Abstract
Background
MAdCAM-1 is an adhesion molecule expressed in Peyer's patches and lymphoid tissues which is mobilized by cytokines like TNF-α and is a major determinant of lymphocyte trafficking to the gut in human inflammatory bowel disease (IBD). It has been suggested that both reactive oxygen and nitrogen metabolites participate in regulating adhesion molecule expression in response to TNF-α.
Methods
To examine how exogenous and endogenous sources of NO modulate MAdCAM-1 induction by TNF-α, we pre-treated mouse lymphatic endothelial cells with either long or short acting NO donors prior to TNF-α-stimulation, and measured MAdCAM-1 induction at 24 h.
Results and Discussion
DETA-NO, a long-acting NO donor, and SperNO, a rapid releasing NO donor both inhibited TNF-α-stimulated MAdCAM-1 expression in a concentration dependent manner. Both NO donors also reduced a4b7-dependent lymphocyte endothelial adhesion. Inhibition of endogenous NO production by either L-NAME, a non-selective NOS inhibitor, or by 1400 w, a selective iNOS inhibitor failed to induce, or potentiate TNF-α regulated MAdCAM-1 expression.
Conclusions
Exogenous NO donors may be beneficial in the treatment of IBD, while endogenous nitric oxide synthases may be less effective in controlling adhesion molecule expression in response to cytokines.
Background & Aims
MAdCAM-1 is a 60 kD endothelial cell adhesion molecule expressed on the surface of high endothelial venules in the gut, and in Peyers patches. MAdCAM-1 is expressed basally in these tissues and is dramatically increased in inflammatory bowel disease (IBD). In IBD, especially Crohn's disease, MAdCAM-1 acts as the main ligand for a4b7-expressing lymphocytes and recruits these lymphocytes into the intestine where they initiate and sustain chronic inflammation. Several animal models and human studies support an absolute requirement for both MAdCAM-1 and a4b7 in the production of immune models of colitis. MAdCAM-1 is expressed on the surface of lymphoid endothelial cells in response to several cytokines including TNF-α and IL-lb, however, the signal transduction pathways involved in MAdCAM-1 are still not well understood. However, since MAdCAM-1 is induced by Th1 cytokines, like TNF-α and IL-1b, it is likely that its induction is mechanistically similar to that of adhesion molecules like ICAM-1 and VCAM-1. These adhesion molecules are also induced by Th1 cytokines and require activation of the NF-kB/PARP. The activation of these transcription factors also requires the formation of intracellular oxidants, since mobilization of these adhesion molecules in response to cytokines can be prevented by antioxidants like PDTC or NAC. Physiologically, the expression of these cell adhesion molecules also appears to be limited by the formation of NO through either constituitive, or inducible forms of nitric oxide synthase (eNOS, iNOS). It has been suggested that NO could inhibit the transcription/translation of adhesion molecules through either scavenging of signal oxidants produced in response to cytokines, or through covalent modification of polypeptides in the signaling pathway, like IkB.
Here, we examine the induction of MAdCAM-1 by TNF-α, and evaluated whether endogenous nitric oxide (from eNOS and iNOS), or exogenous NO (from rapid or slow-releasing NO donors) affect the expression of MadCAM-1. Our data suggest that in this model, endogenous NO (derived from either iNOS or eNOS), does not significantly influence MAdCAM-1 expression, however, both rapid and slow releasing NO donors can potently inhibit the expression of MAdCAM-1 and reduce lymphocyte endothelial adhesion.
Materials and Methods
Reagents
Recombinant mouse TNF-α was purchased from ENDOGEN (Stoughton, MA). DETA-NO, SperNO and 1400 w were purchased from Alexis corp. (San Diego, CA). L-NAME was purchased from Sigma (St. Louis, MO).
Cell culture
SVEC4-10 is an endothelial cell line derived by SV40 (strain 4A) transformation of murine small vascular endothelial cells originally isolated from axillary lymph node vessels of an adult male C3H/Hej mouse [1]. These cell types were all maintained in DMEM with 10% fetal calf serum with 1% antibiotic/ antimycotic, and seeded onto 24-well tissue culture plates at approximately 20,000 cells/cm2; cultures were used immediately after reaching confluency.
