Abstract
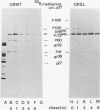
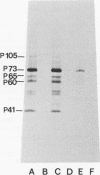
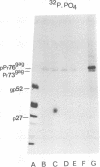
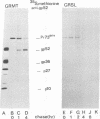
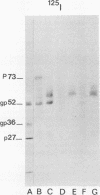
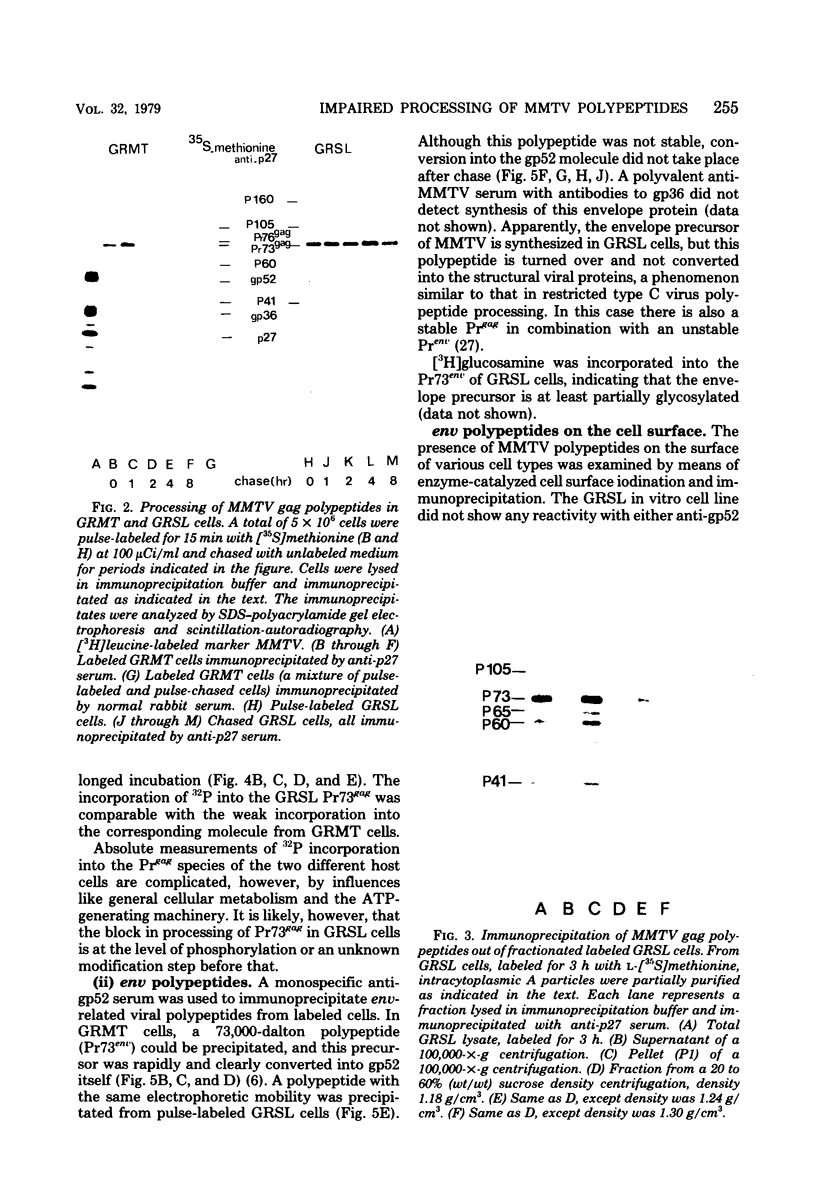
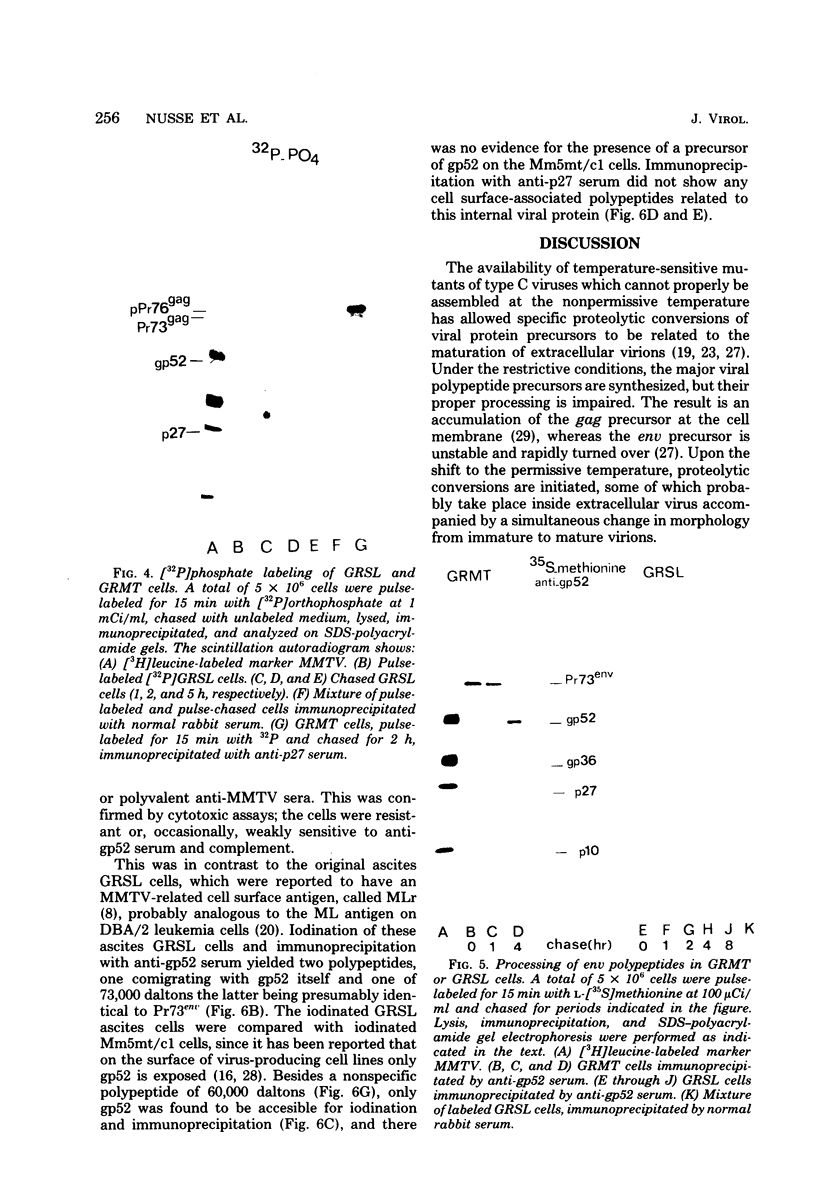
Processing of polypeptides of the mouse mammary tumor virus, a type B retrovirus, was investigated in a transplanted thymic lymphoma cell line of the GR strain (GRSL). This cell line was maintained in vivo in ascites form and in vitro as a suspension culture. GRSL cells produce clusters of intracytoplasmic A particles and are virtually deficient in the production of mature extracellular B-type particles. As control, a mammary tumor cell line of the same mouse strain capable of complete virion synthesis was used. The kinetics of viral polypeptide synthesis were studied by pulse labeling with various isotopes (including 35S and 32P), followed by immunoprecipitation of cell lysates with monospecific antisera to the major mouse mammary tumor virus gag and env proteins, p27 and gp52, respectively. Both the primary gag and env precursor polypeptides were synthesized in the GRSL cells, but their conversion into viral proteins was impaired. The major gag precursor, Pr73gag, was stable over a period of 8 h, and mature viral core polypeptides could not be detected. Also, the highly phosphorylated intermediates in the proteolytic processing of Pr73gag in virus-producing cells were absent in GRSL cells. By immunoprecipitation, Pr73gag was detected in a GRSL particle fraction with the density of intracytoplasmic A particles. The precursor for envelope proteins, Pr73env, was turned over without the generation of mature viral envelope components gp52 and gp36. The in vivo-transplanted ascites GRSL cells, however, were shown to express gp52 on the cell surface together with a 73,000-dalton polypeptide, as indicated by cell surface iodination and immunoprecipitation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHARD W. Electron microscopy of tumor cells and tumor viruses; a review. Cancer Res. 1958 Jun;18(5):491–509. [PubMed] [Google Scholar]
- Bolognesi D. P., Montelaro R. C., Frank H., Schäfer W. Assembly of type C oncornaviruses: a model. Science. 1978 Jan 13;199(4325):183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Calafat J., Buijs F., Hageman P. C., Links J., Hilgers J., Hekman A. Distribution of virus particles and mammary tumor virus antigens in mouse mammary tumors, transformed BALB-c mouse kidney cells, and GR ascites leukemia cells. J Natl Cancer Inst. 1974 Oct;53(4):977–992. doi: 10.1093/jnci/53.4.977. [DOI] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Polyproteins related to the major core protein of mouse mammary tumor virus. J Virol. 