Abstract
Brain arteriovenous malformation (BAVM), a rare but important cause of intracranial hemorrhage, has increased angiogenesis and inflammation as key components of the nidus of abnormal vessels and stroma that form the resected surgical specimen. Accordingly, vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGFβ) have both been implicated in BAVM pathology for their pro-angiogenic and vascular-regulating roles. The c-terminal fragment of the extracellular matrix component perlecan (domain V, DV) has been shown to be increased and to, via the α5β1 integrin, increase VEGF levels in and around areas of cerebral ischemic injury, another pro-angiogenic condition. We sought to determine whether the concentrations of DV, DV’s proangiogenic receptor α5β1 integrin, or DV’s anti-angiogenic receptor α2β1 integrin are elevated in human BAVM tissue. DV levels were increased in BAVM compared to control brain tissue from epileptic resection, as was α5β1 integrin. Additionally, α5β1 integrin was preferentially increased and localized to endothelial cells compared to α2β1 integrin. VEGF and TGFβ levels were also increased in BAVM compared to control tissue. Furthermore, increases in all components were strongly correlated. Excessive generation of pro-angiogenic DV in BAVM suggests that DV may participate in its pathology and may represent a future therapeutic target.
Keywords: Arteriovenous Malformation, AVM
Introduction
A brain arteriovenous malformation (BAVM) is an abnormal tangled mass of arteries and veins, devoid of a true intervening capillary bed, that results in shunting of blood from the arterial to venous circulations. [1,2] These abnormal vessels, along with intervening stromal elements that are predominantly glial and inflammatory cells, is termed the BAVM nidus. It is this nidus that is the target for surgical resection. Traditionally viewed as some kind of embryological failure to develop normally, there is an emerging view that a post-natal event occurs which triggers BAVM formation and evolution into the lesion that is seen on imaging studies and that forms the bulk of the resected surgical specimen. This view has been termed the response-to-injury hypothesis, and presupposes that there is some kind of genetic variation that predisposes one to formation of a BAVM if some sort of local pro-angiogenic stimulation occurs. Such stimulation could be, for example, a minor degree of traumatic injury or localized microvascular thrombosis that otherwise escapes clinical detection. [2] In the case of Hereditary Hemorrhagic Telangiectasia, the genetic variation is known, e.g., defective endoglin or ALK1, both members of the TGFβ superfamily pathways. In the case of sporadic BAVM, the existence of underlying genetic variation is not currently understood but is the subject of investigation. [3]
The association of vascular endothelial growth factor (VEGF) with abnormal vasculature of the BAVM nidus [4] led us to hypothesize that the extracellular matrix heparan sulfate proteoglycan perlecan’s C-terminal domain V (DV), a protein fragment endogenously cleaved and solubilized [5] that increases VEGF production and secretion following stroke, might also play a role in the pathology of BAVM. [6] As BAVM is an important cause of intracranial hemorrhage, but the specific molecular mechanisms of its etiology, growth, and rupture remain unclear, further characterization of the abnormal signaling molecules within resected BAVM is a potentially important way to identify potential therapeutic targets. [4,7] Here we investigated the relationship between levels of DV, total and endothelial cell expressed levels of its pro-angiogenic α5β1 integrin [6] and anti-angiogenic α2β1 integrin (normally absent from brain microvasculature) receptors, [8] and total levels of VEGF and TGFβ in human BAVM.
Materials and methods
Western blot analysis
After IRB approval and informed consent, surgical specimens from either BAVM resection of non-embolized nidal tissue (cases, n=6), or temporal lobe samples obtained from the surgical treatment of epilepsy (controls, n=3) were frozen and homogenized in RIPA buffer (G Biosciences). Western blots were performed with specific antibodies to DV (R&D systems, MAB2364), α5 (Millipore, AB1928), α2 (Chemicon International, AB1936), VEGF (Abcam, ab9570), TGFβ (Abcam, ab66043), CD31 (Abcam, ab54211), and β-actin (Abcam, ab13822). Blots were quantified using ImageJ software.
Immunohistochemistry
Coimmunohistochemistry was performed on frozen, acetone fixed human brain slices from BAVM or control (epilepsy) tissue with antibodies for α5 or α2 and CD31 with the appropriate Alexa Fluor conjugated secondary antibodies. Tissue slices were imaged with a BD Biosystems Carv II spinning disk confocal mounted on a Zeiss Axioplan. Fluorescent images were obtained using the same exposure time to allow for comparison of relative fluorescent intensities between different images and were analyzed using iVision-Mac™ Image Acquisition and Analysis Software and Image J software.