Lymphocytes
Mouse CD8+ T cell lymphoma TK-1 cells which constituitively express α4β7 were obtained from Dr. Eugene Butcher (Stanford University). These cells were cultured in RPMI medium supplemented with 10% FCS, 2 mM L-glutamine, and 0.05 mM 2-mercaptoethanol without antibiotic/antimycotic.
RT-PCR analysis
MAdCAM-1 message in response to TNF-α and blockers was measured by RT-PCR. When NO donors were used they were given as co-treatments, NO synthase blockers (L-NAME) were pre-treated for 60 minutes prior to incubation. Total cell RNA was extracted from SVEC cells using the RNeasy Kit (QIAGEN Inc., Valencia, CA) according to manufacturers instructions. First-strand cDNAs were prepared from 6 μg of total RNA using a mixture of oligo(dT)12-18 and random hexamer primers with Superscript reverse transcriptase (Promega, Madison, WI). The following oligonucleotides were synthesized and used as primers: PI; 5'-CCTAGTACCCTACCAGCTCA-3' P2; 5'-ATCTCCTCTTCTTGCTCTGG-3' (P1-P2; 474 bp)
As controls, a 307 bp (sense; 5'-CGGTGTGAACGGATTTGGCCGTAT-3', antisense; 5'-GGCCTTCTCCATGGTGGTGAAGAC-3') fragment of murine GAPDH was also amplified in a same tube with primers 1 and 2. Primer sequences for GAPDH were separated by introns to control for potential sample contamination by genomic DNA. PCR amplification was performed at 95°C for 3 min; followed by 30 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 1 min, and then at 72°C for the final 5 min. The PCR products were separated on 1.2% agarose gels. To normalize mRNA levels, the density of the MAdCAM-1 and GAPDH bands from the same lane were measured by scanning the three replicate gels (n = 3), using a HP ScanJet™ flatbed scanner. Images were analyzed for density using Image Pro Plus™ image analysis software (Media Cybernetics, Silver Springs, MD). The data are calculated as the rations of the optical density values to those of GAPDH, and are presented as a persentage of TNF-α stimulated density ration. Significant changes in MAdCAM-1 densities were determined using the Instat™ statistical package (Graphpad Software, San Diego, CA).
Western analysis of cell lysates
Monolayers were pretreated (NO synthase blockers) or co-treated (NO donors) with cytokines and harvested at 24 hours. Equal quantities of protein (75 μg) from each sample were electrophoretically separated on 7.5% SDS-PAGE gels. Gels were transferred to nitrocellulose membranes (Sigma) and blocked with 5% milk powder in PBS at 4°C (overnight). These membranes were washed twice for 10 min with wash buffer (0.1% milk powder in PBS). Primary rat anti-mouse MadCAM-1 mAb (MECA 367, Pharmingen, San Diego, CA) [2] was added at a concentration of 10 μg/ml and incubated at room temperature for 2 h. These membranes were washed twice with wash buffer. Secondary goat anti-rat horseradish peroxidase conjugated secondary antibody (Sigma) was added at a 1: 2000 dilution for 2 h. Lastly, membranes were washed 3 times and developed using the enhanced chemiluminescence (ECL) detection system (Amersham, La Jolla, CA). The density of MAdCAM-1 staining was measured by scanning the 60 kD band, using a HP ScanJet™ flatbed scanner. Images were analyzed for density using Image Pro Plus™ image analysis software (Media Cybernetics). The data are expressed as a percentage of TNF-α-induced level of density. Significant changes in MAdCAM-1 densities were determined using the Instat™ statistical package (Graphpad Software). For NF-κB p65 blotting, rabbit anti-p65 polyclonal (Rockland, Germany) and goat anti-rabbit horseradish peroxidase conjugated antibody (Sigma) were used as primary and secondary antibody respectively at a 1:1000 dilution. For giving experiment, each treatment was performed at least in triplicate.
p65 immunoblotting
For NF-κB p65 blotting, SVEC were pretreated for 1 h with or without inhibitors, and then incubated for 1 h with either vehicle or TNF-α (20 ng/ml). Nuclear extracts were prepared as described previously [3]. rabbit anti-p65 polyclonal (Rockland, Germany) and goat anti-rabbit horseradish peroxidase conjugated antibody (Sigma) were used as primary and secondary antibody respectively at a 1:1000 dilution. For giving experiment, each treatment was performed at least in triplicate.
