Abstract
Here we review a unique aspect of CNS research on biologically active peptides that started against a background of prevalent dogmas but ended by exerting considerable influence on the field. During the course of refuting some doctrines, we introduced several concepts that were unconventional and paradigm-shifting at the time. We showed that (1) hypothalamic peptides can act ‘up’ on the brain as well as ‘down’ on the pituitary, (2) peripheral peptides can affect the brain, (3) peptides can cross the blood-brain barrier, (4) the actions of peptides can persist longer than their half-lives in blood, (5) perinatal administration of peptides can exert actions persisting into adulthood, (6) a single peptide can have more than one action, (7) dose-response relationships of peptides need not be linear, (8) the brain produces antiopiate as well as opiate peptides, (9) there is a selective high affinity endogenous peptide ligand for the mu-opiate receptor, (10) a peptide’s name does not restrict its effects, and (11) astrocytes assume an active role in response to metabolic disturbance and hyperleptinemia. The evolving questions in our laboratories reflect the diligent effort of the neuropeptide community to identify the roles of peptides in the CNS. The next decade is expected to see greater progress in the following areas: (a) interactions of peptides with other molecules in the CNS; (b) peptide involvement in cell-cell interactions; and (c) peptides in neuropsychiatric, autoimmune, and neurodegenerative diseases. The development of peptidomics and gene silencing approaches will expedite the formation of many new concepts in a new era.
Keywords: Peptide, antiopiate, mu-opiate, astrocytes, leptin, MIF-1, MSH, behavior, learning, memory, Parkinson’s disease, mental depression, ObR
INTRODUCTION
As in sociology, the birth and growth of a new idea require a suitably receptive environment. Young neuropeptide researchers nowadays may find it difficult to imagine that a time ever existed in which it was inconceivable that a peptide in the periphery could affect CNS function. Even today some old-timers do not acknowledge that selective peptides can cross the blood-brain barrier (BBB) in intact form. Only a few years ago when we started to elucidate the regulation and functions of astrocytic leptin receptors, there was resistance arising in part from neuron-centric training and experience. The ability and attempt to challenge existing paradigms is presumably being encouraged, but reality reveals unnecessary delay most of the time. The developmental history of neuropeptide research is part of the forty-year legacy and ongoing efforts of our laboratories. Although this represents only a drop in what is now a vast ocean of neuropeptide research, the reiteration of peptide concepts may be helpful in current pharmaceutical design.
CONCEPTS
1. Hypothalamic Peptides can Act “Up” as Well as “Down”
In the classical model of the hypothalamic-pituitary-target organ axis, the story begins with the production of a hypothalamic releasing or inhibiting hormone. The hypothalamic hormone is transported to the anterior pituitary via the hypophyseal portal circulation. There, a corresponding pituitary hormone is produced and released into the circulation. The pituitary hormone then induces the synthesis and release of a target gland hormone, which feeds back negatively to inhibit the production of the corresponding pituitary and hypothalamic hormones. This pathway, although shown by the endocrine pioneer George Sayers and others since the late 1940’s, did not take into account the rest of the brain as a target for the hypothalamic hormone.
We thought that it would be more efficient for the body to use the same hypothalamic hormones to act on brain regions “higher up” than the hypothalamus than to create new ones. The hypothesis that a peptide hormone from the hypothalamus can act on the rest of the brain was first proven by studies with MIF-1 [1,2]. Later, tract tracing studies from many neuroanatomy groups confirmed the presence of complicated projection pathways that certainly involve hypothalamic peptides [3–6]. Studies after injection of hypothalamic peptides show effects not only confined to hypothalamusrelated behavior. Numerous examples of hypothalamic peptides acting on higher CNS centers are provided in the Brain Peptides Section of the Handbook of Biologically Active Peptides [7].
MIF-1 is a tripeptide (Pro-Leu-Gly-NH2) first isolated from bovine hypothalamus. The peptide sequence is seen in the side ring of oxytocin and in Tyr-MIF-1. Its endogenous concentration is highest in the hypothalamus; however, it may or may not be a derivative of the oxytocin gene. The isolation of MSH-release-inhibiting factor-1 (MIF-1, Pro-Leu-Gly-NH2, or PLG) almost four decades ago involved the 11000-fold concentration of 5000 cow hypothalami, followed by what is now considered the rather imprecise method of thin layer chromatography [8,9]. Hypothalamic extracts were more active than cerebral cortical extracts in inhibiting MSH release. Recently, we applied a sensitive and specific triple quadrupole linear ion trap mass spectrometer analyzing three multiple reaction monitoring transitions for MIF-1 that clearly shows the presence of this tripeptide in the hypothalamus, striatum, and elsewhere in the mouse brain [10]. Furthermore, MIF-1 treatment induces region-specific activation of the immediate early gene c-Fos, the highest activation being in the cingulate cortex, infralimbic cortex, nucleus accumbens, paraventricular hypothalamic nucleus, medial basal amygdaloid nucleus, fiber tract in the piriform cortex, paraventricular thalamic nucleus, and some other thalamic nuclei [11]. These are regions involved in the regulation of mood, anxiety, depression, and memory.
MIF-1 has effects on CNS activity, including learning and behavior. When tested in a 12-choice Warden maze for a palatable food reward, rats injected with MIF-1 have shorter latencies and make fewer errors than controls during learning, but not extinction [12]. After acquisition of a visual task in an extradimensional spatial shift problem, MIF-1 facilitates acquisition of brightness discrimination, presumably by increasing attention [13]. MIF-1 also increases passive avoidance retention and decreases shock-suppressed water intake [14], and it can attenuate the amnesia induced by puromycin [15] and electroconvulsive shock [16]. It also can enhance information storage received through olfactory cues in social investigatory behavior [17]. Thus, the general effects of MIF-1 on learning seem beneficial.
In addition, MIF-1 has secondary effects on body temperature. Although it does not alter basal colonic temperature or motor activity of rats at 4 or 20 °C, it increases d-amphetamine-induced hypothermia at 4 °C and decreases the hyperthermic effect of the amphetamine at 20 °C [18]. Similarly, MIF-1 blocks the hypothermic effects of chlorpromazine at both temperatures in normal and hypophysectomized animals, another example of an extra-endocrine action not mediated by the pituitary. In addition, the hypothermia induced by reserpine [19], β-endorphin, or morphine are all antagonized by MIF-1 [20].
MIF-1 shows therapeutic effects in animal models of Parkinson’s disease [1,2]. It is active in tests of dopa-potentiation [1,21–23] and oxotremorine antagonism in mice [24–26], and deserpidine antagonism in monkeys as well as mice [27]. It exerts these effects even when given orally to mice and monkeys [27–29], and does not require the presence of the pituitary gland [1,24,28]. MIF-1 is also effective against the neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) that induces many of the clinical symptoms of Parkinson’s disease [30,31]. In rats with unilateral substantia nigra lesions induced by 6-OHDA, peripheral injection of MIF-1 (2 ng/kg) potentiates the effect of L-dopa on apomorphine-induced turning, and it decreases endorphin levels in the caudate nucleus at a dose of 20 mg/kg [32].
In clinical investigations of the efficacy of MIF-1 in Parkinson’s disease, the first study tested 16 Canadian patients and found improvement in rigidity and tremor [33]. A similar pattern was then observed in 10 German patients [34]. Akinesia and rigidity were the symptoms most improved in a study with Austrian patients, and this was evident only 2 days after treatment began, reached a maximum at 1 week, and persisted for several more weeks [35]. In some later studies of parkinsonian patients, MIF-1 reduced the side-effects and prolonged the efficacy of Madopar (levodopa + benserazide) for 2 – 6 weeks after drug cessation [36–38]. The significant potentiation by MIF-1 of the effects of Madopar in reducing dyskinesia, rigidity, and tremor in these studies is consistent with that shown in earlier studies, particularly by Barbeau [39]. This expert in clinical trials of anti-parkinsonian drugs reported that “The improvement obtained in almost all (5/6) cases was of such magnitude that it far surpassed the clinical effect of any of the numerous antiparkinson drugs which we have tested in our laboratory over the past 15 years, including levodopa itself.” These findings indicate that the actions of MIF-1 are not confined to the hypothalamus.
In the Austrian patients with Parkinson’s disease [35], depressive symptoms also improved, as they did in a study of 12 German parkinsonian patients receiving MIF-1 in a double-blind crossover design [40]. A small group of Canadian patients with Parkinson’s disease showed a 55% reduction in the classic Hamilton score of mental depression [39]. These observations of improvement of depressive as well as parkinsonian symptoms were intriguing.
In animal models of mental depression, MIF-1 was also effective. Some of the early animal models for Parkinson’s disease discussed above are also considered animal models of depression [1,2]. The more selective Porsolt forced swim test of behavioral despair in rats [41] is a classical model of depression in which MIF-1 is also active [42–44]. A peripheral dose as low as 0.01 mg/kg of MIF-1 showed anti-immobility effects, and this was antagonized by blockade of brain dopamine receptors [43]. It is consistent with allosteric modulation of the dopamine receptor by MIF-1 [45]. In the Nomura water wheel modification [46] of the Porsolt test for mice, MIF-1 is also active in doses as low as 0.01 mg/kg ip [47]. In other models in which antidepressants are active, Niesink and van Ree found that MIF-1 reversed isolation-induced increase in social activity [48], and Dutch researchers also reported that MIF-1 antagonized melatonin-induced behavioral changes [49]. MIF-1 is also effective in the labor-intensive Katz test of unpredictable chronic stress [50,51].
