding, dendritic spine
Introduction
MMPs are a family of zinc dependent endopeptidases that were named for their ability to process proteins of the extracellular matrix including collagens and laminins (McCawley and Matrisian, 2001, Sternlicht and Werb, 2001). It is now well established that these proteinases can also process a variety of non matrix proteins, including varied soluble molecules and cell surface receptors (McCawley and Matrisian, 2001). The family includes soluble secreted forms as well as transmembrane members, and activity is controlled at many levels including expression, cellular release, and activation. The MMPs are also bound by endogenous inhibitors including the tissue inhibitors of metalloproteinases (TIMPs), which can interact with both pro- and active forms.
MMP expression in the CNS can be dramatically upregulated by a variety of noxious stimuli including infection, hypoxia, and excitotoxins (Milward et al., 2007, Rosenberg, 2009). Many studies of MMPs in the CNS have therefore focused on their role in pathology. In excess, MMPs likely contribute to neuronal and synaptic injury.
Less is known about the role of MMPs in normal CNS physiology, and thus whether critical physiological processes are likely disrupted when MMP levels are pathologically elevated. Studies to date have, however, determined that MMPs may play a critical role in learning and memory. For example, a broad spectrum MMP inhibitor has been shown to influence hippocampal CA1 plasticity (Meighan et al., 2007), and MMP-9 activity in particular has been implicated in the maintenance of late LTP (Nagy et al., 2006). In addition, antisense to MMPs has been shown to prevent acquisition in the Morris water maze test (Meighan et al., 2006), and methamphetamine-induced behavioral sensitization is reduced in mice lacking MMP-2 –or MMP-9 (Mizoguchi et al., 2007a). Similarly, proteases have been shown to contribute to cocaine associated conditioned place preference (Brown et al., 2007, Brown et al., 2008, Maiya et al., 2009). Recent studies also suggest that some MMPs may selectively modulate NMDA or AMPA receptor function (Cho et al., 2008, Pauly et al., 2008, Szklarczyk et al., 2008, Michaluk et al., 2009), and that they may influence long term depression (Meighan et al., 2007, Cho et al., 2008).
Consistent with their potential to influence learning and memory, several MMPs are expressed in cells of the central nervous system including neurons and astrocytes (Yong et al., 2001, Milward et al., 2007). In addition, studies suggest that at least two of the soluble MMPs and one of the transmembrane forms can be expressed, released or activated in a neuronal activity dependent manner (Meighan et al., 2006, Michaluk et al., 2007b, Cho et al., 2008, Pauly et al., 2008). In one study, MMP-3 and -9 levels were shown to increase with hippocampal dependent learning and memory while MMP-2 was not (Meighan et al., 2006). A more recent study showed that NMDA stimulation of spinal cord neurons was linked to an increase in MMP-3 activity (Pauly et al., 2008). In addition, one of the transmembrane MMPs, tumor necrosis factor alpha converting enzyme (TACE), was recently shown to be regulated in an activity dependent manner through a mechanism involving metabotropic glutamate receptor activation (Cho et al., 2008).
The localization of MMPs also suggests they may be important to activity dependent changes at the synapse. For example, recently published data have shown that these enzymes can be localized to synaptic terminals (Sbai et al., 2008). It is tempting to speculate that, as may be the case in non CNS cell types, MMP release from preformed vesicular stores might quickly follow the activation of particular signaling pathways (Kean et al., 2009). The mechanisms by which MMPs may contribute to synaptic changes that underlie learning and memory are likely multiple and at this point, not completely understood. Remodeling of the extracellular matrix has been posited, as has an MMP dependent change in integrin signaling with potential effects on signaling pathways affecting glutamate receptor channels (Nagy et al., 2006). Consistent with a role for integrins are studies in which integrin receptor antagonists have blocked MMP dependent changes in dendritic spine shape or LTP (Nagy et al., 2006, Meighan et al., 2007, Wang et al., 2008).
An additional mechanism by which MMPs might rapidly modulate synaptic structure and function would be through their ability to cleave specific synaptic cell adhesion molecules. Of particular interest is their potential to stimulate the shedding of ICAM-5, an adhesion molecule that is expressed only by spiny neurons in the telencephalon. The C terminal intracytoplasmic domain of ICAM-5 is linked to ezrin-radixin-moiesin proteins that can influence actin polymerization (Furutani et al., 2007), and an association between reduced expression of ICAM-5 and spine enlargement has been demonstrated (Matsuno et al., 2006). In addition, in a study focused on developmental plasticity, long term NMDA treatment (16 h) of neurons was associated with spine enlargement and MMP dependent shedding of ICAM-5 (Tian et al., 2007).
In the present study, we tested the possibility that NMDA might stimulate MMP dependent ICAM-5 shedding within minutes, as might be expected if ICAM-5 shedding were to play a role in LTP. In addition, because tetanic stimulation might stimulate release of glutamate and/or MMPs in a relatively localized manner, we tested the possibility that ICAM-5 shedding would indeed occur with a stimulus known to induce LTP.
Experimental Procedures
Reagents and antibodies
Recombinant active MMP-2, -3, -7 and -9 were obtained from Calbiochem/EMD Biosciences (San Diego, CA). Of note, while the sequence of a given MMP may vary depending on the species of origin, substrate specificity is usually conserved so that an MMP from one species will typically cleave a substrate from its own species and from a variety of others. The MMP inhibitors (2R)-[(4-Biphenylylsulfonyl)amino]-N-hydroxy-3-phenylpropionamide (BiPS) and N-Isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhydroxamic Acid (NNGH) were also obtained from Calbiochem, while N-methyl-D-aspartic acid (NMDA) and the NMDA receptor antagonist (2R)-amino-5-phosphovaleric acid (APV) were from Sigma (St. Louis, MO). The anti-N terminal ICAM-5 antibody (AF1173) was from R and D systems (Minneapolis, MN). Recombinant ICAM-5 was also obtained from R and D systems. This recombinant construct contains amino acids 31-828 of the extracellular domain of mouse ICAM-5 fused to the Fc region of human IgG. The rabbit polyclonal ICAM-5 cytoplasmic specific antibody was created using two synthetic peptide antigens that correspond to amino acids 889-902 and 905-917 of mouse ICAM-5. These synthetic peptides were conjugated to keyhole limpet hemocyanin and co-injected into rabbits for production of antisera. The antisera specific for ICAM5 were then affinity purified. The anti-β-actin and anti-β-tubulin were purchased from AbCam (Cambridge, MA), and anti-Nav1.2 was purchased from NeuroMab (Davis, CA). Anti postsynaptic density-95 (PSD95) was purchased from NeuroMab, while the anti-glutamic acid decarboxylase (GAD) and anti- synaptophysin (SYP) were purchased from Sigma. Texas-Red conjugated phalloidin was purchased from Molecular Probes.
Dissociated Neuronal Cultures
Animals from which cultures were prepared were humanely euthanized in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals prior to the harvesting of tissue. Every effort was made to reduce the number of animals used. For figures 1c–d, 1f, 2, and 3a, rat hippocampal neurons were plated on polylysine-coated (1 mg/ml) 12 well tissue culture plates at density of 2 × 105 cells/well in the absence of an astrocyte feeder layer. Hippocampal neurons were maintained in neurobasal medium (Gibco, Grand Island, NY) containing 2% B27 supplement (Gibco), and 500 μM L-glutamine (Sigma), and the medium was changed every 3 to 4 days. These neurons were also treated with 5 μM cytosine arabinoside to inhibit the proliferation of non-neuronal cells. Immunostaining indicates that less than 5% of cells were non-neuronal cells at 7 days in vitro (DIV) in this culture system. For other figures, primary cortical neurons were derived from E18-20 rats and prepared as previously described (Haughey et al., 1999). Briefly, tissue was dissociated by gentle trituration in a calcium-free Hank’s balanced salt solution and was centrifuged at 1000 g. Cells were resuspended in minimal essential media containing 10% fetal bovine serum (FBS) and 1% antibiotic solution (104 U of penicillin G/mL, 10 mg streptomycin/mL and 25 μg amphotericin B/mL; Sigma). Neurons were then plated at a density of 100 000 cells/mL on poly-D-lysine coated tissue culture ware. Three hours after plating the media was replaced with serum-free Neurobasal media containing 1 X B-27 supplement (Gibco). Cultures were used between 10 and 14 days in vitro.