TK-1 lymphocyte adhesion assay
Lymphocyte adhesion assays were performed as previously described [4] with modifications. Briefly, TK-1 cells were suspended in Hank's Balanced salt solution (HBSS) and radiolabeled by incubating TK-1 cells at 2 × 107 cells/ml with 30 μCi Na51CrO4/ml (New England Nuclear, Natick, MA) at 37°C for 60 min. The cells were then washed twice with ice-cold HBSS, spun at 250 g for 8 min to remove unincorporated radioactivity and resuspended in HBSS. The TK-1 lymphocyte cell line used in this assay expresses high levels of the alpha 4 beta 7 integrin, which can interact with multiple ligands including mucosal addressin-1 (MAdCAM-1), as well as VCAM-1, L-selectin and fibronectin [5]. To activate endothelium, the monolayers were incubated with TNF-α (20 ng/ml) for 24 h as described. Cytokine-treated endothelial cells were washed three times with media. Labeled TK-1 cells were then added to the endothelium at a 5:1 lymphocyte: endothelial cell ratio [6] and allowed to bind for 30 min under static conditions. At the end of the incubation period, the supernatant was removed and the monolayers were washed twice with HBSS. The remaining endothelial cells and adherent TK-1 cells were solubilized with IN NaOH. The 51Cr activity of the supernatant, washed fluid, and lysate were assessed in a gamma counter. The percent of added TK-1 cells that adhered to the SVEC monolayers was quantified as follows: %adhesion = [cpm in lysate /(cpm in lysate + cpm in supernatant and wash)] × 100. the average of four identically treated monolayers.
Statistical Analysis
All values are expressed as mean ± SE. Data were analyzed using one-way ANOVA with Bonferroni's correction for multiple comparisons. Significance was accepted at p < 0.05.
Results
Effect of NO on MAdCAM-1 in SVEC
We investigated the effect of DETA-NONOate on TNF-α-induced MAdCAM-1 message in SVEC by RT-PCR. Figure 1 shows the PCR products obtained with the promers, which amplify the first and second IgG-like domain of MAdCAM-1 [7]. A strong band is detected as MAdCAM-1 transcript after stimulated with TNF-α (20 ng/ml) for 12 h. Pretreatment of DETA-NONOate (100 μM) blocked the TNF-α (20 ng/ml, 12 h) induced MAdCAM-1 transcript (37.3% of TNF-α treated) while there was no equivalent change in GAPDH (Figure 1).
Figure 1.
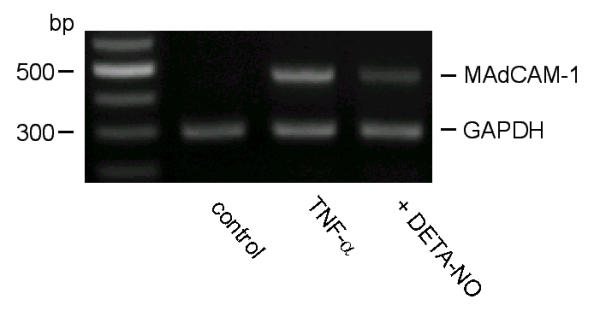
RT-PCR analysis of MAdCAM-1 and GAPDH in SVEC exposed to TNF-α with or without DETA-NONOate. In controls, only a faint MAdCAM-1 transcript is detected (474 bp). MAdCAM-1 band is easily detected after TNF-α stimulation (20 ng/ml). DETA-NONOate (100 μM) blocked TNF-α-induced MAdCAM-1 transcript. In each panel, 307 bp bands indicate GAPDH transcripts, used to evaluate amount and quality of RNA. Similar results were obtained in 3 separate experiments.