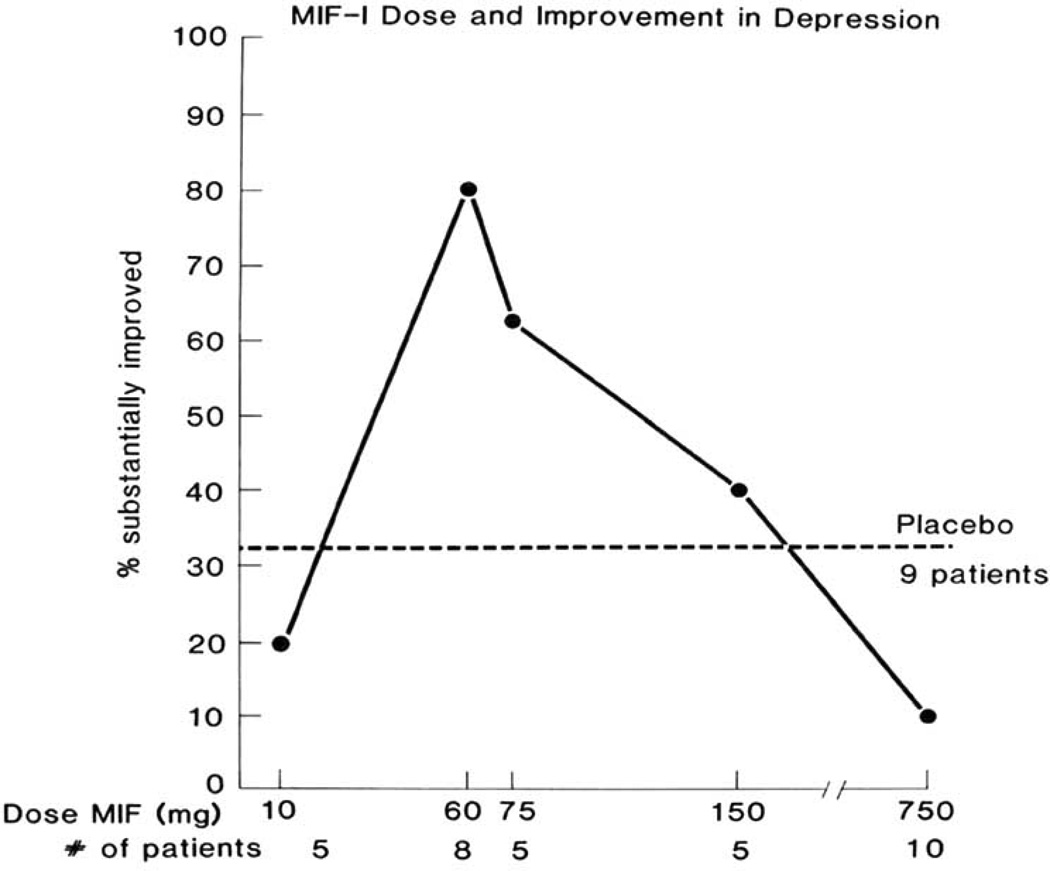
Clinical studies of mental depression showed the inverted-U shaped dose-response relationship noted in animal studies. In the first two small studies of oral MIF-1 in women with major depressive illness, marked improvement was seen with single daily doses of 60 or 75 mg/day by mouth, but not the large doses (Fig. 1) [52,53]. Van der Velde then studied 20 depressed patients, half of whom received 75 mg of the tricyclic antidepressant imipramine po and the other half 60 mg of MIF-1 po daily for 4 weeks. His conclusion was that MIF-1 was “as least as effective as imipramine in this study, and that its anti-depressive effect was a rapid and often dramatic one” [54]. Another 20 patients with major depression received a single subcutaneous injection of either 10 mg MIF-1 or placebo for 5 days [55]. The treatments were reversed for a second week of 5 consecutive daily injections. At the end of the first week, 8 of 9 patients receiving MIF-1 showed marked improvement on the Hamilton test as compared with only 2 of 11 patients receiving the saline placebo. Administration of MIF-1 during the second week to the patients who had received placebo during the first week resulted in a similar improvement. A graphic summary of the studies with oral MIF-1 by R.H. Ehrensing is shown in Fig. (1). Considering the rapid onset of action after MIF-1, it is hoped that the hormesis effect [56] will not prevent its development as an antidepressant.
Fig. 1.
The inverted-U shaped dose-response curve seen in investigations by R.H. Ehrensing et al. of the effects of oral MIF-1 in patients with mental depression.
The contribution of endogenous MIF-1 within the hypothalamus to actions “higher” in the brain, perhaps through axonal transport along projection pathways and molecular diffusion along fiber tracts within the CNS, has not been determined. Regardless, as shown in several animal models of Parkinson’s disease and depression discussed in this section, these actions are not mediated “down” on the pituitary. Clearly, a hypothalamic peptide can act “up” to affect functions typically mediated by the cerebral cortex or the striatum.
2. Peptides in the Periphery can Act on the Brain
The prevailing view four decades ago was that peptides in the periphery could not have any effect whatsoever on the brain. This was not limited to those investigators who were sure that peptides could not cross the BBB directly, but extended to researchers who could not conceive of even indirect effects of peptides on the brain. Perhaps a contributing factor in this persistent disbelief was the failure of some early studies with adrenocorticotropin (ACTH) and vasopressin to consider the secondary effects of these pituitary hormones (e.g., on adrenal steroid release and blood pressure) or of the vehicle itself [57]. It was not realized that a peptide’s actions are not completely dependent on its half-life in blood (see Section 4), so that a vehicle prolonging the half-life of the peptide is not necessary to demonstrate the CNS effects of a peripherally injected peptide.
The first unconfounded studies showing an effect of a peripherally administered peptide on the brain used the pituitary hormone melanocyte-stimulating hormone (MSH; melanocortin), rather than ACTH or vasopressin. These studies demonstrated behavioral and electrophysiological (EEG) responses in rodents as well as human beings [58–65]. Supporting studies with MSH, extending their earlier studies with ACTH and vasopressin, were then provided by the large capable Utrecht group headed by David deWied [66]. Rather than being satisfied with revealing these effects on the general process of learning and memory, we further showed in a series of what then were considered sophisticated studies that the process of attention was a key component [67–69]. Unfortunately, Organon pharmaceuticals chose for clinical studies an MSH analog whose efficacy was determined by the conditioned avoidance response in rats, rather than by tests of the native MSH itself with its broader actions. This point was emphasized in studies showing that different analogs of MSH can have differing dose-related responses in different behavioral paradigms [70,71].
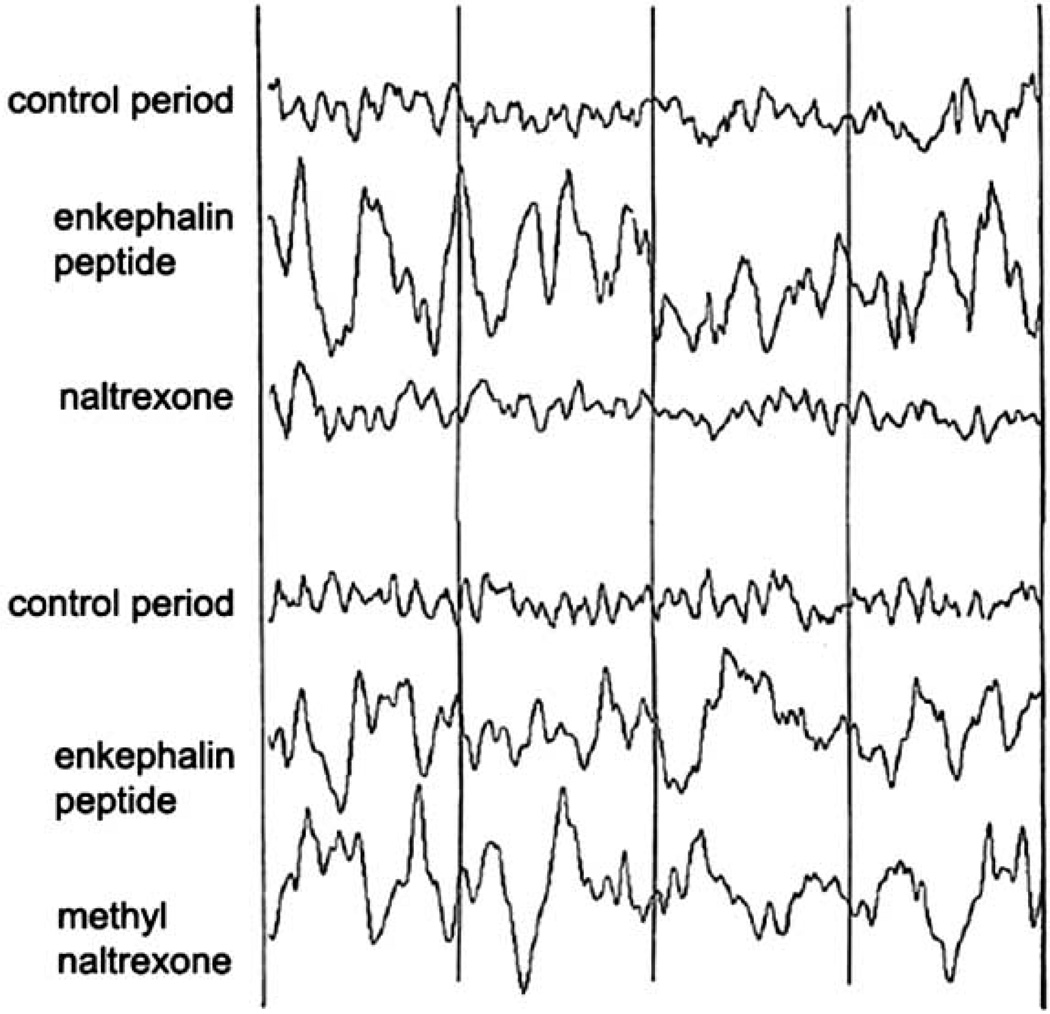
A good example of a peptide injected peripherally acting on the brain and not mediated by peripheral effects was shown with a Met-enkephalin analog. In this study, naltrexone, an opiate antagonist that is able to cross the BBB, blocked the EEG effects of the Met-enkephalin analog whereas methyl naltexone, which does not readily cross the BBB, was unable to do so [72]. This is illustrated in Fig. (2).
Fig. 2.
Demonstration that the electrophysiological effects of an analog of the opiate Met-enkephalin (enkephalin peptide) administered peripherally can be blocked by an opiate antagonist (naltexone) that crosses the bloodbrain barrier but not by an opiate antagonist (methyl naltrexone) that does not readily cross the blood-brain barrier (modified from Kastin et al. [72]).
3. Peptides can Cross the Blood-Brain Barrier
The regional distribution of c-Fos activation is different when MIF-1 is administered intravenously and when it is administered intracerebroventricularly. Although the concentration and disappearance kinetics are not identical in the two experimental regimens, intravenous MIF-1 activates more areas [11]. One possible mechanism mediating the CNS effects of MIF-1 after peripheral injection is its passage across the blood-brain barrier (BBB). The BBB provides the largest surface area for efficient exchange of information, particularly for substances coming from the periphery. The surface area of the BBB is 100–150 cm2/g, and the microvessels composing the BBB are less than 40 µm apart [73–75]. The vast BBB contrasts with the circumventricular organs (CVOs) that provide specialized areas for rapid transmission of peripheral input to certain localized CNS regions. As for interleukin-1α, the uptake by CVOs accounts for less than 5% of total brain permeation [76]. Indeed, MIF-1 rapidly enters the brain by a saturable transport system [77]. After peripheral injection, tritiated MIF-1 is rapidly distributed to rat brain, with the highest concentration in the pituitary, followed by the striatum, hypothalamus, and midbrain [78], although occipital and frontal cortex also ranked high in an earlier study [79].
The pharmacokinetic BBB permeation of the Tyr-MIF-1 family of peptides has been quantified in mice. MIF-1 is rapidly transported into brain from blood by a partially saturable system whereas Tyr-MIF-1 only enters slowly by passive diffusion. By contrast, Tyr-MIF-1 has a saturable transport system out of the brain that is not shared by MIF-1, illustrating its selectivity structurally; but surprisingly, it is shared by a structurally dissimilar pentapeptide, Met-enkephalin that does, however, share an N-terminal tyrosine [80–82]. This can be interpreted as sharing functions, since Tyr-MIF-1 is an antiopiate and Met-enkephalin is an opiate. As shown with knockout mice lacking P-glycoprotein (Pgp) or inhibited by cyclosporin A, Pgp is not involved in the efflux of Tyr-MIF-1 or the endomorphins [83,84].
There are two principal ways by which peptides and proteins interact with the BBB: (a) transport or diffusion in intact form across the endothelial cells that are the structural backbone of the BBB [85–88] and (b) their actions on these endothelial cells which result in altered endothelial function, cytotoxicity, or cell proliferation [89–92]. We have quantified the BBB permeability of a series of bioactive peptides and polypeptides, including neurotrophic peptides, larger neurotrophins, and several classes of cytokines. Many small proteins cross the BBB (Tables 1 and 2) [93–95]. The mechanisms include saturable transport or passive diffusion based on physicochemical properties. Even for peptides and larger proteins that do not cross, they may still act on cerebral endothelial cells to change their functions. The distinctive characteristics of different peptides and proteins in their interactions with the BBB have broad therapeutic implications. For example, saturation of the transport of leptin across the BBB explains much of the phenomenon of ‘leptin resistance’ [96–99].