Figure 1. NMDA mediates rapid shedding of ICAM-5.
For figure 1a, dissociated neuronal cultures were treated for 30 minutes with medium or medium containing100 μM NMDA. Lysates were subsequently analyzed by Western blot with an antibody to the N terminal domain of ICAM-5. An NMDA associated reduction in full length ICAM-5 (arrow) can be appreciated. Figure 1b shows results from an experiment in which a novel C terminal antibody to ICAM-5 was tested for its ability to detect ICAM-5. Lysates from HEK cells transfected with 1 μg ICAM-5 or vector alone were analyzed by Western blot. A band at approximately 148 kDa, representing mature glycosylated ICAM-5 is detected in ICAM-5 transfectants but not in vector only transfectants (left most lanes as indicated), and detection of this band is eliminated when the Ab was first incubated with those peptides used to generate the antibody (1:10 ratio Ab to peptides). A lower molecular weight band representing non-glycosyalted ICAM-5 is also present in the ICAM-5 transfectants. Figure 1c shows representative results of an experiment, performed in triplicate, in which cultures were stimulated for 15 minutes with100 μM NMDA in the presence or absence of APV (50 μM), as indicated. Lysates were subsequently analyzed by Western blot with anti- C terminal ICAM-5. A putative16 kDa CTF can be appreciated in the NMDA stimulated culture lysates (arrowhead). Figure 1d shows a time course experiment in which NMDA was tested for its ability to generate the 16 kDa CTF. There is an increase in CTF immunoreactivity in lysates within 5 minutes of NMDA administration. Figure 1e is a graphic representation of ICAM-5 showing the transmembrane domain which spans amino acids 834-854, 9 IgC2 like domains (yellow), and the approximate regions to which peptides were generated (orange). The 9.5 kDa portion of the molecule representing the region extending from the intracytoplasmic C terminus to the extracellular side of the transmembrane region, and the 16 kDa portion that likely represents the putative 16 kDa CTF, are also indicated. Figure 1f shows NTFs in supernatants from NMDA treated cultures. Such fragments can be appreciated in 2/3 supernatants from cultures that were stimulated for only 5 minutes, and in 2/2 that were stimulated for 15 minutes.
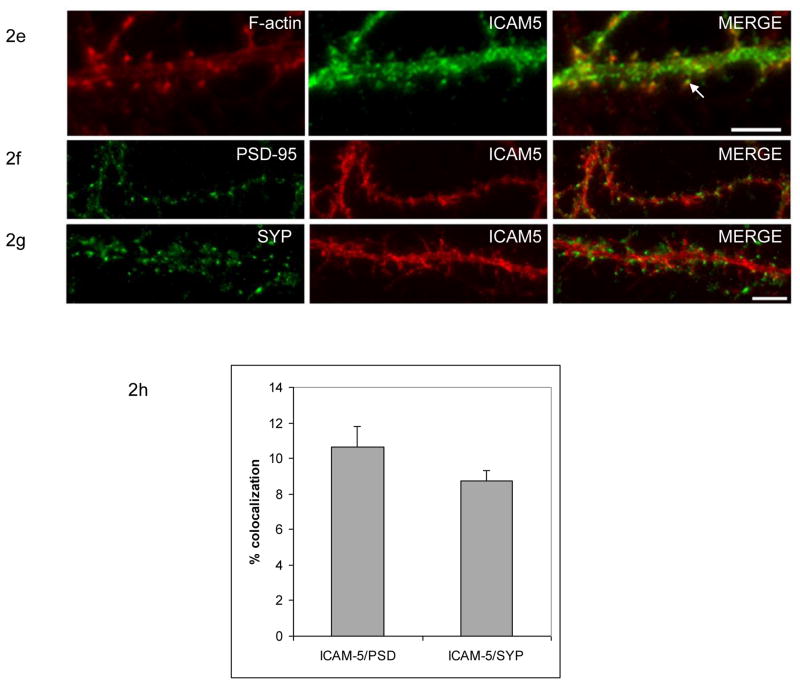
Figure 2. Postsynaptic expression of ICAM-5.
Figures 2a–c show neuronal immunostaining for ICAM-5, MAP2, and the sodium channel Nav1.2. While ICAM-5 and MAP2 immunoreactivity colocalize to some extent, ICAM-5 staining is less apparent along the process that shows immunoreactivity for the sodium channel. The scale bar represents 10 μm. Figure 2d shows that a GAD positive cell (arrow) is negative for ICAM-5. The scale bar again represents 10 μm. Figure 2e shows a higher power image demonstrating both F-actin and ICAM-5 staining. The latter can be appreciated along thin processes (arrow), consistent with its known expression along filopodia and the necks of thin spines. The scale bar represents 5 μm. Figures 2f–g shows a neuronal process immunostained for ICAM-5, PSD95 and synaptophysin (SYP). There is relatively little colocalization with PSD95 or SYP, markers of relatively mature synapses. Quantified results are shown in 2h. The difference between ICAM-5 that was colocalized with SYP or PSD95 and that which was not is significant (P< 0.0001, Student’s t test). The scale bar represents 10 μm.
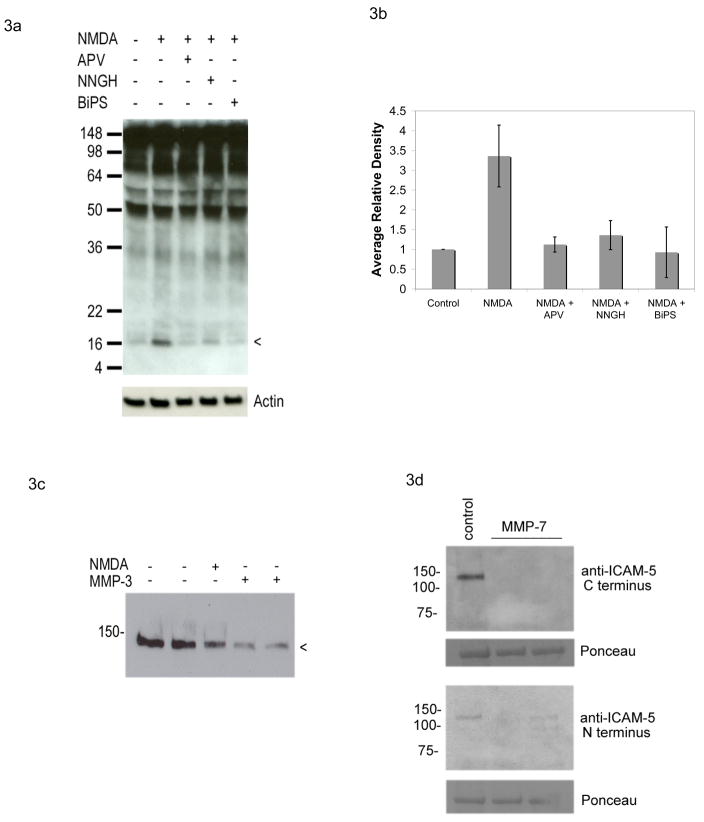
Figure 3. MMPs mediate rapid NMDA dependent shedding of ICAM-5.