TNF-α induces MAdCAM-1 protein expression which is reduced by NO donors
TNF-α (20 ng/ml) induced MAdCAM-1 expression is blocked both by the short-acting and long-acting NO donors, SperNO and DETA-NO respectively. SperNO significantly reduces MAdCAM-1 expression at 100 and 1000 uM (Fig. 2), while the long acting NO donor DETA-NO significantly reduced MAdCAM-1 at a only 1/10 the concentration of SperNO needed to reduce MAdCAM-1 expression (10 uM and 100 uM significantly attenuated MAdCAM-1 expresssion).
Figure 2.
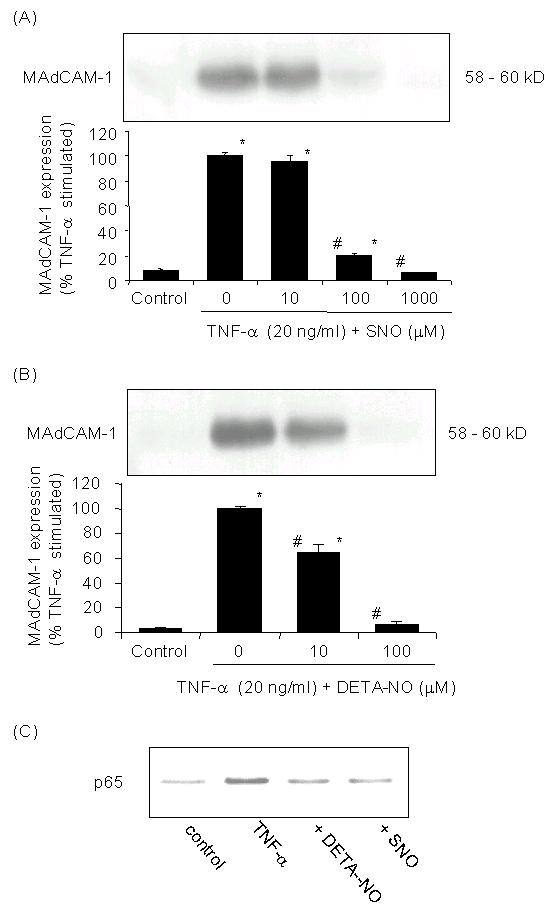
Effect of NO on MAdCAM-1 in SVEC. Cells were pretreated with (A) DETA-NONOate or (B) Spermine-NONOate (SNO) with indicated concentrations and then exposed to TNF-α (20 ng/ml) stimulation in the continued presence of NO donors. MAdCAM-1 expression is significantly increased after TNF-α. DETA-NONOate or Spermine-NONOate (SNO) blocked the TNF-α induced MAdCAM-1 expression dose dependently. Values represent mean ± SE; each group (n = 3). * p < 0.001 vs. untreated controls, # p < 0.001 vs. TNF-α treatment. (C) Effect of NO on p65 NF-κB translocation. Immunoblots of NF-κB p65 in nuclear extracts from SVEC. Nuclear extracts (40 μg) were loaded in each well. (B) Inhibition of TNF-α activation of NF-κB in SVEC. After 1 h of TNF-α (20 ng/ml) treatment nuclear p65 of NF-κB is induced. Pretreatment of the cells with DETA-NONOate (100 μM) or Spermine-NONOate (100 μM) reduced the TNF-α induced nuclear translocation of the NF-κB p65 subunit.
NO donors block TNF-α induces nuclear translocation of p65
Figure 2C shows that TNF-α (20 ng/ml) significantly increased nuclear translocation of p65, a subunit of the p50/p65 NF-kB complex. In the presence of DETA-NO (100 uM) or SperNO (100 uM), p65 nuclear translocation was significantly blocked as visualized by its appearance in western blotting of nuclear samples.