Table 1.
BBB Transport Systems of Endogenous Ingestive Peptides
| Category | Representative Peptides | References |
|---|---|---|
| Saturable | Leptin | [96] |
| Insulin | [150,151] | |
| TNFα | [86] | |
| Mahogany protein | [152] | |
| Pancreatic polypeptide | [153] | |
| Human ghrelin | [154] | |
| CRH | [155] | |
| Saturable, but requires activation |
Urocortin - no entry unless activated by leptin |
[100] |
| by TNFα | [156] | |
| by glucose | [157] | |
| Passive diffusion (non- saturable) |
Orexin A | [158] |
| NPY | [159] | |
| AgRP | [160] | |
| CART | [161] | |
| α-MSH | [79,162] | |
| GLP-1 | [163] | |
| Amylin | [164] | |
| Urocortin II | [155] | |
| cHis-Pro | [165] | |
| Nesfatin | [166,167] | |
| FGF21 | [168] | |
| PYY3-36 | [169] | |
| No entry | Orexin B | [158] |
| MCH | [170] | |
| Obestatin | [171] | |
| Adiponectin | [171] | |
| Mouse ghrelin | [154,171] | |
Table 2.
BBB Transport of Other Endogenous Peptides/Polypeptides, Mostly Cytokines and Neurotrophins
| Category | Representative Peptide/Polypeptide |
References |
|---|---|---|
| Saturable | CNTF | [172] |
| EGF | [173] | |
| GM-CSF | [174] | |
| IFNγ | [175] | |
| IGF1 | [176] | |
| IL1α/IL1β | [85] | |
| IL1RA | [177] | |
| LHRH | [178] | |
| LIF | [179] | |
| Neuregulin1β1 | [180] | |
| PACAP38 | [181] | |
| Passive diffusion (non- saturable) |
CINC1 | [182] |
| GH | [183] | |
| IFNα | [175] | |
| IL2 | [184] | |
| IL6 | [185] | |
| IL8 | [186] | |
| PACAP27 | [181] | |
| TGFα | [187] | |
| No entry | GDNF | [188] |
| IL10 | [189] | |
| MIP1α, MIP1β | [190] | |
| PDGF-AA, PDGF-BB | [191] | |
| TGFβ | [192] | |
There are also peptide-peptide interactions at BBB transport. Urocortin is a 4 kD peptide that binds to corticotropin releasing hormone receptors (CRHR) and has multiple effects on CNS functions, including enhancing concentration, memory, inhibiting food intake, and providing neurotrophic support. Although of relatively low molecular weight, urocortin does not show significant penetration across the BBB in the basal state. However, the addition of leptin, or glucose, can greatly enhance the permeation of urocortin across the BBB, apparently activating the transport system from blood to brain. Once activated, urocortin transport is saturable [100]. This unusual facilitation is also seen in cultured cerebral microvessel endothelial cells, where urocortin undergoes rapid trafficking after internalization [101].
The interactions between urocortin and leptin occur not only at the level of transport but also at the level of intracellular signaling. Both CRH receptors can mediate the saturable transport of urocortin [102]. Urocortin binding activates adenylate cyclase and induces cAMP production. While leptin enhances the endocytosis of urocortin, urocortin potentiates leptin-induced transcriptional activation of signal transducer and activator of transcription (STAT) -3. The interactions are not reciprocal, since leptin fails to further increase urocortin-mediated cAMP production [103].
Melanocortin and leptin also show signaling interactions at the BBB. In the cerebral microvessels that compose the BBB, receptors for both melanocortin and leptin are present. For the central effects of feeding, ObRb, which is not a G-protein coupled receptor, is the main signaling receptor for leptin while MC3R and MC4R, G-protein coupled receptors, are the main signaling receptors for αMSH (melanocortin). In cellular models with overexpression of murine ObRb, MC3R, or MC4R, leptin induces activation of Stat3 whereas αMSH stimulates the production of cAMP. After costimulation, αMSH potentiates the STAT3 activation induced by leptin, while leptin has only a minor effect in increasing αMSH-initiated cAMP production downstream to MC3R [104]. This novel potentiation in cellular signaling may have considerable biological implications.
4. The Actions of Peptides can Persist Longer That Their Half-Lives in Blood
Among the many possible ways by which peripheral peptides can exert CNS effects [105], the most direct route is to cross the BBB. Skepticism about the ability of peptides to cross the BBB was exemplified by a report from a “blue-ribbon” advisory panel to NIH that stated “Since the half-life of (peptides) is short, it is unlikely that significant entry occurs into brain” [106].
As mentioned in Section 2 of this review, some vehicles for the administration of peptides designed to prolong their actions can exert that action themselves [57]. But a short half-life in blood does not mean that a peptide cannot exert long-acting effects without the presence of a vehicle to prolong its actions. Even though the biological action of a substance frequently correlates with its half-life in blood, this is less applicable to peptides. Peptides in blood usually remain intact for only a few minutes, whereas the central actions mentioned above for MIF-1 and MSH can persist for several hours after peripheral administration. This was clearly demonstrated many years ago for luteinizing hormone-releasing hormone [107,108]. An exception to the rule of short disappearance times for small peptides is MIF-1. Its half-life in human plasma is 5 days although in rat plasma it lasts only a few minutes, like other small peptides including the related Tyr-MIF-1 and endomorphins [109–112]. Moreover, unlike most peptides, many studies have shown that MIF-1 is active orally in rodents [27–29,113] as well as human beings [33,52–54,114]. It is yet to be determined whether MIF-1 is a substrate for oligopeptide transporter (PEPT)-1 in the intestine and PEPT-2 in the choroid plexus, or both in the kidney. Overall, signaling cascades and secondary effects of a peptide can last longer than the half-life of the intact peptide.
5. Perinatal Administration of Peptides Exerts Long-Lasting Effects
In comparison with other peptide concepts that we introduced, the first demonstration that perinatal administration of peptides can exert long-lasting effects did not encounter the same skepticism or derision. It was presented after it had been well established that perinatal administration of sex steroids could affect adult rodents, and provided further evidence supporting the view that a peptide’s half-life in blood does not necessarily correlate with its biological actions (Concept 4 above). The ability of perinatally administered peptides to affect adult behavior was first shown with MSH [115,116] and TRH [117], and then with endorphin [118,119], Metenkephalin [120], MIF-1 [121], and Tyr-MIF-1 [122,123]. Later, perinatal administration of Tyr-MIF-1 was also found to alter its own transport across the BBB [124], and similar confirmation of this principle continues to be shown with other peptides.
6. One Peptide can Exert more than One Action
Although it is now evident that a peptide can bind to different receptor subtypes, recruit different signaling elements, and show a variety of effects in different tissues, its name tended to confine its perception at one time. Two articles in Nature [125,126] and one in Lancet [60] were the first to use the term “extra-pigmentary” to describe the effects of MSH in mammals. Persistence of the presence of MSH in rodents without much of a pigmentary function suggested to us that it may have acquired new functions consistent with the principle of peptide conservation. Since melanocytes, the target organ of MSH in lower vertebrates, are of neural crest origin, the brain seemed to us a logical target for MSH in mammals. This concept of one peptide exerting more than one action was so novel at the time it was introduced that it was inscribed in the 1982 William S. Middleton Award, the major research award of the Veteran Affairs Administration, to one of us (AJK).
In addition to different behavioral and EEG effects in rats and human beings shown for both MSH (Concept 2) and MIF-1 (Concept 1), these peptides can also exert antiopiate effects, as discussed below (Section 8). Moreover, LHRH is now frequently called gonadotropin-releasing hormone (GnRH) because of its ability to release both LH and FSH [127,128]. Whereas MIF-1 exerts antiopiate but not opiate activity, addition of a single amino acid to the N-terminus of this tripeptide yields Tyr-MIF-1 (Tyr-Pro-Leu-Gly-amide) that can exert both opiate and antiopiate actions [129]. An additional amino acid substitution results in Tyr-W-MIF-1 (Tyr-Pro-Trp-Gly-amide) [130,131] which also has dual opiate and antiopiate effects. More recent examples of many peptides exerting multiple actions have buried the “one hormone-one action theory”.
7. Increased Peptide Doses can Result in Decreased Effects
Frequent criticisms from referees of our early papers were that we were using unfamiliar statistical techniques (analysis of variance followed by a multiple comparisons test) and that some of the effects we observed did not follow a linear dose-response relationship. Even now the mechanism for the inverted-U, bell-shaped dose-response relationship remains elusive, but it has become so generalized a phenomenon that Calabrese published 14 papers in Crit Rev Toxicol (volume 38, 2008) describing this “hormesis”. These reports nicely summarize the occurrence of non-linear dose-response relationships in a variety of experimental and clinical situations, to which we added a commentary about peptides [56]. With this emphasis on hormesis, there now exists an International Dose-Response Society and an online Dose-Response journal.
Non-linear dose-response relationships may be one of the reasons that “big pharma” has not pursued the promising early results with the clinical use of MIF-1 to treat mental depression. Unlike other antidepressants, MIF-1 exerts its maximal effects in less than a week, usually within 3–5 days, and these effects can persist for months even though larger doses may not be effective [52,53,55], as also was observed in several animal studies [42,47,132]. One may consider “cellular plasticity”, where a higher dose or persistent presence of a peptide activates alternative/additional pathways, downregulates signaling elements by desensitization, or induces gene silencing. Any of the mechanistic studies will have to stem from the original observation that peptides have biphasic and pleiotropic effects.
8. The Brain Contains Antiopiate Peptides
The existence of endogenous antiopiates could be considered consistent with a type of short-loop negative feedback mentioned in Section 1. Rather than representing a negative feedback from a peripheral target organ (e.g., adrenal gland) to the pituitary/ hypothalamus, antiopiates within the brain would be more efficient to regulate the actions of brain opiates. Incidentally, we avoid use of the term opioid coined for enkephalins and endorphins because its evolving meaning is no longer confined to peptides.
MIF-1 was the first peptide recognized as showing antiopiate activity [133]. It functions as an antiopiate by itself, and reverses the effects of morphine in a large variety of experimental conditions, as well as in a clinical study [114]. The many confirmatory studies have been reviewed recently [132]. In the first paper describing the antiopiate effects of MIF-1, it was predicted that antiopiate activity would be found for other endogenous peptides [133]. This prediction has been validated for peptides that had already been described, like cholecystokinin (CCK)-8, MSH, somatostatin, angiotensin II, hemorphins, and morphiceptin, as well as for peptides such as members of the NPFF family, nociceptin/orphanin FQ, and enterostatin that were discovered after the antiopiate concept was introduced.
9. There is a Specific Peptide Ligand for the Mu-Opiate Receptor
The mu (μ) opiate receptor is the main subtype for the analgesic actions of morphine. In the early 1990’s, specific endogenous peptides were already known to selectively bind to the delta and kappa opiate receptors, but an endogenous peptidergic ligand specific for the mu opiate receptor had not been identified, as endorphin is relatively non-selective. It was anticipated for quite a while that a peptide selectively binding to mu opiate receptors would eventually be found in brain. The arduous work leading to the discovery of the mu receptor specific endormorphin-1 and endomorphin-2 by Zadina and colleagues started from painstaking extraction and structural function analyses. Although MIF-1 does not bind to opiate receptors, Tyr-MIF-1, comprising the MIF-1 tripeptide with an N-terminal tyrosine, had some affinity for the mu-opiate site, in addition to its own high affinity site. Tyr-W-MIF-1, isolated from bovine [134] and human [130] brain tissue, is identical to Tyr-MIF-1 except for substitution of leucine in the third position by tryptophan. This tetrapeptide has about 200-fold selectivity for mu over delta and kappa opiate receptors [135]. Additional single amino acid substitutions resulted in endomorphin-1 (Tyr-Pro-Trp-Phe-NH2) and endomorphin-2 (Tyr-Pro-Phe-Phe-NH2), isolated from bovine [136] and human [137] brain tissue with high selectivity and affinity for the mu opiate receptor (Table 3). Their structure is quite different from that of the enkephalins, endorphins, and dynorphins. More than 500 papers with the endomorphins have substantiated their role.