Dissociated neuronal cultures were stimulated for 15 minutes with 100 μM NMDA, in the presence or absence of the NMDA receptor blocker APV or the MMP activity inhibitors NNGH or BiPS as indicated. Lysates were then prepared and analyzed by Western blot with an antibody to the C terminal domain of ICAM-5. As shown in Figure 3a, which is representative of 3 experiments, NMDA was again associated with generation of the 16 kDa CTF of ICAM-5 and this was inhibited by pretreatment of cells with 50 mM APV as well as by either 3.2 μM NNGH or 25 μM BiPS. Figure 3b represents densitometric analysis of results, for which differences between control and NMDA were significant (P< 0.05) while differences between control and other treatment groups were not. For figure 3c, neurons were stimulated for 30 minutes with 100 μM NMDA or 100 ng/ml MMP-3, as indicated, and lysates analyzed by Western blot with an antibody to the exodomain. A reduction in immunoreactivity can be appreciated with both NMDA and MMP-3. For figure 3d, neurons were stimulated for 30 minutes with 100 ng/ml MMP-7, as indicated, and lysates analyzed by Western blot with N or C terminal specific antibodies as noted. The major band at approximately 50 kDa seen with Ponceau staining is shown as a loading control. A reduction in ICAM-5 immunoreactivity can be appreciated with MMP-7.
HEK 293A cell culture and DNA transfection
HEK293A cells were purchased from American Type Culture Collection (Manassas, VA). Cells were grown in high glucose Dulbecco’s Modified Eagle Medium (DMEM, MediaTech, Herdon, VA) supplemented with 10% FCS (Hyclone Laboratories, Logan, UT), 50 U/ml penicillin, 50 U/ml streptomycin in a humidified incubator at 37°C under 5% CO2. Cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. HEK293A cells were cotransfected with 1 μg of the construct. After 1 h incubation at 37°C, the media was changed with fresh DMEM. Cells were lysed in reducing sample buffer for Western blot analysis after 48 h transfection.
Constructs
Mouse ICAM-5 cDNA (Entrez nucleotide database number NM0083192) was purchased from Genscript (Piscataway, NJ). The sequence fidelity of ICAM5 was verified by DNA sequencing. This was cloned into pcDNA 3.1 (+) (Invitrogen, Carlsbad CA), with flanking HindIII and XbaI sites.
Hippocampal slice preparation for electrophysiology
Standard procedures for preparing and maintaining hippocampal slices were used as described previously (Wang et al., 2004). All experiments were performed on transverse hippocampal slices (350 mm) prepared from 4–6 week-old male C57/Bl6 mice. Slices were allowed to recover for at least 1h in a holding chamber at room temperature and were then transferred to a submerged recording chamber and perfused continuously (2–3 ml/min) with artificial cerebrospinal fluid (ACSF) which consisted of (in mM): NaCl, 120; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 26; MgSO4, 1.3; CaCl2, 2.5; glucose, 10, pH 7.4. The osmolality was adjusted to 290 mmol/kg, using a 5520 Vapor Pressure Osmometer (Wescor, Inc). Media temperature was maintained at 30–32°C. All solutions for LTP studies contained 50 mM picrotoxin to block GABAA receptor activity. The quality of the slices was confirmed by visualizing the neurons with an upright microscope (Nikon E600FN) using Nomarski-type differential interference contrast optics combined with infrared videomicroscopy (DAGE-MT1), and by performing an input-output curve on each slice.
Cell stimulation, lysate preparation, Western blotting, and densitometric analyses
Unless otherwise indicated, neurons were treated for 15 min. with 100 μM NMDA, with or without a 15 min pretreatment with 50 μM APV, cells were subsequently lysed in reducing sample buffer. Samples were loaded and fractionated on 12% SDS-PAGE gels and transferred onto nitrocellulose membrane. After blocking, the membranes were incubated with anti-ICAM5 cytoplasmic pAb (1:1000), anti-tubulin pAb (1:1000), and anti-actin pAb (1:5000), followed by peroxidase-conjugated secondary antibodies. Membranes were washed with PBS three times and developed with an ECL kit (GE healthcare, Arlington, VA).
For other Western blot figures, lysates from cultured cells were prepared via the addition of lysis buffer [50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% NP-40, 0.5% sodium deoxycholate, 0.2 mM phenylmethylsulfonyl fluoride [PMSF], 0.5 mM dithiothreitol [DTT], 1x protease inhibitor cocktail (Sigma P8340), and added GM-6001 (10 μM)]. The mixture was placed into an eppendorf tube, sonicated for 10 seconds, kept on ice for 20 minutes, and then spun at 14,000 rpm for 15 minutes at 4° C in an eppendorf centrifuge. For lysates from hippocampal slices, each slice was placed into 200 μl of lysis buffer and similarly processed. Supernatants were then saved and used in Western blot experiments. Western blot was performed using 20 μg of protein per lane, as determined by the Qubit assay (Invitrogen). Prior to analysis, samples were mixed with sample buffer containing 5% β-mercaptoethanol and boiled for 5 minutes at 95°C. Electrophoresis was performed on Tris-glycine polyacrylamide gradient gels (Biorad, Hercules, CA). Following electrophoretic transfer of the protein to a polyvinylidene difluoride (PVDF) membrane (Biorad), Hercules, CA), membranes were stained with Ponceau to approximate loading and transfer. Membranes were then blocked in 5% nonfat dry milk in phosphate buffered saline with 0.1% Tween (PBST) for 1 hr. The blot was then probed with the indicated primary antibody, at a dilution recommended by the manufacturer, for 1.5 hr at room temperature. After washing the membrane three times (15 minutes each) in PBST, it was incubated with an appropriate secondary antibody for 1/2 hour at room temperature. The membrane was then washed again in PBST and immunoreactive bands were visualized using electrochemiluminescence (Amersham).
Western blot of recombinant protein digests were performed similarly, except that the entire reaction, rather than 20 μg of total protein, was analyzed. In these experiments, 3 μg of the recombinant protein had been incubated with 400 ng enzyme, and the digestion had been carried out for 60 min in 30 μl of buffer (50 mM Tris, 10 mM CaCl2, 150 mM NaCl, 1 mM ZnCl2, pH 7.5) at 37° C.
Western blot of concentrated supernatants was performed using the 20 μl protein suspension mixed with sample loading buffer (Biorad Laemmli buffer).
Where indicated, densitometric analysis of data was performed using Vision Works Acquisition and Analytical Software, version 6.7.1 (Upland, CA) in accordance with the manufacturer’s instructions.
Concentration of cell culture supernatants
Supernatants were concentrated with VWR centrifugal filters (cat no. 82031-344). 500 μl of supernatant from culture wells containing 2 × 105 cells/ml medium was spun at full speed in an eppendorf centrifuge for 20 min at 4° C. 20 μl lysis buffer [50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% NP-40, 0.5% sodium deoxycholate, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 0.5 mM dithiothreitol (DTT), 1x protease inhibitor cocktail (Sigma P8340) ] was then added to the top of the filter to suspend retained proteins and this suspension was used for Western blot analysis.