Statistical analysis
Western blot values were normalized by dividing by the average control value × 100. Two sample t-tests were used to investigate differences in average protein expression levels between cases and controls. To account for possible differences in total protein loading and endothelial cell mass, we performed analysis of covariance of protein levels and case-control status, adjusting for both β-actin and CD31.
Results
Table 1 shows the demographic and BAVM characteristics of the study cohort. Protein levels of DV, α5, α2, VEGF, TGFβ, and CD31 were all elevated (p < .01) in BAVM compared to controls by western blot analysis (Fig. 1). DV had the largest difference between BAVM cases and controls (~14 fold) and α5 differed more between BAVM cases and controls than did α2 (~6 and 2.5 fold, respectively). Furthermore, VEGF and TGFβ were also elevated in BAVM compared to control tissue (both ~9 fold). DV, α5, VEGF, and TGFβ protein levels in both BAVM cases and controls were strongly correlated (r = DV:TGFβ = 0.86; DV:VEGF = 0.79; DV:α5 = 0.78; TGFβ:VEGF = 0.92; TGFβ:α5 = 0.86; VEGF:α5 = 0.76). After adjusting for β-actin and CD31, protein levels remained higher in BAVM cases compared to controls for all five proteins (p ≤ .05). β-actin was not associated with protein expression in any of the models (p > .1), while CD31 was only associated with VEGF (p = .009).
TABLE 1.
Baseline Characteristics of Study Cohort
| Characteristics | Cases (n = 6) | Controls (n = 3) | P Value |
|---|---|---|---|
| Age | 43 ± 18 | 31 ± 8 | 0.200 |
| Sex | 0.524 | ||
| Male | 2 (33%) | 2 (67%) | |
| Female | 4 (67%) | 1 (33%) | |
| Presentation | |||
| Hemorrhage | 2 (33%) | NA | |
| Headache | 3 (50%) | NA | |
| Incidental | 1 (17%) | NA | |
| Size (cm) | 1.8 ± 0.8 | NA | |
| Venous Drainage | |||
| 1 | 4 (66%) | NA | |
| 2 | 1 (17%) | NA | |
| 3 | 1 (17%) | NA | |
| Location | |||
| Cortical | 0 (0%) | NA | |
| Frontal | 4 (66%) | NA | |
| Occipital | 1 (17%) | NA | |
| Parietal | 1 (17%) | NA | |
| Spetzler-Martin Score* | |||
| 1 | 2 (33%) | NA | |
| 2 | 3 (50%) | NA | |
| 3 | 1 (17%) | NA | |
| 4 | 0 (0%) | NA | |
| 5 | 0 (0%) | NA | |
age represents SD
size represents SD (cm)
The Spetzler-Martin Score is an estimation of the risk of neurosurgery to an AVM patient based on the AVM size, venous drainage, and brain location. Higher values represent a more at-risk patient and less operable AVM. [11]
Fig. 1.
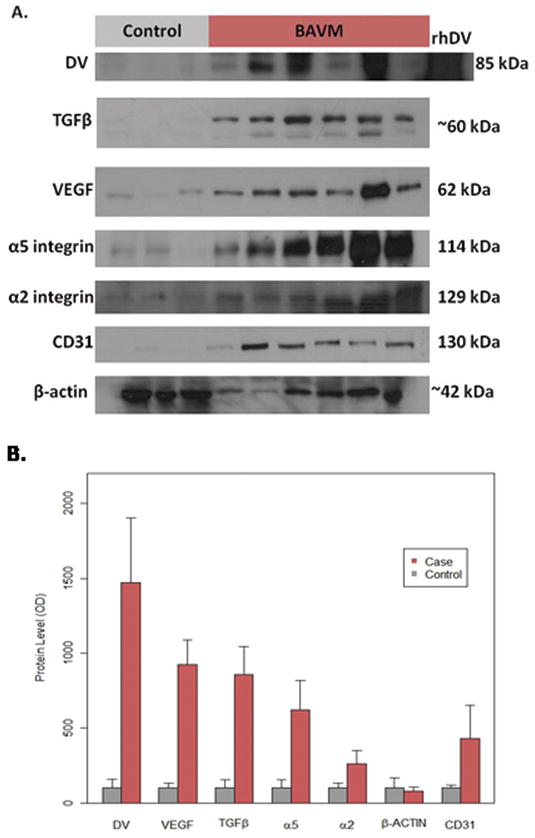
Protein levels of DV, VEGF, TGFβ, α5, α2, and CD31 are increased in human BAVM compared to control (epilepsy) tissue. (A) Representative western blots for each protein investigated. Note that recombinant human DV (rhDV) was used as a positive control for DV blots. (B) Bar graph displaying the mean amounts of DV (p < .001), VEGF (p < .001), TGFβ (p < .001), α5 (p = .001), α2 (p = .006), and CD31 (p = .02) were increased in BAVM patients compared to controls, while β-actin was not (p = .649). These relationships remained the same after adjusting for CD31 and β-actin in the ANCOVA models for DV (p = .04), VEGF (p < .001), TGFβ (p = .008), α5 (p = .05), and α2 (p = .03).