Table 3.
Structures of Opiate Mediating Members of the Tyr-Mif-1 Family of Peptides
10. A Peptide’s Name does Not Restrict its Actions
Recognition of the idea that substances can have multiple actions seemed to have eluded early investigators in the peptide field. Once a peptide is named for the action by which it was first discovered, why would a young researcher bother to explore new functions? Before the availability of crystallography and domain analyses, the nomenclature became a hindrance for thinking outside the box. We’ve already mentioned that MSH does more than stimulate melanocytes in mammals and LHRH releases FSH as well as LH (Sections 2 and 6). Moreover, orexin affects sleep as well as food ingestion [138] and somatostatin inhibits more peptides than just growth hormone (somatotropin) [139,140]. Not only are peptides remaining to be discovered, but new functions for already discovered peptides can be predicted, so there needs to be frequent reminders of this obvious concept for peptides.
11. Astrocytes are Involved in Obesity
Just as some of the concepts discussed above involve new functions for peptides with previously described actions, it is reasonable to predict that new functions will be described for anatomical constituents, such as astrocytes, known to serve other purposes.
As mentioned in Section 3, saturation of the transport system for leptin explains much of leptin resistance, in which excess circulating leptin no longer exerts its effect to reduce food intake and adiposity. In the past few years, we have taken several unique approaches in understanding the regulatory changes that direct leptin transport from blood to brain in rodents. The leptin receptor, ObR, is a member of the class I cytokine receptor family. Although the leptin receptor subtype ObRa is most abundantly expressed in cerebral microvessels and it efficiently mediates leptin endocytosis, Koletsky rats lacking ObRa because of a Tyr763 stop mutation in the extracellular domain [141] still transport some leptin into the brain [142,143]. Besides the ObRa isoform, we have shown that ObRb, ObRc, ObRd, and even the tailless form of ObR can endocytose leptin and release it from receptor-overexpressing cells in intact form [144]. We also found unexpected changes of ObR subtypes in cerebral microvessels and hypothalamus during development [145].
For about 15 years, CNS leptin research has focused on neuronal signaling. It was not until 2008 that astrocytic leptin receptors gained recognition from the demonstration that agouti viable yellow (Avy) mice with adult-onset obesity show specific upregulation of astrocytic ObR [146]. In the BBB microvessels of Avy mice, the expression of abundant ObRa is unchanged while that of the sparse ObRb is mildly upregulated. This, the shift of cellular distribution of ObR from BBB endothelia to astrocytes may represent a dynamic defensive mechanism of the CNS against metabolic disturbance. The upregulation of astrocytic ObR and concurrent reduction of neuronal ObR are also seen in B6 wildtype mice with diet-induced obesity [147]. The astrocytes respond to leptin treatment with robust cell signaling, including calcium influx [147] and induction of pSTAT3 and pERK [148]. Sadly, despite the undisputable multilevel evidence of astrocytic production of ObR, including confocal microscopic analysis of immunohistochemistry with a variety of antibodies, RT-PCR, and double-labeling in-situ hybridization [149], it took nearly two years for the neuron-centric community to accept the presence of ObR in astrocytes. Fortunately, acceptance of this concept took less time than that for some of the other concepts discussed here, undoubtedly facilitated by further demonstration of functional changes of mice after astrocytic ObR functions are modulated.
There are two aspects of the experimental proof that astrocytic ObR functions to antagonize neuronal leptin activities in pathological conditions. First, in mice pretreated with fluorocitrate, an inhibitor of astroglial metabolic activity, there is redistribution of leptin in neurons and increased pSTAT3 activation (Pan W et al., unpublished observations). Second, in mice with specific knockout of astrocytic ObR that we recently generated, there is partial resistance to diet-induced obesity, and reduced susceptibility to pilocarpine-induced seizures (Pan W et al., unpublished observations) and experimental autoimmune encephalomyelitis (Wu X et al., unpublished observations). Overall, the improvement of behavioral outcome in these disease models indicates that the astrocytic leptin system may serve to worsen neuropathology in reactive astroglia.
It is reasonable to speculate that the astrocytic leptin system serves its normal function in the resting state. For example, it might be essential for the normal development of the hippocampus where essentially all astrocytes express ObR. Work is ongoing to determine whether astrocytic ObR signaling serves to promote neurogenesis, synaptogenesis, and hippocampal related memory and cognitive functions during the course of development and aging.
THE CONTEXT OF PEPTIDE CONCEPTS IN THE NEUROPEPTIDE FIELD, AND FUTURE DIRECTIONS
The line of research in which we continue to engage originated at a time in which peptides were not yet considered neuromodulators or hormones. The concepts were built upon experimental evidence from somewhat unconventional approaches. They contributed to the transformation from regional to global, from single to multifocal, and from cross-sectional to longitudinal views of the peptide world. Now, neuropeptide research can take more drastic turns. The origin and fate of a peptide are better understood by use of molecular and cellular approaches, and studies of the interactions of peptides are aided by peptidomic methods. Comparative studies across species and time have been particularly valuable. Like the discovery of small interfering RNAs, it is possible that peptides will soon be deemed to have silencing functions to modulate biological activities. The broad functions of peptides are already demonstrated by the trophic, vasogenic, and immunomodulatory roles of many that are implicitly involved in neurodegenerative, oncogenic, and autoimmune diseases. Interacting with classical neurotransmitters such as monoamines, peptides play critical roles and may provide novel therapeutic targets for neuropsychiatric disorders. Peptides also serve as carriers for drug delivery, including lytic peptides and polycation peptides that penetrate cell membranes. Like amino acids and much larger extracellular matrix components, peptides can be essential for cell-cell interactions. There will also be more studies of peptides interacting with other classes of molecules. The ever expanding field is rewarding for all who have contributed a few paradigm-shifting concepts as structural backbones or building blocks.
CONCLUSIONS
As these concepts have become established, some lessons might be gleaned from the course of their progress. Richard Conniff puts it in perspective in the June 2008 issue of the Smithsonian: “Ideas that seem obvious in retrospect are anything but, in real life”. Good advice was provided by Alexander Fleming: “Don’t let your mind be cluttered up with prevailing doctrine” and by Kiekegaard: “There are two ways to be fooled: one is to believe what isn’t so; the other is to refuse to believe what is so”. Even earlier, Schopenhauer realized that discovery frequently undergoes three stages: ridicule, opposition, and acceptance as self-evident, with JBS Haldane adding a fourth stage: “I always said so”.
Even in the 21st century, there was resistance to our last concept about astrocytic leptin, but publications in Endocrinology, Brain, and Peptides facilitated its acceptance. By summarizing this and earlier concepts in this issue of Current Pharmaceutical Design, we hope that it represents the beginning of successful efforts to identify novel targets for neuropsychiatric, autoimmune, and neurodegenerative diseases.
ACKNOWLEDGMENTS
Current grant support to the authors is provided by NIH (DK54880 and NS62291). We thank our former laboratory members and collaborators for much of the work discussed here, particularly Drs. Curt A. Sandman, Richard and Gayle Olson, Rudolph H. Ehrensing, James E. Zadina, and William A. Banks.
ABBREVIATIONS
- BBB
Blood-brain barrier
- CNS
Central nervous system
- CRH
Corticotropin (ACTH) releasing hormone
- CRHR
CRH receptor
- MSH
Melanocyte-stimulating hormone (melanocortin)
- MIF-1
MSH release inhibitory factor-1 (Pro-Lys-Gly-NH2)
REFERENCES
- 1.Plotnikoff NP, Kastin AJ, Anderson MS, Schally AV. DOPA potentiation by a hypothalamic factor, MSH release-inhibiting hormone (MIF) Life Sci. 1971;10:1279–1283. doi: 10.1016/0024-3205(71)90326-2. [DOI] [PubMed] [Google Scholar]
- 2.Kastin AJ, Coy DH, Schally AV, Miller LH. Peripheral administration of hypothalamic peptides results in CNS changes. Pharmacol Res Commun. 1978;10(4):293–312. doi: 10.1016/s0031-6989(78)80025-3. [DOI] [PubMed] [Google Scholar]
- 3.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153(1):1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 4.Wiegand SJ, Price JL. Cells of origin of the afferent fibers to the median eminence in the rat. J Comp Neurol. 1980;192(1):1–19. doi: 10.1002/cne.901920102. [DOI] [PubMed] [Google Scholar]
- 5.Hokfelt T, Johansson O, Ljungdahl A, Lundberg JM, Schultzberg M. Peptidergic neurones. Nature. 1980;284(5756):515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- 6.van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224(1):1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- 7.Vaudry H. Section X: Brain Peptides. In: Kastin AJ, editor. Handbook of biologically active peptides. Amsterdam: Elsevier; 2006. pp. 621–628. [Google Scholar]