Immunocytochemistry
Neurons were fixed in 4% paraformaldehyde, 4% sucrose in phosphate-buffered saline (PBS) for 30 min at room temperature, permeabilized in 0.05% triton X-100 for 30 min, and rinsed in PBS, containing 0.05% triton X-100. Non-specific binding sites were blocked by incubation for 30 min at room temperature in Blotto-T (4% non-fat dry milk powder in 20mM Tris, pH7.5, 150mM NaCl, 0.05% Triton X-100). Double immunostaining was performed with anti-ICAM5 (rabbit polyclonal) and anti-Nav1.2 (mouse monoclonal) for 1 h at room temperature. Cells were washed three times in Blotto-T to remove excess primary antibodies. Immunostaining was visualized by incubation with the Alexa Fluor 488 goat anti-mouse and Alexa Fluor 594 goat anti-rabbit IgGs.
F-actin viualization with phalloidin was performed as previously described (Lim et al., 2008). Colocalization studies of ICAM-5 and synaptic proteins (PSD95 and synaptophysin) were also performed as previously described (Lim et al., 2008). Briefly, neurons were randomly chosen for image acquisition (5–10 cells from 3–6 experiments). A single threshold was chosen for each image so that clusters would correspond to spots having approximately 2 fold or greater intensity above the diffuse fluorescence of the parent dendrite. The percentage of colocalized ICAM-5 was determined as the percentage of punctate synaptophysin or PSD95 clusters with which ICAM-5 immunoreactivity was also observed. 50–100 μm of the dendritic length from 3–4 dendrites per cell were evaluated and analyzed.
Peptide sequencing
The products of recombinant ICAM-5/MMP reactions were resolved on a 4–15% Tris-glycine polyacrylamide gradient gel (Biorad, Hercules, CA), and then transferred to a polyvinylidene difluoride (PVDF) membrane (Biorad). Recombinant protein and MMPs were run on the same gel and similarly transferred so that bands unique to the digests could be identified. Following protein transfer, the PVDF membrane was stained with sequencing grade Coomassie blue (BioRad 1610436). Appropriate bands were identified and cut out for N terminal sequencing.
N terminal sequencing was performed with the Perkin-Elmer/Applied Biosystems Procise Protein Sequencing System. This method is compatible with samples that have been electroblotted onto PVDF.
Electrophysiology
Slices were preincubated with the MMP inhibitor for 15 min. as indicated. Field excitatory post synaptic potentials (fEPSPs) were recorded in CA1 stratum radiatum, and recording electrodes (1–2 MΩ) were filled with bubbled ACSF. Stimuli were delivered through fine bipolar tungsten electrodes to activate Schaffer collaterals/commissural afferents. Data were collected and analyzed using an Axopatch 200B and pCLAMP 8 software (Axon Instruments, Union City, CA). All signals were recorded and filtered at 2 kHz and digitized at 10 kHz. Data are presented at the mean ± SEM, and Student’s t-test was used for statistical comparison.
Statistics
While Student’s t test was used for pairwise comparisons including control versus MMP inhibitor treated EPSP results, and control versus NMDA effects on ICAM-5 ectodomain immunostaining along filopodia, ANOVA with a Bonferroni post hoc test was used to compare the multiple groups examined by densitometric analysis.
Results
I. NMDA stimulates rapid ectodomain shedding of ICAM-5
In previously published work, it was elegantly shown that treatment of cortical neurons for 16 hours with NMDA was followed by the shedding of ICAM-5 (Tian et al., 2007). While neuronal activity may stimulate increased expression of varied MMPs at the transcriptional level, it is also possible that pre-formed MMPs might be rapidly activated and/or released from vesicular stores in association with neuronal activity. To determine whether ICAM shedding might occur in a more rapid manner, we treated cultured neurons for 15–30 minutes as indicated in figure 1 and then prepared lysates for analysis by Western blot. Blots were probed with antibodies to the N- terminal domain of ICAM-5. NMDA was associated with a loss of N terminal immunoreactivity (Fig. 1a).
For reasons that likely included antibody sensitivity, we did not detect an N terminal fragment (NTF) in lysates from dissociated cultures (data not shown). We therefore generated our own antibody, choosing peptide antigens from regions proximal to the C terminus of ICAM-5. As shown (Fig. 1b), this antibody was sensitive and specific. It recognized mature glycosylated ICAM-5 at about 148 kDa. In addition, based on experiments with endoglycosidases (not shown), it recognized an immature non glycosylated form of ICAM-5 at 100 kDa.
When the C terminal antibody was subsequently used to probe Western blots of control and NMDA stimulated neuronal culture lysates, a fragment of approximately 16 kDa was observed in association with NMDA (Fig. 1c). Blots were overexposed to see the C terminal fragment (CTF) and thus changes in full-length ICAM-5 immunoreactivity cannot be appreciated. Pretreatment of cells with the NMDA receptor antagonist APV blocked the generation of this fragment, which was detectable as early as 5 minutes following NMDA stimulation (Fig. 1d). Of note is that, assuming an average molecular weight of 115 Da per amino acid, the portion of ICAM-5 that extends from the intracytoplasmic C terminus of the 917 amino acid protein to the juxtamembrane start of the extracellular domain at amino acid 835 would be approximately 9.5 kDa. A CTF of 16 kDa is therefore consistent with ectodomain cleavage, and would include amino acid segments to which the antibody was raised (see diagram, Fig. 1e).
We also examined supernatants for the presence of an NTF or NTFs. As shown (Fig. 1f), NTFs could be detected in 2/3 supernatants tested at 5 minutes following NMDA stimulation and in 2/2 tested at 15 minutes. Of note is that there is more than one band. Tian and colleagues have described a secondary cleavage at the extreme N terminus of ICAM-5 (Tian et al., 2007), and thus one of the bands likely represents ectodomain in which 2 cleavage events have occurred.
II. Post synaptic expression of ICAM-5
Since altered interactions between the C terminus and cytoplasmic elements may follow ICAM-5 cleavage to influence spine volume (Furutani et al., 2007), shedding from post synaptic processes in particular would likely be important to a phenomenon such as LTP. To better localize ICAM-5, double immunostaining studies were performed. In cultured hippocampal neurons, immunostaining for ICAM-5 using the C terminal antibody showed a punctuate pattern on the cell body and MAP2 positive processes (Fig. 2a). When the C terminal ICAM5 antibody was preincubated with the synthetic peptides to which it was raised (1:10 ratio), specific labeling was abolished (Fig. 2b). Staining neurons with the axonally localized sodium channel, Nav1.2 (Fig. 2c), shows little colocalization, suggesting that ICAM-5 immunoreactivity is predominantly post synaptic, as has been suggested in prior studies (Yoshihara and Mori, 1994). Specificity of the antibody was also supported by its inability to stain a GAD positive neuron (Fig. 2d).
Higher power images, that include phalloidin staining to visualize F-actin, show that ICAM-5 immunoreactivity can be observed along the necks of thin spines (Fig. 2e), and colocalization studies suggest that ICAM-5 is rarely detected in association with PSD95 or synaptophysin (Figs. 2f–g). This is consistent with previous reports showing that while ICAM-5 is expressed along filopodia and the necks of thin spines, it shows a relative lack of colocalization with GluR2 clusters in more mature spines (Matsuno et al., 2006).
III. MMPs mediate rapid NMDA-induced shedding of ICAM-5
We next tested the possibility that select MMPs might mediate rapid NMDA dependent shedding of ICAM-5. While typically released as pro-forms and activated extracellularly, recent data suggest active forms of select MMPs may be present in vesicular stores (Taraboletti et al., 2002) and that vesicles might be present in proximity to the synapse (Sbai et al., 2008). Recent work also suggests that at least one MMP associates with dendritic spines of excitatory synapses (Wilczynski et al., 2008).