NO synthase inhibitors do not induce or enhance TNF-α induced MAdCAM-1 expression
TNF-α induced MAdCAM-1 was not affected by either a non-selective NO synthase inhibitor, L-NAME (1 mM) or by a relatively selective iNOS inhibitor, 1400-ω (100 μM) (Fig. 3).
Figure 3.
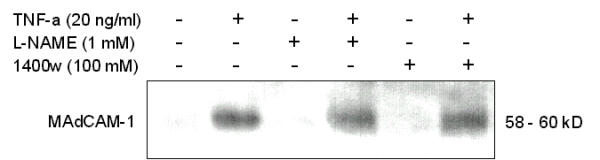
Effect of NOS inhibitors on MAdCAM-1 in SVEC. Cells were pretreated with vehicle, L-NAME or 1400ω with indicated concentrations and then exposed to TNF-α (20 ng/ml) stimulation in the continued presence of the NOS inhibitor. NOS inhibitors themselves did not induce MAdCAM-1 expression nor did they increase MAdCAM-1 in TNF-α treated cells. Similar results were obtained in 3 separate experiments.
Adhesion of a4b7 expressing lymphocytes to MAdCAM-1 expressing cells
Having established a role of NO donors in the regulation of MAdCAM-1 expression by endothelial cells, we examined effects of NO donors on the adhesion of a4b7 expressing mouse lymphocyte cell line TK-1 to TNF-α-treated endothelial cultures. Figure 4 showed that control adhesion of TK-1 cells was 12.3 ± 0.98%; TNF-α induced maximal adhesion (29.8 ± 1.30%, p < 0.001 vs. untreated control) at 24 h. This relatively high level of adhesion was inhibited by pre-incubation of endothelial monolayers with anti-MAdCAM-1 antibody. MAdCAM-1 antibody reduced TK-1 adhesion to 17.0 ± 1.18% (p < 0.001 vs. TNF-α treatment), showing that most of the TNF-α induced lymphocyte adhesion in this model was MAdCAM-1 dependent. Similarly, treatment of endothelial monolayers with DETA-NONOate significantly reduced adhesion to 19.6 ± 0.72% of that induced by TNF-α alone (p < 0.001). DETA-NONOate did not affect lymphocyte adhesion to untreated endothelial monolayers (data not shown).
Figure 4.
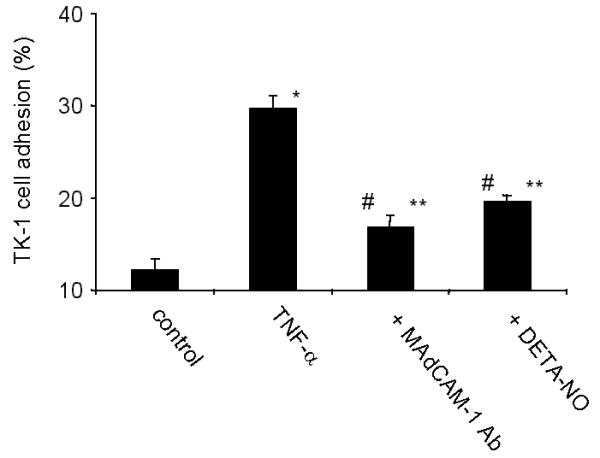
Adhesion of TK-1 cells on SVEC. TNF-α induced significant increase of TK-1 cells adhesion. This increased adhesion was significantly inhibited by pretreatment with MAdCAM-1 Ab. Pretreatment of DETA-NONOate (100 μM) also significantly inhibited TNF-α (20 ng/ml, 24 h) -induced TK-1 adhesion on SVEC. Each value represents the mean ± SE; each group (n = 4). * p < 0.001, ** p < 0.05 vs. untreated control, # p < 0.001 vs. TNF-α treatment.