1978 Jun;26(3):660–672. doi: 10.1128/jvi.26.3.660-672.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Puma J. P., Nandi S. Identification of a precursor protein to the major glycoproteins of mouse mammary tumor virus. J Virol. 1975 Jan;17(1):275–282. doi: 10.1128/jvi.17.1.275-282.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Hilgers J., Haverman J., Nusse R., van Blitterswijk W. J., Cleton F. J., Hageman P. C., van Nie P., Calafat J. Immunologic, virologic, and genetic aspects of mammary tumor virus-induced cell-surface antigens: presence of these antigens and the Thy 1.2 antigen on murine mammary gland and tumor cells. J Natl Cancer Inst. 1975 Jun;54(6):1323–1333. doi: 10.1093/jnci/54.6.1323. [DOI] [PubMed] [Google Scholar]
- Kohno M., Tanaka H. Characterization of an RNA-directed DNA polymerase found in association with murine intracytoplasmic A-particles. J Virol. 1977 May;22(2):273–280. doi: 10.1128/jvi.22.2.273-280.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nusse R., Asselbergs F. A., Salden M. H., Michalides R. J., Bloemendal H. Translation of mouse mammary tumor virus RNA: precursor polypeptides are phosphorylated during processing. Virology. 1978 Nov;91(1):106–115. doi: 10.1016/0042-6822(78)90359-8. [DOI] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. Phosphorylation of murine mammary tumor virus precursor polypeptides. J Virol. 1979 Apr;30(1):241–247. doi: 10.1128/jvi.30.1.241-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. Synthesis and processing of precursor polypeptides to murine mammary tumor virus structural proteins. J Virol. 1978 Jan;25(1):374–383. doi: 10.1128/jvi.25.1.374-383.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUECK B., BOYSE E. A., OLD L. J., CARSWELL E. A. ML: A NEW ANTIGEN FOUND IN LEUKAEMIAS AND MAMMARY TUMOURS OF THE MOUSE. Nature. 1964 Sep 5;203:1033–1034. doi: 10.1038/2031033a0. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Whittington E. S. Identification of the structural proteins of the murine mammary tumor virus that are serologically related to the antigens of intracytoplasmic type-A particles. Virology. 1977 Aug;81(1):91–106. doi: 10.1016/0042-6822(77)90061-7. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Fine D. L., Massey R. J. Mouse mammary tumor virus and murine leukemia virus cell surface antigens on virus producer and nonproducer mammary epithelial tumor cells. Virology. 1978 Jul 15;88(2):379–383. doi: 10.1016/0042-6822(78)90294-5. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Long C. W., Oroszlan S., Arthur L., Fine D. L. Isolation of separate precursor polypeptides for the mouse mammary tumor virus glycoproteins and nonglycoproteins. Virology. 1978 Mar;85(1):168–174. doi: 10.1016/0042-6822(78)90421-x. [DOI] [PubMed] [Google Scholar]