We focused on MMPs known to be expressed in neurons and/or astrocytes, and to play a role in hippocampal dependent learning and memory. We therefore tested inhibitors that potently inhibit MMPs including MMPs 2, 3 and 9. NNGH has been used as an inhibitor of MMP-3 (Pauly et al., 2008), though it cleaves a variety of other soluble MMPs as well (Tamura et al., 1998, Calderone et al., 2006, Arendt et al., 2007). BiPS is used as a relatively selective inhibitor of MMPs 2 and 9, though it may target other soluble MMPs when used at typical concentrations of greater than 1 μM (Tamura et al., 1998). As shown (Fig. 3a), both inhibitors reduced NMDA dependent processing of ICAM-5 as determined by Western blot analysis. Densitometric analysis of results from three similar experiments is also shown (Fig. 3b).
Consistent with the ability of MMP inhibitors to suppress NMDA dependent cleavage of ICAM-5, we observed that exogenous MMP-3 can also cause rapid shedding of ICAM-5 (Fig. 3c). MMP-7, which shares substrate similarity with MMP-3 (Wilson and Matrisian, 1996, Agnihotri et al., 2001), also affected shedding (Fig. 3d). This latter figure also compares the N and C terminal antibodies for their ability to detect reductions in the immunoreactivity for full length ICAM-5.
IV. MMPs cleave recombinant ICAM-5 at an extracellular site that is proximal to the transmembrane domain
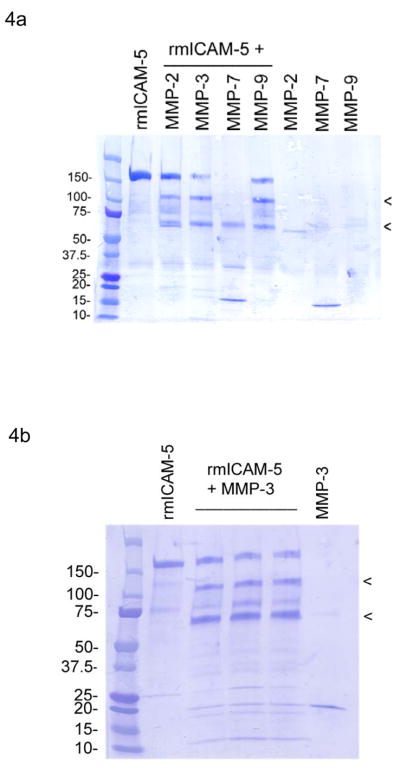
To identify potential cleavage sites within the ectodomain of ICAM-5, in vitro digests were performed using a recombinant construct in which the segment containing amino acids 31-828 of the extracellular domain of mouse ICAM-5 was fused to the Fc region of human IgG. Digest products were resolved via polyacrylamide gel electrophoresis, transferred to a PVDF membrane, and stained with coomassie blue as shown (Figs. 4a and b). Select fragments were then subjected to N terminal sequencing. Two major fragments (arrowheads) were observed in association with MMP-2, -3 and -9. N terminal sequencing of the MMP-3 generated product denoted by the uppermost arrowhead showed that this product contained the N terminus of the original construct. Sequencing of the product denoted by the lowermost arrowhead instead revealed a new N terminus beginning with amino acid 780. This same novel N terminus was observed in association with the MMP-9 digestion, suggesting it is a site targeted by more than one MMP.
Figure 4. In vitro digests of recombinant ICAM-5.
Recombinant mouse ICAM-5 was incubated with select MMPs as indicated, and the digestion products resolved by electrophoresis. Following transfer to a PVDF membrane, specific products were isolated for N terminal sequencing (arrowheads). Products denoted by the arrowheads in the MMP-3 and -9 digests in particular were analyzed (4a and b). Both MMPs generated a fragment having the construct’s original N terminus (uppermost arrowhead), as well as a fragment showing a new N terminus beginning with amino acid 780 (lowermost arrowhead).
Of note is that the cleavage site is approximately 50 amino acids from the transmembrane region. If cleavage of ICAM-5 occurs at a similar site in vivo, the NTF generated would be approximately 783 amino acids and the initial CTF would be 141 amino acids. With 115 daltons (Da) as the average weight of an amino acid, cleavage at this site would generate a CTF of 16.2 kDa, which is consistent with the length of the CTF seen in cell culture lysates.
V. MMP dependent ICAM cleavage occurs with tetanic stimulation of hippocampal slices
Previous studies have shown that reduced expression of ICAM-5 is correlated with an increase in the volume of dendritic spines (Matsuno et al., 2006). It is possible that full length ICAM-5 is important to the maintenance of filopodia and that following ectodomain shedding the interaction of the CTF with phosphorylated ezrin-radixin-moiesin proteins is altered so that spine expansion can occur (Furutani et al., 2007). Since an increase in dendritic spine volume may be important to LTP, we sought to determine whether ICAM-5 cleavage may occur with tetanic stimulation. This was important in that NMDA stimulation of dissociated neurons in culture does not accurately represent events occurring with LTP, in which release of the NMDA receptor agonist and/or MMPs may be relatively localized and, because of slice complexity, ICAM-5 may be more difficult to target.
Previous studies have also shown that while MMP inhibitors may influence varied forms of plasticity, MMP inhibitors have a robust ability to influence LTP (Nagy et al., 2006, Meighan et al., 2007). In our studies, we therefore used a tetanic stimulus that has been shown to stimulate LTP (Huang et al., 2006), a post synaptic form of plasticity to which cytoskeletal changes likely contribute.
As shown (Fig. 5a), this protocol stimulated an increase in the slope of excitatory post synaptic potentials (EPSPs) that was reduced from control in slices pretreated with the MMP inhibitor NNGH. Of note is that NNGH did not change the input-output relationship, which suggests that the compound did not alter slice viability or basic electrical properties (Fig. 5b). For the majority of slices, recordings were taken out to 30 minutes. For a subset of slices, however, recordings were stopped at 15 minutes and lysates prepared for subsequent analysis by Western blot. These results (Fig. 5c) demonstrate that tetanic stimulation caused an MMP dependent increase in immunoreactivity for the 16 kDa CTF. Densitometric analysis of results shows an approximate doubling of 16 kDa band intensity in association with LTP, and an abrogation of this increase in slices pretreated with NNGH (Fig. 5d). The difference between control and LTP densitometric results were significant (P< 0.01) as were the differences between LTP and LTP/NNGH (P< 0.05).
Figure 5. MMP-dependent shedding of ICAM-5 occurs during early LTP.
Hippocampal slices were stimulated (two 1s trains of 100 Hz separated by 20s) in the presence or absence of the MMP inhibitor NNGH (10 μM). Recordings were taken from 5 –10 control and MMP inhibitor treated slices, and confirm that LTP occurred with this protocol (5a). Control EPSP traces were taken in the first 10 minutes of recording and post tetanus traces taken in the last 10 minutes. Input-output data comparing control and NNGH treated slices is shown in figure 5b. For figure 5c, lysates were prepared from a subset of slices 15 minutes following their stimulation, and compared to lysates from control slices that had been similarly maintained in ACSF but not stimulated. Lysates were subsequently tested for the 16 kDa C terminal cleavage product (arrowhead) by Western blot. Densitometric results are shown in figure 5d, and demonstrate that the density of the 16 kDa band is increased in association with LTP. This increase is abrogated when slices were pretreated with NNGH. The difference between control and LTP densitometric results were significant (P< 0.01) as were the differences between LTP and LTP/NNGH results (P< 0.05).