Discussion
Human IBD is characterized by the extravasation of leukocytes, especially lymphocytes, into the gut, where these cells induce and sustain chronic intestinal inflammation. [8]. Lymphocyte homing to both normal tissues, and to sites of inflammation is, regulated in part, by differential expression of cell surface homing receptors, and their bonds with tissue specific vascular addressins that are sites of lymphocyte recruitment from blood [9,10,11]. Lymphocyte homing to mucosal lymphoid tissues such as Peyer's patches, and the intestinal lamina propria involves a single-chain 60-Kd adhesion glycoprotein, the mucosal vascular addressin MAdCAM-1 [12,13].
MAdCAM-1 is expressed on endothelial cells in mesenteric lymph nodes, lamina propria of the small and large intestine, and the lactating mammary gland. Increased expression of MAdCAM-1 on murine endothelial cells can be induced after stimulation with IL-1 and TNF-α. [13]. MAdCAM-1 has also been detected at high levels on colonic lamina propria venules from mice with hapten induced colitis [14], mice injected with TNF-α [15], or colitic IL-2 knockout mice [16]. The interaction of the α4β7 integrin on a subset of T cells, with its ligand, MAdCAM-1, on high venular and lymphoid endothelial cells has been shown to be support the entry of of these lymphocytes into the gut in IBD [8]. However, this process is still not well understood.
Immune-models of colitis in mice show that the MAdCAM-1/B7 integrin bond is essential to produce colitis. CD45RBhigh CD4+ T lymphocytes (which normally home to, and populate the colons of severe-combined immune deficient ('SCID') mice) will not enter the gut after treatment of recipient animals with either MAdCAM-1, or α4β7 specific antibody. This shows that this pair of molecules is clearly involved in cell traffic into the inflamed gut [17]. Therefore, it is possible that there may be unregulated MAdCAM-1 expression in IBD, and likely that the control of MAdCAM-1 expression may be an effective potential treatment for IBD.
With respect to adhesion, adhesion of polymorphonuclear leukocytes has been shown to be enhanced by treatment with NOS inhibitors, similarly Hokari et al. [18] have also demonstrated that L-NAME promotes the adhesion of T-lymphocytes cells to endothelium in vivo.
NO is an important modulator of adhesion molecule expression in both acute and chronic inflammatory states, and may influence the course of IBD. It is known that NO can function both as an oxidant, or antioxidant depending on the availability of reactive oxygen species [19]. Similarly, the concentration of NO can also augment [20,21] or inhibit [22,23] oxygen-radical mediated tissue damage and lipid peroxidation. It has also been reported that inhibition of endothelial nitric oxide synthases (using the non-selective NOS inhibitor L-NAME), induces endothelial adhesion molecules (ICAM-1 and VCAM-1) in HUVEC [24,25].
With respect to NO in inflammatory bowel disease. Singer et al., [26] found that iNOS expression was significantly increased in colonic epithelia from individuals with IBD. In iNOS deficient mice, McCafferty et al. (1997) [27] reported that acetic acid-induced colitis was more severe in iNOS-/- deficient animals, consistent with a protective role of iNOS derived NO in colitis.
De Caterina et al. [28] reported that in another model, NO blocked the TNF-α or IL-1β induced expression of VCAM-1, E-selectin, and ICAM-1. Similarly, GSNO also reduced NF-kB activation (as measured by EMSA). Further, Binion et al. [29] reported that iNOS expression in human intestinal micro vascular endothelial cells inhibited leukocyte adhesion, indicating that iNOS derived NO may control inflammation in human IBD. Further support of this hypothesis comes from a more recent report by Binion's group showing that individuals afflicted with Crohn's colitis have an apparent inability to express iNOS in their intestinal microvascular endothelium [29]. McCafferty et al., [27,30] suggest that in IBD, iNOS blocks an early, obligatory phase of inflammation, but perhaps not the chronic phase. Kubes and McCafferty [31] suggest in IBD, NO may be either beneficial or detrimental and may reflect the tissue source (cell type), enzymatic source of NO (eNOS vs. iNOS) and rate of NO production, as well as the bioavailability of oxidants in these different models.