- 8.Nair RMG, Kastin AJ, Schally AV. Isolation and structure of hypothalamic MSH release-inhibiting hormone. Biochem Biophys Res Commun. 1971;43:1376–1425. doi: 10.1016/s0006-291x(71)80026-8. [DOI] [PubMed] [Google Scholar]
- 9.Schally AV, Kastin AJ. Purification of a bovine hypothalamic factor which elevates pituitary MSH levels in rats. Endocrinology. 1966;79(4):768–772. doi: 10.1210/endo-79-4-768. [DOI] [PubMed] [Google Scholar]
- 10.Kheterpal I, Kastin AJ, Mollah S, Yu C, Hsuchou H, Pan W. MIF-1 in mouse brain. Peptides. 2008;30:1276–1281. doi: 10.1016/j.peptides.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan RS, Yu C, Kastin AJ, He Y, Ehrensing RH, Hsuchou H, Stone KP, Pan W. Brain activation by peptide Pro-Leu-Gly-NH2 (MIF-1) Int J Peptides. 2010 doi: 10.1155/2010/537639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stratton LO, Kastin AJ. Increased acquisition of a complex appetitive task after MSH and MIF. Pharmacol Biochem Behav. 1975;3(5):901–904. doi: 10.1016/0091-3057(75)90124-0. [DOI] [PubMed] [Google Scholar]
- 13.Beckwith BE, Sandman CA, Kastin AJ. Influence of 3 short chain peptides (α-MSH, MSH/ACTH 4-10, MIF- I) on dimensional attention. Pharmacol Biochem Behav. 1976;5(Suppl 1):17–21. doi: 10.1016/0091-3057(76)90322-1. [DOI] [PubMed] [Google Scholar]
- 14.Pucilowski O, Plaznik A, Kostowski W. MIF-1 facilitates passive avoidance retention. Pol J Pharmacol Pharm. 1982;34:107–113. [PubMed] [Google Scholar]
- 15.Walter R, Hoffman PL, Flexner J, Flexner L. Neurohypophyseal hormones, analogs, and fragments: Their effect on puromycin-induced amnesia. Proc Natl Acad Sci USA. 1975;72:4180–4184. doi: 10.1073/pnas.72.10.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovacs G, Szabo G, Telegdy G, Balaspiri L, Palos E, Szpornyi L. Antiamnesic effects of D-pipecolic acid and analogues of Pro-Leu-Gly-NH2 in rats . Pharmacol Biochem Behav. 1989;31:833–837. doi: 10.1016/0091-3057(88)90392-9. [DOI] [PubMed] [Google Scholar]
- 17.Hlinak Z, Krejci I. Social recognition in male rats: age differences and modulation by MIF-1 and Alaptide. Physiol Res. 1991;40:59–67. [PubMed] [Google Scholar]
- 18.Yehuda S, Kastin AJ. Interaction of MIF-I or á-MSH with D-amphetamine or chlorpromazine on thermoregulation and motor activity of rats maintained at different ambient temperatures. Peptides. 1980;1:243–248. doi: 10.1016/0196-9781(80)90061-3. [DOI] [PubMed] [Google Scholar]
- 19.Kastin AJ, Honour LC, Coy DH. Effects of MIF-1 and three related peptides on reserpine-induced hypothermia in mice. Pharmacol Biochem Behav. 1981;15:983–985. doi: 10.1016/0091-3057(81)90066-6. [DOI] [PubMed] [Google Scholar]
- 20.Yehuda S, Kastin AJ, Coy DH. Antagonistic actions of MIF-1 on the hypothermia and hypomotility induced by β-endorphin or morphine. Int J Neurosci. 1980;11:317–320. doi: 10.3109/00207458009147596. [DOI] [PubMed] [Google Scholar]
- 21.Huidobro-Toro JP, Scotti de CA, Longo VG. Intensification of central catecholaminergin and serotonergic processes by the hypothalamic factors MIF and TRF and by angiotensin II. Pharmacol Biochem Behav. 1975;3(2):235–242. doi: 10.1016/0091-3057(75)90153-7. [DOI] [PubMed] [Google Scholar]
- 22.Huidobro-Toro JP, De Carolis AS, Longo VG. Action of two hypothalamic factors (TRH, MIF) and of angiotensin II on the behavioral effects of L-DOPA and 5-hydroxytryptophan in mice. Pharmacol Biochem Behav. 1974;2(1):105–109. doi: 10.1016/0091-3057(74)90141-5. [DOI] [PubMed] [Google Scholar]
- 23.Voith K. Synthetic MIF analogues. Part II: Dopa potentiation and fluphenazine antagonism. Arzneimittelforschung. 1977;27(12):2290–2293. [PubMed] [Google Scholar]
- 24.Plotnikoff NP, Kastin AJ, Anderson MS, Schally AV. Oxotremorine antagonism by a hypothalamic hormone, melanocyte-stimulating hormone release-inhibiting factor, MIF. Proc Soc Exp Biol Med. 1972;140:811–814. doi: 10.3181/00379727-140-36558. [DOI] [PubMed] [Google Scholar]
- 25.Castensson S, Sievertsson H, Lindeke B, Sum CY. Studies on the inhibition of oxotremorine induced tremor by a melanocyte-stimulating hormone release-inhibiting factor, thyrotropin releasing hormone and related peptides. FEBS Lett. 1974;44(1):101–105. doi: 10.1016/0014-5793(74)80315-7. [DOI] [PubMed] [Google Scholar]
- 26.Bjorkman S, Castensson S, Sievertsson H. Tripeptide analogues of melanocyte-stimulating hormone release-inhibiting hormone (Pro-Leu-Gly-NH2) as inhibitors of oxotremorine-induced tremor. J Med Chem. 1979;22(8):931–935. doi: 10.1021/jm00194a009. [DOI] [PubMed] [Google Scholar]
- 27.Plotnikoff NP, Kastin AJ, Anderson MS, Schally AV. Deserpidine antagonism by a tripeptide, L-prolyl-L-leucylglycinamide. Neuorendocrinology. 1973;11:67–71. doi: 10.1159/000122119. [DOI] [PubMed] [Google Scholar]
- 28.Plotnikoff NP, Minard FN, Kastin AJ. DOPA potentiation in ablated animals and brain levels of biogenic amines in intact animals after prolyl-leucylglycinamide. Neuroendocrinology. 1974;14:271–279. doi: 10.1159/000122270. [DOI] [PubMed] [Google Scholar]
- 29.Plotnikoff NP, Kastin AJ. Oxotremorine antagonism by prolyl-leucyl-glycine amide administered by different routes and with several anticholinergics. Pharmacol Biochem Behav. 1974;2:417–419. doi: 10.1016/0091-3057(74)90090-2. [DOI] [PubMed] [Google Scholar]
- 30.Marcotte ER, Chugh A, Mishra RK, Johnson RL. Protection against MPTP treatment by an analog of Pro-Leu-Gly-NH2 (PLG, MIF-1) Peptides. 1998;19(2):403–406. doi: 10.1016/s0196-9781(97)00321-5. [DOI] [PubMed] [Google Scholar]
- 31.Sheng JG, Xu DL, Yu HZ, Xu XR, Tang QM. Partial protection from the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) by Pro-Leu-Gly-NH2(PLG; MIF-1) Life Sci. 1987;40(20):2007–2010. doi: 10.1016/0024-3205(87)90291-8. [DOI] [PubMed] [Google Scholar]
- 32.Pan J, Xu D, Zhao Y, Yu H. Mechanisms of the anti-Parkinson's effect of PLG. Chin J Neurol Psychiatry. 1985;18:205–208. [Google Scholar]
- 33.Kastin AJ, Barbeau A. Preliminary clinical studies with L-prolyl-L-leucyl-glycine amide in Parkinson's disease. Can Med Assoc J. 1972;107:1079–1081. [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer PA, Schneider E, Jacobi P, Maxion H. Effect of melanocyte-stimulating hormone-release inhibiting factor (MIF) in Parkinson's syndrom. Eur Neurol. 1974;12(5–6):360–368. doi: 10.1159/000114633. [DOI] [PubMed] [Google Scholar]
- 35.Gerstenbrand F, Poewe W, Aichner F, Kozma C. In: Central nervous system effects of hypothalamic hormones and other peptides. Collu R, editor. New York: Raven Press; 1979. p. 415. [Google Scholar]
- 36.Pan J. Effect of PLG on Parkinson's disease. J Int Neurol Neurosurg. 1982;5:2. [Google Scholar]
- 37.Xu D, Yu H, Chen S, Pan J. Preliminary report on the effect of PLG (MIF-1) in the treatment of Parkinson's disease. Acta Univ Med Sec Shanghai. 1986;4:328–331. [Google Scholar]
- 38.Wang Z, Xu D, Yu H, Chen S, Tang G, Pan J. Therapeutic effects of PLG in Parkinson's disease. Shanghai Med J. 1992;3:15. [Google Scholar]
- 39.Barbeau A. Potentiation of levodopa effect by intravenous L-prolyl-L-leucyl-glycine amide in man. Lancet. 1975;2(7937):683–684. doi: 10.1016/s0140-6736(75)90778-3. [DOI] [PubMed] [Google Scholar]
- 40.Schneider E, Fischer PA, Jacobi P, Reh W. The influence of MIF (melanocyte-stimulating hormone-release inhibiting factor) on psychomotor function and mood in parkinsonian patients. Preliminary report (author's transl) Arzneimittelforschung. 1978;28(8):1296–1297. [PubMed] [Google Scholar]
- 41.Porsolt RD, Le PM, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 42.Kastin AJ, Scollan EL, Ehrensing RH, Schally AV, Coy DH. Enkephalin and other peptides reduce passiveness. Pharmacol Biochem Behav. 1978;9:515–519. doi: 10.1016/0091-3057(78)90051-5. [DOI] [PubMed] [Google Scholar]
- 43.Pulvirenti L, Kastin AJ. Blockade of brain dopamine receptors antagonizes the anti-immobility effect of MIF-1 and Tyr-MIF-1 in rats. Eur J Pharmacol. 1988;151:289–292. doi: 10.1016/0014-2999(88)90810-2. [DOI] [PubMed] [Google Scholar]
- 44.Kostowski W, Danysz W, Dyr W, Jankowska E, Krzascik P, Palejko W, Stefanski R, Plaznik A. MIF-1 potentiates the action of tricyclic antidepressants in an animal model of depression. Peptides. 1991;12(5):915–918. doi: 10.1016/0196-9781(91)90037-p. [DOI] [PubMed] [Google Scholar]
- 45.Fisher A, Mann A, Verma V, Thomas N, Mishra RK, Johnson RL. Design and synthesis of photoaffinity-labeling ligands of the L-prolyl-L-leucylglycinamide binding site involved in the allosteric modulation of the dopamine receptor. J Med Chem. 2006;49(1):307–317. doi: 10.1021/jm050644n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nomura S, Shimizu J, Kinjo M, Kametani H, Nakazawa T. A new behavioral test for antidepressant drugs. Eur J Pharmacol. 1982;83(3–4):171–175. doi: 10.1016/0014-2999(82)90248-5. [DOI] [PubMed] [Google Scholar]
- 47.Kastin AJ, Abel DA, Ehrensing RH, Coy DH, Graf MV. Tyr-MIF-1 and MIF-1 are active in the water wheel test for antidepressant drugs. Pharmacol Biochem Behav. 1984;21:767–771. doi: 10.1016/s0091-3057(84)80017-9. [DOI] [PubMed] [Google Scholar]
- 48.Niesink RJ, van Ree JM. Neuropeptides and social behavior of rats tested in dyadic encounters. Neuropeptides. 1984;4(6):483–496. doi: 10.1016/0143-4179(84)90092-1. [DOI] [PubMed] [Google Scholar]
- 49.Hatta K, Wolterink G, Van Ree J. Prolyl-leucyl-glycinamide, thyrotropin-releasing hormone and beta-endorphin-(10–16), like antidepressants, antagonize melatonin-induced behavioural changes in rats. Eur J Pharmacol. 1995;284:327–330. doi: 10.1016/0014-2999(95)00473-x. [DOI] [PubMed] [Google Scholar]
- 50.Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5(2):247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- 51.Pignatiello MF, Olson GA, Kastin AJ, Ehrensing RH, McLean JH, Olson RD. MIF-1 is active in a chronic stress animal model of depression. Pharmacol Biochem Behav. 1989;32:737–742. doi: 10.1016/0091-3057(89)90027-0. [DOI] [PubMed] [Google Scholar]