In addition to the higher levels of α5 over that of α2 (Fig. 1), α5 (Fig. 2A) also exhibited greater colocalization to CD31-labeled endothelial cells compared to α2 (Fig. 2B) by immunohistochemistry. Although α2 was increased in BAVM compared to controls, it was more diffusely distributed than α5.
Fig. 2.
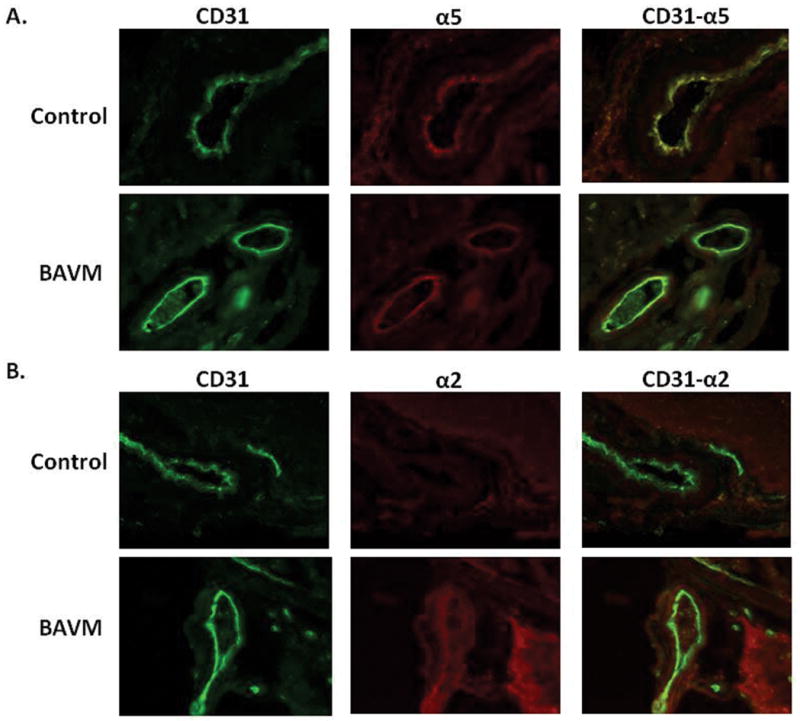
α5 integrin, compared to α2 integrin, appears more greatly increased and localized to endothelial cells in human BAVM compared to control (epilepsy) tissue. Co-immunohistochemistry of CD31 (Abcam) with A. α5 (Millipore) or B. α2 (Chemicon International). Original magnification × 200. Note that the increased α2 in BAVM displays a more diffuse staining pattern than α5 in BAVM.
Discussion
We have demonstrated in BAVM tissue that (1) Total DV protein is increased; (2) DV’s pro-angiogenic receptor α5β1 integrin, compared to DV’s anti-angiogenic receptor α2β1 integrin, has higher protein expression and is more localized to endothelial cells; and (3) Total VEGF and TGFβ levels are elevated and are both correlated with DV expression. Because DV is known to potentiate an angiogenic cascade involving VEGF, and VEGF appears to be an important part of the BAVM nidus, DV may contribute to AVM pathology and represent a potential therapeutic target. In this study, we employed resected brain tissue from epilepsy patients as our control human brain tissue. Although the optimal control would be completely unaffected tissue, as epilepsy pathology or drugs that epileptic individuals take could potentially confound our investigation, epilepsy tissue is the otherwise most appropriate and available “normal” human tissue control.