In this present study, we investigated how MAdCAM-1 expression in cultured endothelial cells is altered by NO donors and by NO synthase inhibitors. We have also examined how cytokines and NO-modifying agents control adhesion of α4β7-expressing mouse lymphocytes in response to TNF-α.
We observed that both a short and long-acting NO donor significantly reduce TNF-α-induced expression of MAdCAM-1 expression in lymphatic endothelial cells. The effects of NO donors appear to reflect their ability to prevent the nuclear translocation of NF-kB. The MAdCAM-1 promoter has several NF-kB binding sites [32], and are necessary to induce MAdCAM-1 expression. Both a short and long acting NO donor blocked MAdCAM-1 induction, but the slow-releasing NO DETA-NO was apparently at least 10 times more effective than SperNO (on a molar basis). This probably reflects the need for persistently elevated NO to block cytokine signaling in this system.
In this model, the mechanism through which NO donors limit inflammation is by NF-kB inhibition, since these NO donors effectively block p65 translocation into the nucleus. Khan et al. [33] demonstrated that NO donors blocked TNF-α induced NF-κB activation in human umbilical vein endothelial cells as measured by EMSA. Similarly, Spiecker [34] showed that GSNO, blocked TNF-α induced NF-κB activation by EMSA in human saphenous vein endothelium. However in that model IκBα, the inhibitor of the NF-kB complex was still degraded. Therefore, NO might limit NF-kB activation through pathways independent of IkB-a. In that report, NO was found to induce IκB synthesis through activation of its promoter, therefore, increased IkB-a might also contribute to NF-kB inhibition . Several other possible pathways, e.g. p38, p42/44 MAPK and transcription factors, e.g. SP1 and possibly AP2, Adh1 (ETF), PEA3 sites [32] which could also contribute to NF-kB dependent gene activation and might participate in NO mediated MAdCAM-1 regulation by cytokines. [35] have reported that an NO-releasing derivative of mesalamine, (one agent currently used to treat gut inflammation), exhibits enhanced anti-inflammatory effects compared to the parent compound. While NO donors are not currently used for treatment of IBD, the literature and our data here suggest that NO donors could be beneficial.
An improved understanding of the interactions of these signals may improve our ability to understand the role of MAdCAM-1 in IBD and design effective therapies to treat it.
Competing interests
None declared
Abbreviations
MAdCAM-1 (mucosal addressin cell adhesion molecule-1)
Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/content/backmatter/1471-230X-1-5-b1.pdf
Acknowledgments
Acknowledgements
The authors acknowledge John Elrod, Dr. Makotoh Sasaki and Merilyn Jennings for outstanding technical assistance and valuable scientific input. This work was supported by NIH grants HL47615, DK43785 and DK47663
Contributor Information
Tadayuki Oshima, Email: toshim@lsumc.edu.
Paul Jordan, Email: pjorda1@lsuhsc.edu.
Matthew B Grisham, Email: mgrish@lsumc.edu.
Jonathan S Alexander, Email: jalexa@lsumc.edu.
Merilyn Jennings, Email: mjenni@lsumc.edu.
Makotoh Sasaki, Email: msasak@lsumc.edu.
Kenneth Manas, Email: kmanas@lsumc.edu.