- Smith G. H. Evidence for a precursor-product relationship between intracytoplasmic A particles and mouse mammary tumour virus cores. J Gen Virol. 1978 Oct;41(1):193–200. doi: 10.1099/0022-1317-41-1-193. [DOI] [PubMed] [Google Scholar]
- Tanaka H. Precursor-product relationship between nonglycosylated polypeptides of A and B particles of mouse mammary tumor virus. Virology. 1977 Feb;76(2):835–850. doi: 10.1016/0042-6822(77)90263-x. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk W. J., Emmelot P., Hilgers J., Kamlag D., Nusse R., Feltkamp C. A. Quantitation of virus-induced (MLr) and normal (Thy.1.2) cell surface antigens in isolated plasma membranes and the extracellular ascites fluid of mouse leukemia cells. Cancer Res. 1975 Oct;35(10):2743–2751. [PubMed] [Google Scholar]
- Vitetta E. S., Baur S., Uhr J. W. Cell surface immunoglobulin. II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. J Exp Med. 1971 Jul 1;134(1):242–264. doi: 10.1084/jem.134.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Mechanism of formation of pseudotypes between vesicular stomatitis virus and murine leukemia virus. Cell. 1977 Jul;11(3):505–511. doi: 10.1016/0092-8674(77)90068-x. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Tang R., Nandi S. Identification of the mammary tumor virus envelope glycoprotein (gp52) on mouse mammary epithelial cell surface. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1044–1050. doi: 10.1016/0006-291x(77)90961-5. [DOI] [PubMed] [Google Scholar]
- Yeger H., Kalnins V. I., Stephenson J. R. Type-C retrovirus maturation and assembly: post-translational cleavage of the gag-gene coded precursor polypeptide occurs at the cell membrane. Virology. 1978 Aug;89(1):34–44. doi: 10.1016/0042-6822(78)90037-5. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Properties of a P70 proteolytic factor of murine leukemia viruses. Cell. 1977 Nov;12(3):709–719. doi: 10.1016/0092-8674(77)90271-9. [DOI] [PubMed] [Google Scholar]
- van de Ven W. J., van Zaane D., Onnekink C., Bloemers H. P. Impaired processing of precursor polypeptides of temperature-sensitive mutants of Rauscher murine leukemia virus. J Virol. 1978 Feb;25(2):553–561. doi: 10.1128/jvi.25.2.553-561.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]