- 52.Ehrensing RH, Kastin AJ. Melanocyte-stimulating hormone-release inhibiting hormone as an anti-depressant: a pilot study. Arch Gen Psychiatry. 1974;30:63–65. doi: 10.1001/archpsyc.1974.01760070047007. [DOI] [PubMed] [Google Scholar]
- 53.Ehrensing RH, Kastin AJ. Dose-related biphasic effect of prolyl-leucyl-glycinamide (MIF-I) in depression. Am J Psychiatry. 1978;135:562–566. doi: 10.1176/ajp.135.5.562. [DOI] [PubMed] [Google Scholar]
- 54.van der Velde CD. Rapid clinical effectiveness of MIF-I in the treatment of major depressive illness. Peptides. 1983;4(3):297–300. doi: 10.1016/0196-9781(83)90136-5. [DOI] [PubMed] [Google Scholar]
- 55.Ehrensing RH, Kastin AJ, Wurzlow GF, Michell GF, Mebane AH. Improvement in major depression after low subcutaneous doses of MIF-1. J Affect Disord. 1994;31:227–233. doi: 10.1016/0165-0327(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 56.Kastin AJ, Pan W. Peptides and hormesis. Crit Rev Toxicol. 2008;3(7):629–631. doi: 10.1080/10408440802026372. [DOI] [PubMed] [Google Scholar]
- 57.Ley F, Corsen J. Effects of ACTH and zinc phosphate vehicle on shuttlebox CAR. Psychon Sci. 1970;20:307–309. [Google Scholar]
- 58.Sandman CA, Kastin AJ, Schally AV. Melanocyte-stimulating hormone and learned appetitive behavior. Experientia. 1969;25:1001–1002. doi: 10.1007/BF01898118. [DOI] [PubMed] [Google Scholar]
- 59.Kastin AJ, Miller LH, Nockton R, Sandman CA, Schally AV, Stratton LO. Behavioral aspects of MSH. Prog Brain Res. 1973;39:461–470. doi: 10.1016/s0079-6123(08)64100-x. [DOI] [PubMed] [Google Scholar]
- 60.Kastin AJ, Kullander S, Borglin N, et al. Extrapigmentary effects of MSH in amenorrheic women. Lancet 1. 1968;1:1007–1010. doi: 10.1016/s0140-6736(68)91113-6. [DOI] [PubMed] [Google Scholar]
- 61.Sandman CA, Denman PM, Miller LH, Knott JR, Schally AV, Kastin AJ. Electroencephalographic measures of melanocyte stimulating hormone activity. J Comp Physiol Psychol. 1971;76:103–109. doi: 10.1037/h0031046. [DOI] [PubMed] [Google Scholar]
- 62.Kastin AJ, Miller LH, Gonzalez-Barcena D, et al. Psychophysiologic correlates of MSH activity in man. Physiol Behav. 1971;7:893–896. doi: 10.1016/0031-9384(71)90060-6. [DOI] [PubMed] [Google Scholar]
- 63.Kastin AJ, Sandman CA, Stratton LO, Schally AV, Miller LH. Behavioral and electrographic changes in rat and man after MSH. Prog Brain Res. 1975;42:143–150. doi: 10.1016/S0079-6123(08)63655-9. [DOI] [PubMed] [Google Scholar]
- 64.Sandman CA, George JM, Nolan JD, Van Riezen H, Kastin AJ. Enhancement of attention in man with ACTH/MSH 4-10. Physiol Behav. 1975;15:427–431. doi: 10.1016/0031-9384(75)90209-7. [DOI] [PubMed] [Google Scholar]
- 65.Sandman CA, George J, McCanne TR, Nolan JD, Kaswan J, Kastin AJ. MSH/ACTH 4-10 influences behavioral and physiological measures of attention. J Clin Endocrinol Metab. 1977;44:884–891. doi: 10.1210/jcem-44-5-884. [DOI] [PubMed] [Google Scholar]
- 66.de Wied D, Bohus B, van Wimersma Greidanus TB. The hypothalamo-neurohypophyseal system and the preservation of conditioned avoidance behavior in rats. Prog Brain Res. 1974;41:417–428. doi: 10.1016/s0079-6123(08)61922-6. [DOI] [PubMed] [Google Scholar]
- 67.Sandman CA, Miller LH, Kastin AJ, Schally AV. A neuroendocrine influence on attention and memory. J Comp Physiol Psychol. 1972;80:54–58. doi: 10.1037/h0032827. [DOI] [PubMed] [Google Scholar]
- 68.Sandman CA, Beckwith W, Gittis M, Kastin AJ. Melanocyte-stimulating hormone (MSH) and overtraining effects on extradimensional shift (EDS) learning. Physiol Behav. 1974;13:163–166. doi: 10.1016/0031-9384(74)90320-5. [DOI] [PubMed] [Google Scholar]
- 69.Miller LH, Kastin AJ, Sandman CA, Fink M, Van Veen WJ. Polypeptide influence on attention, memory, and anxiety in man. Pharmacol Biochem Behav. 1974;2:663–668. doi: 10.1016/0091-3057(74)90035-5. [DOI] [PubMed] [Google Scholar]
- 70.Miller L, Fischer S, Groves G, Rudrauff M, Kastin AJ. MSH/ACTH 4-10 influences on the CAR in human subjects: a negative finding. Pharmaol Biochem Behav. 1977;7:417–419. doi: 10.1016/0091-3057(77)90208-8. [DOI] [PubMed] [Google Scholar]
- 71.Sandman CA, Beckwith BE, Kastin AJ. Are learning and attention related to the sequence of amino acids in ACTH/MSH peptides? Peptides. 1980;1:277–280. doi: 10.1016/0196-9781(80)90002-9. [DOI] [PubMed] [Google Scholar]
- 72.Kastin AJ, Pearson MA, Banks WA. EEG evidence that morphine and an enkephalin analog cross the blood-brain barrier. Pharmacol Biochem Behav. 1991;40:771–774. doi: 10.1016/0091-3057(91)90084-f. [DOI] [PubMed] [Google Scholar]
- 73.Strand FL. MIT Press; Cambridge, MA: 1999. p. 89. [Google Scholar]
- 74.Begley DJ. Peptides and the blood-brain barrier: the status of our understanding. Ann NY Acad Sci. 1994;739:89–100. doi: 10.1111/j.1749-6632.1994.tb19810.x. [DOI] [PubMed] [Google Scholar]
- 75.Abbott NJ. The blood-brain barrier. In: Kastin AJ, Pan W, editors. Henry Stewart Talks. 2008. [Google Scholar]
- 76.Plotkin SR, Banks WA, Kastin AJ. Comparison of saturable transport and extracellular pathways in the passage of interleukin-1α across the blood-brain barrier. J Neuroimmunol. 1996;67:41–47. doi: 10.1016/0165-5728(96)00036-7. [DOI] [PubMed] [Google Scholar]
- 77.Banks WA, Kastin AJ. Opposite direction of transport across the blood-brain barrier for Tyr-MIF-1 and MIF-1: comparison with morphine. Peptides. 1994;15:23–29. doi: 10.1016/0196-9781(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 78.Cai H, Xu D, Yu H, Wang Z. The distribution of PLG in rat brain. Chin J Neurol Psychiatry. 1989;5:292–293. [Google Scholar]
- 79.Kastin AJ, Nissen C, Nikolics K, Medzihradszky K, Coy DH, Teplan I, Schally AV. Distribution of [3H]α-MSH in rat brain. Brain Res Bull. 1976;1:19–26. doi: 10.1016/0361-9230(76)90045-9. [DOI] [PubMed] [Google Scholar]
- 80.Banks WA, Kastin AJ. A brain-to-blood carrier-mediated transport system for small, N-tyrosinated peptides. Pharmacol Biochem Behav. 1984;21:943–946. doi: 10.1016/s0091-3057(84)80077-5. [DOI] [PubMed] [Google Scholar]
- 81.Banks WA, Kastin AJ, Fischman AJ, Coy DH, Strauss SL. Carrier-mediated transport of enkephalins and N-Tyr-MIF-1 across blood-brain barrier. Am J Physiol. 1986;251:E477–E482. doi: 10.1152/ajpendo.1986.251.4.E477. [DOI] [PubMed] [Google Scholar]
- 82.Banks WA, Kastin AJ, Michals EA. Tyr-MIF-1 and Metenkephalin share a saturable blood-brain barrier transport system. Peptides. 1987;8:899–903. doi: 10.1016/0196-9781(87)90078-7. [DOI] [PubMed] [Google Scholar]
- 83.Kastin AJ, Fasold MB, Zadina JE. Endomorphins, Met-enkephalin, Tyr-MIF-1, and the P-glycoprotein efflux system. Drug Metab Dispos. 2002;30(3):231–234. doi: 10.1124/dmd.30.3.231. [DOI] [PubMed] [Google Scholar]
- 84.Somogyvari-Vigh A, Kastin AJ, Liao J, Zadina JE, Pan W. Endomorphins exit the brain by a saturable efflux system at the basolateral surface of cerebral endothelial cells. Exp Brain Res. 2004;156(2):224–230. doi: 10.1007/s00221-003-1774-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1α, murine IL-1α and murine IL-1β are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther. 1991;259:988–996. [PubMed] [Google Scholar]
- 86.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47:169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 87.Pan W, Kastin AJ. In: Blood-spinal cord and brain barriers in health and disease. Sharma HS, Westman J, editors. San Diego, CA: Elsevier; 2004. pp. 395–407. [Google Scholar]
- 88.Pan W, Kastin AJ, Daniel J, Yu C, Baryshnikova LM, von Bartheld CS. TNF alpha trafficking in cerebral vascular endothelial cells. J Neuroimmunol. 2007;185:47–46. doi: 10.1016/j.jneuroim.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu C, Kastin AJ, Tu H, Pan W. Opposing effects of proteasomes and lysosomes on LIFR: modulation by TNF. J Mol Neurosci. 2007;32:80–89. doi: 10.1007/s12031-007-0017-4. [DOI] [PubMed] [Google Scholar]
- 90.Yu C, Kastin AJ, Pan W. TNF reduces LIF endocytosis despite increasing NFkappaB-mediated gp130 expression. J Cell Physiol. 2007;213:161–166. doi: 10.1002/jcp.21105. [DOI] [PubMed] [Google Scholar]
- 91.Yu C, Pan W, Tu H, Waters S, Kastin AJ. TNF activates p-glycoprotein in cerebral microvascular endothelial cells. Cell Physiol Biochem. 2007;20:853–88. doi: 10.1159/000110445. [DOI] [PubMed] [Google Scholar]
- 92.Pan W, Yu C, Hsuchou H, Zhang Y, Kastin AJ. Neuroinflammation facilitates LIF entry into brain: role of TNF. Am J Physiol Cell Physiol. 2008;294(6):C1436–C1442. doi: 10.1152/ajpcell.00489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pan W, Kastin AJ. Why study transport of peptides and proteins at the neurovascular interface. Brain Res Rev. 2004;46:32–43. doi: 10.1016/j.brainresrev.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Kastin AJ, Pan W. Blood-brain barrier and feeding: regulatory roles of saturable transport systems for ingestive peptides. Curr Pharm Des. 2008;14(16):1615–1619. doi: 10.2174/138161208784705423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pan W, Kastin AJ. Cytokine transport across the injured blood-spinal cord barrier. Curr Pharm Des. 2008;14(16):1620–1624. doi: 10.2174/138161208784705450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 97.Van Heek M, Compton DS, France CF, et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalra SP. Central leptin insufficiency syndrome: an interactive etiology for obesity, metabolic and neural diseases and for designing new therapeutic interventions. Peptides. 2008;29(1):127–138. doi: 10.1016/j.peptides.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adam CL, Findlay PA. Decreased blood-brain leptin transfer in an ovine model of obesity and weight loss: resolving the cause of leptin resistance. Int J Obes (Lond) 2010;34(6):980–988. doi: 10.1038/ijo.2010.28. [DOI] [PubMed] [Google Scholar]