Our data are in agreement with the view that extracellular matrix undergoes active remodeling as part of the angiogenic and inflammatory vascular environment, likely producing soluble DV from full-length perlecan. It is unlikely that high circulating levels of DV are a sufficient condition for BAVM genesis, as I.P. administration of up to 2 mg/kg in rats or mice do not cause focal brain lesions to form. [6] Rather, it is more likely that the higher levels of DV in BAVM tissue contribute to the progression of BAVM rather than cause BAVM per se. However, while the known functions of DV are of high relevance to BAVM, the mechanistic role of DV in the pathology and progression of BAVM cannot be conclusively determined by this observational study, but warrants additional investigation. The role of increased tissue levels of TGFβ in BAVM are less clear, but TGFβ is involved in many pathways including both vascular stabilization and inflammation, although controversy exists on TGFβ actions in the setting of increased angiogensis. [9] However, the strong correlation between BAVM cases and controls of DV, VEGF, and TGFβ levels suggest that DV is associated with the phenotype of the resected lesion.
The pro-angiogenic brain DV receptor α5β1 integrin appeared to have greater localization to endothelial cells compared to the α2β1 integrin, thereby promoting brain endothelial cell angiogenesis. The more diffuse staining of α2β1 integrin likely represents integrin expression on surfaces of other cell types such as astrocytes or neurons, which are of interest but not the focus of our investigation centered on CD31-positive endothelial cells. It is possible, given the strong correlation between DV and α5β1 integrin, that elevated α5β1 levels in BAVM is partly due to the increased protein levels of DV, as perlecan has been found to increase α5β1 expression in brain endothelial cells. [10] α5β1 is normally absent from mature, non-angiogenic brain blood vessels. The expression pattern that we observed — greatly elevated, endothelial-localized α5β1and slightly elevated but diffusely expressed α2β1 (i.e. not prominently expressed on CD31-positive endothelial cells), an integrin normally absent from brain microvasculature, further underscores the abnormality of the molecular signature of BAVM vascular channels. Finally, as upregulated DV and α5β1 have been identified following cerebral ischemic injury and here in BAVM, this angiogenic protein-receptor association may be of interest to other fields of CNS angiogenic diseases and insults.
Conclusion
Excessive generation of DV and its pro-angiogenic receptor, along with the potentiation of angiogenic and vascular regulatory molecules, suggest that DV could potentially play a role in BAVM pathology and could represent a future therapeutic target.
Acknowledgments
This research was funded by PHS grants R01027713 (WLY), P01NSO44155 (WLY, HS) and R01NS065842-01A01 (GB).
We would like to thank Gregory del Zoppo and L. Gerard Toussaint III
Footnotes
Disclosures and Conflicts of Interest: none declared
References
- 1.Chen Y, Hao Q, Kim H, Su H, Letarte M, Karumanchi SA, et al. Soluble endoglin modulates aberrant cerebral vascular remodeling. Ann Neurol. 2009;66:19–27. doi: 10.1002/ana.21710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H, Pawlikowska L, Chen Y, Su H, Yang GY, Young WL. Brain arteriovenous malformation biology relevant to hemorrhage and implication for therapeutic development. Stroke. 2009;40:S95–7. doi: 10.1161/STROKEAHA.108.533216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H, Pawlikowska L, Young WL. Genetics and vascular biology of brain vascular malformations (Chapter 12) In: Mohr JP, Wolf PA, Grotta JC, Moskowitz MA, Mayberg M, von Kummer R, editors. Stroke: Pathophysiology, Diagnosis, and Management. 5. Philadelphia: Churchill Livingstone Elsevier; 2011. pp. 169–186. [Google Scholar]
- 4.Hao Q, Zhu Y, Su H, Shen F, Yang GY, Kim H, et al. VEGF Induces More Severe Cerebrovascular Dysplasia in Endoglin than in Alk1 Mice. Transl Stroke Res. 2010;1:197–201. doi: 10.1007/s12975-010-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bix G, Iozzo RV. Matrix revolutions: “tails” of basement-membrane components with angiostatic functions. Trends Cell Biol. 2005;15:52–60. doi: 10.1016/j.tcb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest. 2011;121:3005–23. doi: 10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laakso A, Dashti R, Juvela S, Niemela M, Hernesniemi J. Natural history of arteriovenous malformations: presentation, risk of hemorrhage and mortality. Acta Neurochir Suppl. 2010;107:65–9. doi: 10.1007/978-3-211-99373-6_10. [DOI] [PubMed] [Google Scholar]
- 8.Bix G, Iozzo RA, Woodall B, Burrows M, McQuillan A, Campbell S, et al. Endorepellin, the C-terminal angiostatic module of perlecan, enhances collagen-platelet responses via the alpha2beta1-integrin receptor. Blood. 2007;109:3745–8. doi: 10.1182/blood-2006-08-039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20:556–67. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Milner R, Hung S, Wang X, Berg GI, Spatz M, del Zoppo GJ. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008;39:191–7. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476–83. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]