Discussion
Matrix metalloproteinases are known to play a role in several pathological and physiological processes to which facilitated cell migration is critical, including wound healing and cancer metastasis (Sternlicht and Werb, 2001). Consistent with this role, these enzymes process proteins of the extracellular matrix as well as soluble molecules and cell surface receptors that influence cell shape and chemotaxis (Oh et al., 1999, McCawley and Matrisian, 2001, Rosenberg and Mun-Bryce, 2004). In the central nervous system, MMPs have been shown to contribute to cell migration and axon guidance, and emerging data suggest they can also influence the morphology of dendritic spines (Bilousova et al., 2006, Milward et al., 2007, Szklarczyk et al., 2007, Tian et al., 2007). For example, one recent study demonstrated that MMP-9 could induce cofilin phosphorylation and increase the volume of the dendritic spine (Wang et al., 2008), a morphological change associated with LTP (Alvarez and Sabatini, 2007). While diverse MMP dependent activities including direct thrombin receptor activation and the generation of integrin binding matrix fragments might influence the morphology of dendritic spines, the processing of specific synaptic CAMs is also likely to contribute. A recent study showing that long term stimulation of neurons with NMDA (16h) resulted in MMP-9 mediated cleavage of ICAM-5 supports this possibility (Tian et al., 2007), in that reduced expression of ICAM-5 has been associated with the maturation of dendritic spines (Matsuno et al., 2006).
In the present study, we were interested not in developmental synapse formation following long term activation of NMDA receptors, but in the possibility that NMDA might facilitate MMP dependent changes in synaptic CAM integrity that occur in a manner rapid enough to contribute to LTP. We show that both NMDA, and a tetanic stimulation protocol that has been linked to LTP (Huang et al., 2006), stimulate rapid MMP dependent shedding of ICAM-5, and/or a rapid MMP dependent increase in the slope of fEPSPs. We also show that MMP mediated cleavage of ICAM-5 might generate an N terminal fragment with the ability to bind integrins. Previous studies have linked MMP activity to LTP by demonstrating that pretreatment of hippocampal slices with a broad spectrum MMP inhibitor can block this phenomenon (Meighan et al., 2007), and that MMP-9 contributes to the maintenance LTP (Nagy et al., 2006).
From a conceptual standpoint, since MMP mediated synaptic plasticity is likely a critical process, redundancy in terms of the proteases that can mediate such may be involved. We focused on inhibitors that would target MMPs including those expressed by neurons and known to be increased with hippocampal dependent learning and memory. Both MMP-3 and -9 are expressed in neurons and their release may be modulated by neuronal activity (Zhang et al., 1998, Szklarczyk et al., 2002, Pauly et al., 2008). The potential importance of MMP-9 to neuronal activity dependent plasticity is also underlined by several studies which have shown that this proteinase in particular plays a role in LTP, seizure-induced β dystroglycan cleavage, and methamphetamine associated conditioned place preference (Nagy et al., 2006, Michaluk et al., 2007b, Mizoguchi et al., 2007b). With respect to MMP-3, levels of this proteinase are increased with hippocampal dependent learning (Meighan et al., 2006) and with trauma induced synaptogenesis (Kim et al., 2005). Increased levels have been shown to occur in association with NMDA stimulation of neurons. MMP-3 is also known to play a role in developmental plasticity in that it is required for optimal axon extension and is involved in semaphorin 3C-dependent chemoattraction of cortical axons (Gonthier et al., 2007). At the neuromuscular junction, MMP-3 is critical to neuronal activity dependent processing of agrin (VanSaun and Werle, 2000).
The mechanism by which MMP activity is rapidly increased to affect changes contributing to LTP is unknown. The soluble forms of these enzymes are typically released as proforms that are activated extracellularly. One possibility is that neuronal activity is linked to the activation of protein kinases that first increase the activity of a transmembrane MMP. For example, the transmembrane MMP TACE is activated by PKC (Cho et al., 2008). Activated TACE could in turn release an active MMP from a proform anchored by cell surface integrins (Dumin et al., 2001, Conant et al., 2004). Other possibilities include rapid release of MMPs from vesicular stores to be activated by extracellular proteases and/or molecules such as nitric oxide. Rapid release might be calcium dependent, in that a recent study of fibrosarcoma cells demonstrated that phorbol myristate acetate (PMA) induced release of MMP-2 and -9 was soluble NSF attachment protein receptor (SNARE) dependent (Kean et al., 2009).
Of particular interest to the release of MMPs from preformed stores are recent studies suggesting not only that MMPs can be detected in vesicular stores proximal to the synapse (Sbai et al., 2008), but that they may be released from vesicular stores as active enzymes (Taraboletti et al., 2002). In one relevant study, MMP-2 and -9 containing vesicles were observed in the somatodendritic compartment and found in dendritic spines (Sbai et al., 2008). In addition, vesicular secretion of both pro and active forms of MMP-2 was observed (Sbai et al., 2008). In another relevant study, it was shown that at least one MMP associates with dendritic spines of excitatory synapses (Wilczynski et al., 2008). That preformed active MMPs may exist in vesicles is also supported by a study in which active MMP-14 was secreted into the extracellular space via microvesicular exosomes (Hakulinen et al., 2008).
While we have shown that MMPs can cleave ICAM-5, and that inhibition of MMP activity inhibits LTP, we have not established to what extent ICAM-5 cleavage per se contributes to MMP dependent effects on LTP. That such cleavage does contribute, however, is supported by previous studies. For example, reductions in ICAM-5 have been associated with the developmental maturation of dendritic spines (Matsuno et al., 2006), and ICAM-5 mutants have enhanced LTP (Nakamura et al., 2001). Moreover, antibodies to ICAM-5 have been shown to inhibit LTP (Sakurai et al., 1998). It is possible that the antibodies used in this study impaired MMP mediated cleavage of the molecule. We tested two antibodies to the extracellular domain of ICAM-5 (AF 1950 and AF 1173 from R & D Systems), in the hope that they would inhibit MMP dependent ICAM-5 cleavage and could thus be tested for their ability to inhibit LTP. Unfortunately, neither of these antibodies blocked NMDA stimulated ICAM-5 cleavage from cultured neurons when used at concentrations of up to 10 μg/ml (data not shown).
ICAM-5 is an Ig domain containing adhesion molecule with expression limited to the telencephalon. It has a relatively short cytoplasmic domain that can interact with ezrin-radixin-moiesin (ERM) proteins. The interaction of the C terminal domain with phosphorylated ERM proteins may help to maintain the spine in a filopodial form (Furutani et al., 2007). Both the extracellular and intracytoplasmic domains of ICAM-5 are important to filopodial maintenance, and Furutani and colleagues showed that siRNA to ezrin was associated with accelerated spine maturation (Furutani et al., 2007). One possibility is that the full length ICAM-5 ezrin interaction helps to maintain filopodia and thin spines, and that following the shedding of ICAM-5 this interaction is perturbed. It is also possible that only a small or spatially localized portion of total ICAM-5 needs be shed. Since ICAM-5 associates with ERM proteins in thin spines and filopodia, but with α actinin in dendritic shafts and the cell body (Furutani et al., 2007), NMDA dependent cleavage along the former may selectively perturb interactions with ezrin and thus allow these slender processes to mature and potentially contribute to LTP. And while varied spine types may change in response to LTP, it has been suggested that thin plasticity spines may be more sensitive to this phenomenon (Tada and Sheng, 2006). The shedding of ICAM-5 may thus influence LTP predominantly by permitting thin spines to expand, perhaps in response to other MMP independent events initiated by the tetanic stimulus. Of interest, the interaction of an Ig domain containing CAM with ERM proteins has been described in other systems (Haas et al., 2004, Sakurai et al., 2008, Schlatter et al., 2008). For example, the L1 adhesion molecule may interact with ERM proteins at the axonal growth cone (Haas et al., 2004). Thus, while hypothetical, CAM-ERM interactions might contribute to the proper localization of machinery that influences actin dynamics.