- 100.Kastin AJ, Akerstrom V, Pan W. Activation of urocortin transport into brain by leptin. Peptides. 2000;21:1811–1818. doi: 10.1016/s0196-9781(00)00349-1. [DOI] [PubMed] [Google Scholar]
- 101.Tu H, Kastin AJ, Bjorbaek C, Pan W. Urocortin trafficking in cerebral microvessel endothelial cells. J Mol Neurosci. 2007;31:171–182. doi: 10.1385/jmn/31:02:171. [DOI] [PubMed] [Google Scholar]
- 102.Tu H, Kastin AJ, Pan W. CRH-R1 and CRH-R2 are both trafficking and signaling receptors for urocortin. Mol Endocrinol. 2007;21:700–711. doi: 10.1210/me.2005-0503. [DOI] [PubMed] [Google Scholar]
- 103.Pan W, Tu H, Hsuchou H, Daniel J, Kastin AJ. Unexpected amplification of leptin-induced Stat3 signaling by urocortin: implications for obesity. J Mol Neurosci. 2007;33(3):232–238. doi: 10.1007/s12031-007-0071-y. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Y, Wu X, He Y, Kastin AJ, Hsuchou H, Rosenblum CI, Pan W. Melanocortin potentiates leptin-induced STAT3 signaling via MAPK pathway. J Neurochem. 2009;110(1):390–399. doi: 10.1111/j.1471-4159.2009.06144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kastin AJ, Wade LA, Coy DH, Schally AV, Olson RD. In: Brain and pituitary peptides. Wuttke W, Weindl A, Voigt KH, Dries RR, editors. 1980. pp. 71–78. [Google Scholar]
- 106.An evaluation of research needs in endocrinology and metabolic diseases. Advisory Panel Report to the NIH. 1981;IV:495. [Google Scholar]
- 107.Pfaff DW. Luteinizing hormone-releasing factor potentiates lordosis behavior in hypophysectomized ovariectomized female rats. Science. 1973;182:1148–1149. doi: 10.1126/science.182.4117.1148. [DOI] [PubMed] [Google Scholar]
- 108.Moss RL, McCann SM. Induction of mating behavior in rats by luteinizing hormone-releasing factor. Science. 1973;181:177. doi: 10.1126/science.181.4095.177. [DOI] [PubMed] [Google Scholar]
- 109.Kastin AJ, Hahn K, Erchegyi J, et al. Differential metabolism of Tyr-MIF-1 and MIF-1 in rat and human plasma. Biochem Pharmacol. 1994;47:699–709. doi: 10.1016/0006-2952(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 110.Walter R, Neidle A, Marks N. Significant differences in the degradation of Pro-Leu-Gly-NH2 by human serum and that of other species. Proc Soc Exp Biol Med. 1975;148:98–103. doi: 10.3181/00379727-148-38484. [DOI] [PubMed] [Google Scholar]
- 111.Redding TW, Kastin AJ, Nair RMG, Schally AV. The distribution, half-life, and excretion of 14C and 3H-labeled L-prolyl-L-leucyl-glycinamide in the rat. Neuroendocrinology. 1973;11:92–100. doi: 10.1159/000122121. [DOI] [PubMed] [Google Scholar]
- 112.Redding TW, Kastin AJ, Gonzalez-Barcena D, et al. The disappearance, excretion, and metabolism of tritiated prolyl-leucyl-glycinamide in man. Neuroendocrinology. 1974;16:119–126. doi: 10.1159/000122558. [DOI] [PubMed] [Google Scholar]
- 113.Sharma S, Paladino P, Gabriele J, et al. Pro-Leu-glycinamide and its peptidomimetic, PAOPA, attenuate haloperidol induced vacuous chewing movements in rat: A model of human tardive dyskinesia. Peptides. 2003;24(2):313–319. doi: 10.1016/s0196-9781(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 114.Ehrensing RH, Kastin AJ, Michell GF. Antagonism of morphine analgesia by prolyl-leucyl-glycinamide (MIF-1) in humans. Pharmacol Biochem Behav. 1984;21:975–978. doi: 10.1016/s0091-3057(84)80083-0. [DOI] [PubMed] [Google Scholar]
- 115.Beckwith BW, Sandman CA, Hothersall D, Kastin AJ. Influence of neonatal injections of α-MSH on learning, memory and attention in rats. Physiol Behav. 1977;18:63–71. doi: 10.1016/0031-9384(77)90095-6. [DOI] [PubMed] [Google Scholar]
- 116.Beckwith BW, O'Quin RK, Petro MS, Kastin AJ, Sandman CA. The effects of neonatal injections of α-MSH on the open field behavior of juvenile and adult rats. Physiol Psychol. 1977;5:295–299. [Google Scholar]
- 117.Stratton LO, Gibson CA, Kolar KG, Kastin AJ. Neonatal treatment with TRH affects development, learning, and emotionality in the rat. Pharmacol Biochem Behav. 1976;5(Suppl 1):65–67. doi: 10.1016/0091-3057(76)90330-0. [DOI] [PubMed] [Google Scholar]
- 118.Sandman CA, McGivern RF, Berka C, Walker M, Coy DH, Kastin AJ. Neonatal administration of β-endorphin produces "chronic" insensitivity to thermal stimuli. Life Sci. 1979;25:1755–1760. doi: 10.1016/0024-3205(79)90479-x. [DOI] [PubMed] [Google Scholar]
- 119.Moldow RL, Kastin AJ, Hollander C, Coy DH, Sandman CA. Brain β-endorphin-like immunoreactivity in adult rats given β-endorphin neonatally. Brain Res Bull. 1981;7:683–686. doi: 10.1016/0361-9230(81)90118-0. [DOI] [PubMed] [Google Scholar]
- 120.Kastin AJ, Kostrzewa RM, Schally AV, Coy DH. Neonatal administration of Met-enkephalin facilitates maze performance of adult rats. Pharmacol Biochem Behav. 1980;13:883–886. doi: 10.1016/0091-3057(80)90223-3. [DOI] [PubMed] [Google Scholar]
- 121.D'Amore A, Pieretti S, Palazzesi S, et al. MIF-1 can accelerate neuromotor, EEG and behavioral development in mice. Peptides. 1990;11:527–532. doi: 10.1016/0196-9781(90)90054-9. [DOI] [PubMed] [Google Scholar]
- 122.Zadina JE, Kastin AJ, Coy DH, Adinoff BA. Developmental, behavioral, and opiate receptor changes after prenatal or postnatal β-endorphin, CRF, or Tyr-MIF-1. Psychoneuroendocrinology. 1985;10:367–383. doi: 10.1016/0306-4530(85)90078-2. [DOI] [PubMed] [Google Scholar]
- 123.Zadina JE, Kastin AJ, Manasco PK, Pignatiello MF, Nastiuk KL. Long-term hyperalgesia by neonatal β-endorphin and morphiceptin is blocked by neonatal Tyr-MIF-1. Brain Res. 1987;409:10–18. doi: 10.1016/0006-8993(87)90736-0. [DOI] [PubMed] [Google Scholar]
- 124.Harrison LM, Zadina JE, Banks WA, Kastin AJ. Effects of neonatal treatment with Tyr-MIF-1, morphiceptin and morphine on development, tail flick, and blood-brain barrier transport. Dev Brain Res. 1993;75:207–212. doi: 10.1016/0165-3806(93)90025-6. [DOI] [PubMed] [Google Scholar]
- 125.Kastin AJ, Shally AV, Yajima H, Kubo K. Melanocyte stimulating hormone activity of synthetic MSH and ACTH peptides in vivo and in vitro. Nature. 1965;207:978–979. doi: 10.1038/207978a0. [DOI] [PubMed] [Google Scholar]
- 126.Kastin AJ, Arimura A, Schally AV, Miller M. Mass-action type, direct feedback control of pituitary MSH release. Nature. 1971;231:29–30. [PubMed] [Google Scholar]
- 127.Schally AV, Arimura A, Kastin AJ, et al. Gonadotropin-releasing hormone: one polypeptide regulates the secretion of LH and FSH. Science. 1971;173:1036–1038. doi: 10.1126/science.173.4001.1036. [DOI] [PubMed] [Google Scholar]
- 128.Schally AV, Arimura A, Kastin AJ. Hypothalamic regulating hormones. Science. 1973;179:341–350. doi: 10.1126/science.179.4071.341. [DOI] [PubMed] [Google Scholar]
- 129.Zadina JE, Kastin AJ, Kersh D, Wyatt A. Tyr-MIF-1 and hemorphin can act as opiate agonists as well as antagonists in the guinea pig ileum. Life Sci. 1992;51:869–885. doi: 10.1016/0024-3205(92)90615-v. [DOI] [PubMed] [Google Scholar]
- 130.Erchegyi J, Kastin AJ, Zadina JE. Isolation of a novel tetrapeptide with opiate and antiopiate activity from human brain cortex: Tyr-Pro-Trp-Gly-NH2 (Tyr-W-MIF-1) Peptides. 1992;13:623–632. doi: 10.1016/0196-9781(92)90165-y. [DOI] [PubMed] [Google Scholar]
- 131.Yang Y, Chiu T. Opioid and antiopioid actions of Tyr-MIF-1, Tyr-W-MIF-1 and hemorphin-4 on rat locus coeruleus neurons: intracellular recording in vitro. Chin J Physiol. 1997;40:131–135. [PubMed] [Google Scholar]
- 132.Pan W, Kastin AJ. From MIF-1 to endomorphin: the Tyr-MIF-1 family of peptides. Peptides. 2007;28(12):2411–2434. doi: 10.1016/j.peptides.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 133.Kastin AJ, Olson RD, Ehrensing RH, Berzas MC, Schally AV, Coy DH. MIF-1's differential actions as an opiate antagonist. Pharmacol Biochem Behav. 1979;11:721–723. doi: 10.1016/0091-3057(79)90270-3. [DOI] [PubMed] [Google Scholar]
- 134.Hackler L, Kastin AJ, Erchegyi J, Zadina JE. Isolation of Tyr-W-MIF-1 from bovine hypothalami. Neuropeptides. 1993;24:159–164. doi: 10.1016/0143-4179(93)90080-t. [DOI] [PubMed] [Google Scholar]
- 135.Zadina JE, Kastin AJ, Ge L-J, Hackler L. Mu, delta, and kappa opiate receptor binding of Tyr-MIF-1 and of Tyr-W-MIF-1, its active fragments, and two potent analogs. Life Sci. 1994;55:PL461–PL466. doi: 10.1016/0024-3205(94)00533-8. [DOI] [PubMed] [Google Scholar]
- 136.Zadina JE, Hackler L, Ge L-J, Kastin AJ. A potent and selective endogenous agonist for the µ-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 137.Hackler L, Zadina JE, Ge L-J, Kastin AJ. Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex. Peptides. 1997;18:1635–1639. doi: 10.1016/s0196-9781(97)00259-3. [DOI] [PubMed] [Google Scholar]
- 138.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 139.Hokfelt T, Efendic S, Hellerstrom C, Johansson O, Luft R, Arimura A. Cellular localization of somatostatin in endocrine-like cells and neurons of the rat with special references to the A1-cells of the pancreatic islets and to the hypothalamus. Acta Endocrinol Suppl (Copenh) 1975;200:5–41. [PubMed] [Google Scholar]
- 140.Kastin AJ, Redding TW, Hall R, Besser GM, Schally AV. Lipid mobilizing hormones of the hypothalamus and pituitary. Pharmacol Biochem Behav. 1975;3(1 Suppl):121–126. [PubMed] [Google Scholar]
- 141.Wu-Peng XS, Chua SC, Jr, Okada N, Liu SM, Nicolson M, Leibel RL. Phenotype of the obese Koletsky (f) rat due to Tyr763Stop mutation in the extracellular domain of the leptin receptor (Lepr): evidence for deficient plasma-to-CSF transport of leptin in both the Zucker and Koletsky obese rat. Diabetes. 1997;46(3):513–518. doi: 10.2337/diab.46.3.513. [DOI] [PubMed] [Google Scholar]
- 142.Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides. 1999;20:1449–1453. doi: 10.1016/s0196-9781(99)00156-4. [DOI] [PubMed] [Google Scholar]
- 143.Banks WA, Niehoff ML, Martin D, Farrell CL. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Res. 2002;950:130–136. doi: 10.1016/s0006-8993(02)03013-5. [DOI] [PubMed] [Google Scholar]