Also worth considering is the fate of the shed ICAM-5 N terminal domain. It has been suggested that this domain may, through a homophilic interaction with cell surface associated ICAM-5, promote filopodial elongation (Tian et al., 2007). One could imagine that in select situations homophilic interactions might make the full length molecule less sensitive to cleavage. It is tempting to speculate, however, that in some circumstances the shed domain might also influence actin polymerization and thus spine morphology through its potential to bind to postsynaptic integrins that contribute to LTP (Gall and Lynch, 2004). At least two studies have suggested that MMP dependent effects on LTP are in some part integrin dependent, though these studies have implicated RGD binding integrins and/or β1 intergins in particular (Nagy et al., 2006, Meighan et al., 2007). Future studies will be necessary to determine whether the extracellular domain of ICAM-5, which interacts with β2 integrins (Gahmberg et al., 2008), can also interact with other integrins known to influence spine morphology. ICAM-5 might also be but one of several synaptic CAMs shed in response to neuronal activity (Hoffman et al., 1998, Michaluk et al., 2007a, Lim et al., 2008). Thus, while its shedding may increase spine volume via changes in ezrin-radixin-moiesin signaling, β1 integrin dependent changes likely occur as well and might be mediated by the shed N terminal domain of an alternate adhesion molecule.
While cleavage of ICAM-5 occurs within minutes of LTP induction, this event could contribute to later stages of LTP. For example, it could contribute to or allow for changes in actin polymerization that may be required for the activity of PKM and thus to LTP maintenance (Kelly et al., 2007). Given the timing and expected sequelae of ICAM-5 shedding, it would seem unlikely to contribute to other components of synaptic plasticity that are influenced by MMP inhibitors, such as paired pulse facilitation (Meighan et al., 2007).
In summary, we have shown that MMPs cleave a synaptic CAM (ICAM-5) in a rapid neuronal activity dependent manner and that MMP mediated proteolysis is associated with LTP. This contributes to a growing body of literature implicating MMPs as important contributors to learning and memory. Moreover, since the activity of MMPs including MMP-3 and -9 may be substantially increased in the context of inflammation and hypoxia, these data have implications for altered synaptic function in the setting of CNS pathology.
Acknowledgments
This work was supported in part by the Alzheimer’s Association, the National Institutes of Drug Abuse (DA024447) and the National Institutes of Aging (AG029806).
Abbreviations
- MMP
Matrix Metalloproteinase
- ICAM-5
Intercellular adhesion molecule-5
- LTP
long term potentiation
- NMDA
N-methyl-D-aspartatic acid
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- ADAM
A disintegrin and metalloproteinase
- NSF
N-ethylmaleimide-sensitive fusion protein
- PKM
protein kinase M zeta
- CAM
cell adhesion molecule
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2001;276:28261–28267. doi: 10.1074/jbc.M103608200. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Arendt Y, Banci L, Bertini I, Cantini F, Cozzi R, Del Conte R, Gonnelli L. Catalytic domain of MMP20 (Enamelysin) - the NMR structure of a new matrix metalloproteinase. FEBS Lett. 2007;581:4723–4726. doi: 10.1016/j.febslet.2007.08.069. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Rusakov DA, Ethell DW, Ethell IM. Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J Neurochem. 2006;97:44–56. doi: 10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learning & memory (Cold Spring Harbor, NY) 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Harding JW, Wright JW, Sorg BA. Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse. 2008;62:886–889. doi: 10.1002/syn.20562. [DOI] [PubMed] [Google Scholar]
- Calderone V, Fragai M, Luchinat C, Nativi C, Richichi B, Roelens S. A high-affinity carbohydrate-containing inhibitor of matrix metalloproteinases. ChemMedChem. 2006;1:598–601. doi: 10.1002/cmdc.200600020. [DOI] [PubMed] [Google Scholar]
- Cho RW, Park JM, Wolff SB, Xu D, Hopf C, Kim JA, Reddy RC, Petralia RS, Perin MS, Linden DJ, Worley PF. mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron. 2008;57:858–871. doi: 10.1016/j.neuron.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, St Hillaire C, Nagase H, Visse R, Gary D, Haughey N, Anderson C, Turchan J, Nath A. Matrix metalloproteinase 1 interacts with neuronal integrins and stimulates dephosphorylation of Akt. J Biol Chem. 2004;279:8056–8062. doi: 10.1074/jbc.M307051200. [DOI] [PubMed] [Google Scholar]
- Dumin JA, Dickeson SK, Stricker TP, Bhattacharyya-Pakrasi M, Roby JD, Santoro SA, Parks WC. Pro-collagenase-1 (matrix metalloproteinase-1) binds the alpha(2)beta(1) integrin upon release from keratinocytes migrating on type I collagen. J Biol Chem. 2001;276:29368–29374. doi: 10.1074/jbc.M104179200. [DOI] [PubMed] [Google Scholar]
- Furutani Y, Matsuno H, Kawasaki M, Sasaki T, Mori K, Yoshihara Y. Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation. J Neurosci. 2007;27:8866–8876. doi: 10.1523/JNEUROSCI.1047-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg CG, Tian L, Ning L, Nyman-Huttunen H. ICAM-5--a novel two-facetted adhesion molecule in the mammalian brain. Immunology letters. 2008;117:131–135. doi: 10.1016/j.imlet.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Gall CM, Lynch G. Integrins, synaptic plasticity and epileptogenesis. Adv Exp Med Biol. 2004;548:12–33. doi: 10.1007/978-1-4757-6376-8_2. [DOI] [PubMed] [Google Scholar]
- Gonthier B, Nasarre C, Roth L, Perraut M, Thomasset N, Roussel G, Aunis D, Bagnard D. Functional interaction between matrix metalloproteinase-3 and semaphorin-3C during cortical axonal growth and guidance. Cereb Cortex. 2007;17:1712–1721. doi: 10.1093/cercor/bhl082. [DOI] [PubMed] [Google Scholar]
- Haas MA, Vickers JC, Dickson TC. Binding partners L1 cell adhesion molecule and the ezrin-radixin-moesin (ERM) proteins are involved in development and the regenerative response to injury of hippocampal and cortical neurons. Eur J Neurosci. 2004;20:1436–1444. doi: 10.1111/j.1460-9568.2004.03620.x. [DOI] [PubMed] [Google Scholar]
- Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. Journal of cellular biochemistry. 2008;105:1211–1218. doi: 10.1002/jcb.21923. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein tat. J Neurochem. 1999;73:1363–1374. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- Hoffman KB, Martinez J, Lynch G. Proteolysis of cell adhesion molecules by serine proteases: a role in long term potentiation? Brain Res. 1998;811:29–33. doi: 10.1016/s0006-8993(98)00906-8. [DOI] [PubMed] [Google Scholar]
- Huang Z, Shimazu K, Woo NH, Zang K, Muller U, Lu B, Reichardt LF. Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J Neurosci. 2006;26:11208–11219. doi: 10.1523/JNEUROSCI.3526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean MJ, Williams KC, Skalski M, Myers D, Burtnik A, Foster D, Coppolino MG. VAMP3, syntaxin-13 and SNAP23 are involved in secretion of matrix metalloproteinases, degradation of the extracellular matrix and cell invasion. J Cell Sci. 2009;122:4089–4098. doi: 10.1242/jcs.052761. [DOI] [PubMed] [Google Scholar]