- 144.Tu H, Pan W, Feucht L, Kastin AJ. Convergent trafficking pattern of leptin after endocytosis mediated by ObRa - ObRd. J Cell Physiol. 2007;212:215–222. doi: 10.1002/jcp.21020. [DOI] [PubMed] [Google Scholar]
- 145.Pan W, Hsuchou H, Tu H, Kastin AJ. Developmental changes of leptin receptors in cerebral microvessels: unexpected relation to leptin transport. Endocrinology. 2007 doi: 10.1210/en.2007-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pan W, Hsuchou H, He Y, et al. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology. 2008;149(6):2798–2806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hsuchou H, He Y, Kastin AJ, et al. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009;132:889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pan W, Kastin AJ. In: Modern insights into disease - from molecules to man: adipokines. Preedy VR, editor. Enfield, NH, USA: Science Publishers; 2010. [Google Scholar]
- 149.Hsuchou H, Pan W, Barnes MJ, Kastin AJ. Leptin receptor mRNA in rat brain astrocytes. Peptides. 2009;30:2275–2280. doi: 10.1016/j.peptides.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: novel demonstration by species-specific radioimmunoassays. Peptides. 1997;18:1257–1262. doi: 10.1016/s0196-9781(97)00198-8. [DOI] [PubMed] [Google Scholar]
- 151.Woods SC, Porte D., Jr Relationship between plasma and cerebrospinal fluid insulin levels of dogs. Am J Physiol. 1977;233:E331–E334. doi: 10.1152/ajpendo.1977.233.4.E331. [DOI] [PubMed] [Google Scholar]
- 152.Kastin AJ, Akerstrom V. Mahogany (1377–1428) enters brain by a saturable transport system. J Pharmacol Exp Ther. 2000;294:633–634. [PubMed] [Google Scholar]
- 153.Banks WA, Kastin AJ. Regional variation in transport of pancreatic polypeptide across the blood-brain barrier. Pharmacol Biochem Behav. 1995;51:139–147. doi: 10.1016/0091-3057(94)00412-c. [DOI] [PubMed] [Google Scholar]
- 154.Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 155.Kastin AJ, Akerstrom V. Differential interactions of urocortin/corticotropin-releasing hormone peptides with the blood-brain barrier. Neuroendocrinology. 2002;75:367–374. doi: 10.1159/000059433. [DOI] [PubMed] [Google Scholar]
- 156.Pan W, Akerstrom V, Zhang J, Pejovic V, Kastin AJ. Modulation of feeding-related peptide /protein signals by the blood-brain barrier. J Neurochem. 2004;90:455–461. doi: 10.1111/j.1471-4159.2004.02502.x. [DOI] [PubMed] [Google Scholar]
- 157.Kastin AJ, Akerstrom V. Pretreatment with glucose increases entry of urocortin into mouse brain. Peptides. 2001;22:829–834. doi: 10.1016/s0196-9781(01)00397-7. [DOI] [PubMed] [Google Scholar]
- 158.Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. 1999;289:219–223. [PubMed] [Google Scholar]
- 159.Kastin AJ, Akerstrom V. Nonsaturable entry of neuropeptide Y into the brain. Am J Physiol. 1999;276:E479–E482. doi: 10.1152/ajpendo.1999.276.3.E479. [DOI] [PubMed] [Google Scholar]
- 160.Kastin AJ, Akerstrom V, Hackler L. Agouti-related protein (83–132) aggregates and crosses the blood-brain barrier slowly. Metabolism. 2000;49:1444–1448. doi: 10.1053/meta.2000.16556. [DOI] [PubMed] [Google Scholar]
- 161.Kastin AJ, Akerstrom V. Entry of CART into brain is rapid but not inhibited by excess CART or leptin. Am J Physiol. 1999;277:E901–E904. doi: 10.1152/ajpendo.1999.277.5.E901. [DOI] [PubMed] [Google Scholar]
- 162.Wilson JF, Anderson S, Snook G, LLewellyn KD. Quantification of the permeability of the blood-CSF barrier to α-MSH in the rat. Peptides. 1984;5:681–685. doi: 10.1016/0196-9781(84)90006-8. [DOI] [PubMed] [Google Scholar]
- 163.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 164.Banks WA, Kastin AJ, Maness LM, Huang W, Jaspan JB. Permeability of the blood-brain barrier to amylin. Life Sci. 1995;57:1993–2001. doi: 10.1016/0024-3205(95)02197-q. [DOI] [PubMed] [Google Scholar]
- 165.Banks WA, Kastin AJ, Akerstrom V, Jaspan JB. Radioactively iodinated cyclo(His-Pro) crosses the blood-brain barrier and reverses ethanol-induced narcosis. Am J Physiol. 1993;267:E723–E729. doi: 10.1152/ajpendo.1993.264.5.E723. [DOI] [PubMed] [Google Scholar]
- 166.Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides. 2007;28:2223–2228. doi: 10.1016/j.peptides.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 167.Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides. 2007;28(12):2372–2381. doi: 10.1016/j.peptides.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 168.Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28:2382–2386. doi: 10.1016/j.peptides.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nonaka N, Shioda S, Niehoff ML, Banks WA. Characterization of blood-brain barrier permeability to PYY3-36 in the mouse. J Pharmacol Exp Ther. 2003;306:948–953. doi: 10.1124/jpet.103.051821. [DOI] [PubMed] [Google Scholar]
- 170.Kastin AJ, Akerstrom V, Hackler L, Zadina JE. Phe13,Tyr19-Melanin-concentrating hormone and the blood-brain barrier: role of protein binding. J Neurochem. 2000;74:385–391. doi: 10.1046/j.1471-4159.2000.0740385.x. [DOI] [PubMed] [Google Scholar]
- 171.Pan W, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27:911–916. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 172.Pan W, Kastin AJ, Maness LM, Brennan JM. Saturable entry of ciliary neurotrophic factor into brain. Neurosci Lett. 1999;263:69–71. doi: 10.1016/s0304-3940(99)00083-x. [DOI] [PubMed] [Google Scholar]
- 173.Pan W, Kastin AJ. Entry of EGF into brain is rapid and saturable. Peptides. 1999;20:1091–1098. doi: 10.1016/s0196-9781(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 174.McLay RN, Kimura M, Banks WA, Kastin AJ. Granulocyte-macrophage colony-stimulating factor crosses the blood-brain and blood-spinal cord barriers. Brain. 1997;120:2083–2091. doi: 10.1093/brain/120.11.2083. [DOI] [PubMed] [Google Scholar]
- 175.Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J Neuroimmunol. 1997;76:105–111. doi: 10.1016/s0165-5728(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 176.Yu Y, Kastin AJ, Pan W. Reciprocal interactions of insulin and IGF-I in receptor-mediated transport across the blood-brain barrier. Endocrinology. 2006;147:2611–2615. doi: 10.1210/en.2006-0020. [DOI] [PubMed] [Google Scholar]
- 177.Gutierrez EG, Banks WA, Kastin AJ. Blood-borne interleukin-1 receptor antagonist crosses the blood-brain barrier. J Neuroimmunol. 1994;55:153–160. doi: 10.1016/0165-5728(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 178.Barrera CM, Kastin AJ, Fasold MB, Banks WA. Bidirectional saturable transport of LHRH across the blood-brain barrier. Am J Physiol. 1991;261:E312–E318. doi: 10.1152/ajpendo.1991.261.3.E312. [DOI] [PubMed] [Google Scholar]
- 179.Pan W, Kastin AJ, Brennan JM. Saturable entry of leukemia inhibitory factor from blood to the central nervous system. J Neurochem. 2000;106:172–180. doi: 10.1016/s0165-5728(00)00241-1. [DOI] [PubMed] [Google Scholar]
- 180.Kastin AJ, Akerstrom V, Pan W. Neuregulin-1-beta1 enters brain and spinal cord by receptor-mediated transport. J Neurochem. 2004;88:965–970. doi: 10.1046/j.1471-4159.2003.02224.x. [DOI] [PubMed] [Google Scholar]
- 181.Banks WA, Kastin AJ, Komaki G, Arimura A. Passage of pituitary adenylate cyclase activating polypeptide1-27 and pituitary adenylate cyclase activating polypeptide1-38 across the blood-brain barrier. J Pharmacol Exp Ther. 1993;267:690–696. [PubMed] [Google Scholar]
- 182.Pan W, Kastin AJ. Changing the chemokine gradient: CINC1 crosses the blood-brain barrier. J Neuroimmunol. 2001;115:64–70. doi: 10.1016/s0165-5728(01)00256-9. [DOI] [PubMed] [Google Scholar]
- 183.Pan W, Yu Y, Cain CM, Nyberg F, Couraud P, Kastin AJ. Permeation of growth hormone across the blood-brain barrier. Endocrinology. 2005;146:4898–4904. doi: 10.1210/en.2005-0587. [DOI] [PubMed] [Google Scholar]
- 184.Waguespack PJ, Banks WA, Kastin AJ. Interleukin-2 does not cross the blood-brain barrier by a saturable transport system. Brain Res Bull. 1994;34:103–109. doi: 10.1016/0361-9230(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 185.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 186.Pan W, Kastin AJ. Interactions of cytokines with the blood-brain barrier: Implications for feeding. Curr Pharm Des. 2003;9:827–831. doi: 10.2174/1381612033455332. [DOI] [PubMed] [Google Scholar]
- 187.Pan W, Vallance KL, Kastin AJ. TGF alpha and the blood-brain barrier: accumulation in cerebral vasculature. Exp Neurol. 1999;160:454–459. doi: 10.1006/exnr.1999.7215. [DOI] [PubMed] [Google Scholar]
- 188.Kastin AJ, Akerstrom V, Pan W. Glial cell line-derived neurotrophic factor does not enter normal mouse brain. Neurosci Lett. 2003;340:239–241. doi: 10.1016/s0304-3940(03)00007-7. [DOI] [PubMed] [Google Scholar]
- 189.Kastin AJ, Akerstrom V, Pan W. Interleukin-10 as a CNS therapeutic: the obstacle of the blood-brain / blood-spinal cord barrier. Mol Brain Res. 2003;114:168–171. doi: 10.1016/s0169-328x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 190.Banks WA, Kastin AJ. Reversible association of the cytokines MIP-1α and MIP-1β with the endothelia of the blood-brain barrier. Neurosci Lett. 1996;205:202–206. doi: 10.1016/0304-3940(96)12410-1. [DOI] [PubMed] [Google Scholar]
- 191.Kastin AJ, Akerstrom V, Hackler L, Pan W. Different mechanisms influencing permation of PDGF-AA and PDGF-BB across the blood-brain barrier. J Neurochem. 2003;87:7–12. doi: 10.1046/j.1471-4159.2003.01933.x. [DOI] [PubMed] [Google Scholar]
- 192.Kastin AJ, Akerstrom V, Pan W. Circulating TGF beta 1 does not cross the intact blood-brain barrier. J Mol Neurosci. 2003;21(1):43–48. doi: 10.1385/JMN:21:1:43. [DOI] [PubMed] [Google Scholar]
- 193.Horvath A, Kastin AJ. Isolation of tyrosine-melanocyte-stimulating hormone release-inhibiting factor 1 from bovine brain tissue. J Biol Chem. 1989;264:2175–2179. [PubMed] [Google Scholar]