- Kelly MT, Yao Y, Sondhi R, Sacktor TC. Actin polymerization regulates the synthesis of PKMzeta in LTP. Neuropharmacology. 2007;52:41–45. doi: 10.1016/j.neuropharm.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Fillmore HL, Reeves TM, Phillips LL. Elevation of hippocampal MMP-3 expression and activity during trauma-induced synaptogenesis. Exp Neurol. 2005;192:60–72. doi: 10.1016/j.expneurol.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Lim ST, Lim KC, Giuliano RE, Federoff HJ. Temporal and spatial localization of nectin-1 and l-afadin during synaptogenesis in hippocampal neurons. J Comp Neurol. 2008;507:1228–1244. doi: 10.1002/cne.21608. [DOI] [PubMed] [Google Scholar]
- Maiya R, Zhou Y, Norris EH, Kreek MJ, Strickland S. Tissue plasminogen activator modulates the cellular and behavioral response to cocaine. Proc Natl Acad Sci U S A. 2009;106:1983–1988. doi: 10.1073/pnas.0812491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno H, Okabe S, Mishina M, Yanagida T, Mori K, Yoshihara Y. Telencephalin slows spine maturation. J Neurosci. 2006;26:1776–1786. doi: 10.1523/JNEUROSCI.2651-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Meighan PC, Meighan SE, Davis CJ, Wright JW, Harding JW. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J Neurochem. 2007;102:2085–2096. doi: 10.1111/j.1471-4159.2007.04682.x. [DOI] [PubMed] [Google Scholar]
- Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW, Harding JW. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96:1227–1241. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Kolodziej L, Mioduszewska B, Wilczynski GM, Dzwonek J, Jaworski J, Gorecki DC, Ottersen OP, Kaczmarek L. Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J Biol Chem. 2007a;282:16036–16041. doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Kolodziej L, Mioduszewska B, Wilczynski GM, Dzwonek J, Jaworski J, Gorecki DC, Ottersen OP, Kaczmarek L. beta -dystroglycan as a target for MMP-9 in response to enhanced neuronal activity. J Biol Chem. 2007b doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. J Neurosci. 2009;29:6007–6012. doi: 10.1523/JNEUROSCI.5346-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milward EA, Fitzsimmons C, Szklarczyk A, Conant K. The matrix metalloproteinases and CNS plasticity: an overview. J Neuroimmunol. 2007;187:9–19. doi: 10.1016/j.jneuroim.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Mouri A, Niwa M, Mizuno T, Noda Y, Nitta A, Itohara S, Banno Y, Nabeshima T. Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J Neurochem. 2007a;102:1548–1560. doi: 10.1111/j.1471-4159.2007.04623.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Niwa M, Mouri A, Mizuno T, Noda Y, Nitta A, Itohara S, Banno Y, Nabeshima T. Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and -9-deficient mice. J Neurochem. 2007b;100:1579–1588. doi: 10.1111/j.1471-4159.2006.04288.x. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Manabe T, Watanabe M, Mamiya T, Ichikawa R, Kiyama Y, Sanbo M, Yagi T, Inoue Y, Nabeshima T, Mori H, Mishina M. Enhancement of hippocampal LTP, reference memory and sensorimotor gating in mutant mice lacking a telencephalon-specific cell adhesion molecule. Eur J Neurosci. 2001;13:179–189. doi: 10.1046/j.0953-816x.2000.01366.x. [DOI] [PubMed] [Google Scholar]
- Oh LY, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, Yong VW. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci. 1999;19:8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly T, Ratliff M, Pietrowski E, Neugebauer R, Schlicksupp A, Kirsch J, Kuhse J. Activity-dependent shedding of the NMDA receptor glycine binding site by matrix metalloproteinase 3: a PUTATIVE mechanism of postsynaptic plasticity. PLoS ONE. 2008;3:e2681. doi: 10.1371/journal.pone.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet neurology. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S. Matrix metalloproteinases in neuroinflammation and cerebral ischemia. Ernst Schering Research Foundation workshop 1–16; 2004. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Hashikawa T, Yoshihara Y, Kaneko S, Satoh M, Mori K. Involvement of dendritic adhesion molecule telencephalin in hippocampal long-term potentiation. Neuroreport. 1998;9:881–886. doi: 10.1097/00001756-199803300-00022. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Gil OD, Whittard JD, Gazdoiu M, Joseph T, Wu J, Waksman A, Benson DL, Salton SR, Felsenfeld DP. Interactions between the L1 cell adhesion molecule and ezrin support traction-force generation and can be regulated by tyrosine phosphorylation. J Neurosci Res. 2008;86:2602–2614. doi: 10.1002/jnr.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbai O, Ferhat L, Bernard A, Gueye Y, Ould-Yahoui A, Thiolloy S, Charrat E, Charton G, Tremblay E, Risso JJ, Chauvin JP, Arsanto JP, Rivera S, Khrestchatisky M. Vesicular trafficking and secretion of matrix metalloproteinases-2, -9 and tissue inhibitor of metalloproteinases-1 in neuronal cells. Mol Cell Neurosci. 2008;39:549–568. doi: 10.1016/j.mcn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Schlatter MC, Buhusi M, Wright AG, Maness PF. CHL1 promotes Sema3A-induced growth cone collapse and neurite elaboration through a motif required for recruitment of ERM proteins to the plasma membrane. J Neurochem. 2008;104:731–744. doi: 10.1111/j.1471-4159.2007.05013.x. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk A, Conant K, Owens DF, Ravin R, McKay RD, Gerfen C. Matrix metalloproteinase-7 modulates synaptic vesicle recycling and induces atrophy of neuronal synapses. Neuroscience. 2007;149:87–98. doi: 10.1016/j.neuroscience.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Szklarczyk A, Ewaleifoh O, Beique JC, Wang Y, Knorr D, Haughey N, Malpica T, Mattson MP, Huganir R, Conant K. MMP-7 cleaves the NR1 NMDA receptor subunit and modifies NMDA receptor function. Faseb J. 2008;22:3757–3767. doi: 10.1096/fj.07-101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci. 2002;22:920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Watanabe F, Nakatani T, Yasui K, Fuji M, Komurasaki T, Tsuzuki H, Maekawa R, Yoshioka T, Kawada K, Sugita K, Ohtani M. Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-sulfonylamino acid derivatives. Journal of medicinal chemistry. 1998;41:640–649. doi: 10.1021/jm9707582. [DOI] [PubMed] [Google Scholar]
- Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H, Libert C, Itohara S, Mishina M, Rauvala H, Gahmberg CG. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol. 2007;178:687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSaun M, Werle MJ. Matrix metalloproteinase-3 removes agrin from synaptic basal lamina. J Neurobiol. 2000;43:140–149. [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci U S A. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK, Mattson MP, Furukawa K. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2004;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynski GM, Konopacki FA, Wilczek E, Lasiecka Z, Gorlewicz A, Michaluk P, Wawrzyniak M, Malinowska M, Okulski P, Kolodziej LR, Konopka W, Duniec K, Mioduszewska B, Nikolaev E, Walczak A, Owczarek D, Gorecki DC, Zuschratter W, Ottersen OP, Kaczmarek L. Important role of matrix metalloproteinase 9 in epileptogenesis. J Cell Biol. 2008;180:1021–1035. doi: 10.1083/jcb.200708213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996;28:123–136. doi: 10.1016/1357-2725(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, Mori K. Telencephalin: a neuronal area code molecule? Neuroscience research. 1994;21:119–124. doi: 10.1016/0168-0102(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Zhang JW, Deb S, Gottschall PE. Regional and differential expression of gelatinases in rat brain after systemic kainic acid or bicuculline administration. Eur J Neurosci. 1998;10:3358–3368. doi: 10.1046/j.1460-9568.1998.00347.x. [DOI] [PubMed] [Google Scholar]