References
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E, Ringler DJ. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158:2099–2106. [PubMed] [Google Scholar]
- Spiecker M, Darius H, Kaboth K, Hubner F, Liao JK. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J Leukoc Biol. 1998;63:732–739. [PubMed] [Google Scholar]
- Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;20:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hokari R, Miura S, Fujimori H, Tsuzuki Y, Shigematsu T, Higuchi H, Kimura H, Kurose I, Serizawa H, Suematsu M, Yagita H, Granger DN, Ishii H. Nitric oxide modulates T-lymphocyte migration in Peyer's patches and villous submucosa of rat small intestine. Gastroenterology. 1998;115:618–627. doi: 10.1016/s0016-5085(98)70141-6. [DOI] [PubMed] [Google Scholar]
- McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022–1027. doi: 10.1053/gast.1997.v112.pm9041266. [DOI] [PubMed] [Google Scholar]
- Singer II, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Niu XF, Smith CW, Kubes P. Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ Res. 1994;74:1133–1140. doi: 10.1161/01.res.74.6.1133. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Vergnolle N, Muscara MN, Asfaha S, Chapman K, McKnight W, Del Soldato P, Morelli A, Fiorucci S. Enhanced anti-inflammatory effects of a nitric oxide-releasing derivative of mesalamine in rats. Gastroenterology. 1999;17:557–566. doi: 10.1016/s0016-5085(99)70448-8. [DOI] [PubMed] [Google Scholar]
- Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol. 1999;65:349–355. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- Streeter PR, Berg EL, Rouse BT, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- Viney JL, Jones S, Chiu HH, Lagrimas B, Renz ME, Presta LG, Jackson D, Hillan KJ, Lew S, Fong S. Mucosal addressin cell adhesion molecule-1: a structural and functional analysis demarcates the integrin binding motif. J Immunol. 1996;157:2488–2497. [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Sikorski EE, Hallmann R, Berg EL, Butcher EC. The Peyer's patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-alpha and IL-1. J Immunol. 1993;151:5239–5250. [PubMed] [Google Scholar]
- McDonald SA, Palmen MJ, Van Rees EP, MacDonald TT. Characterization of the mucosal cell-mediated immune response in IL-2 knockout mice before and after the onset of colitis. Immunology. 1997;91:73–80. doi: 10.1046/j.1365-2567.1997.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150–158. doi: 10.1016/S0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- Ruegg C, Postigo AA, Sikorski EE, Butcher EC, Pytela R, Erie DJ. Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J Cell Biol. 1992;117:179–189. doi: 10.1083/jcb.117.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew DP, Berlin C, Honda S, Yoshino T, Hamann A, Holzmann B, Kilshaw PJ, Butcher EC. Distinct but overlapping epitopes are involved in alpha 4 beta 7-mediated adhesion to vascular cell adhesion molecule-1, mucosal addressin-1, fibronectin, and lymphocyte aggregation. J Immunol. 1994;153:3847–3861. [PubMed] [Google Scholar]
- Nakache M, Berg EL, Streeter PR, Butcher EC. The mucosal vascular addressin is a tissue-specific endothelial cell adhesion molecule for circulating lymphocytes. Nature. 1989;337:179–181. doi: 10.1038/337179a0. [DOI] [PubMed] [Google Scholar]
- Khan BV, Harrison DG, Olbrych MT, Alexander RW, Medford RM. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci USA. 1996;93:9114–9119. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- O'Connell KA, Edidin M. A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J Immunol. 1990;144:521–525. [PubMed] [Google Scholar]
- Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR, Newman W, Ringler DJ. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willam C, Schindler R, Frei U, Eckardt KU. Increases in oxygen tension stimulate expression of ICAM-1 and VCAM-1 on human endothelial cells. Am J Physiol. 1999;276:H2044–H2052. doi: 10.1152/ajpheart.1999.276.6.H2044. [DOI] [PubMed] [Google Scholar]
- Binion DG, Fu S, Ramanujam KS, Chai YC, Dweik RA, Drazba JA, Wade JG, Ziats NP, Erzurum SC, Wilson KT. iNOS expression in human intestinal microvascular endothelial cells inhibits leukocyte adhesion. Am J Physiol. 1998;275:G592–G603. doi: 10.1152/ajpgi.1998.275.3.G592. [DOI] [PubMed] [Google Scholar]
- Leung E, Berg RW, Langley R, Greene J, Raymond LA, Augustus M, Ni J, Carter KC, Spurr N, Choo KH, Krissansen GW. Genomic organization, chromosomal mapping, and analysis of the 5' promoter region of the human MAdCAM-1 gene. Immunogenetics. 1997;46:111–119. doi: 10.1007/s002510050249. [DOI] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokura S, Wolf RE, Yoshikawa T, Ichikawa H, Granger DN, Aw TY. Endothelial cells exposed to anoxia/reoxygenation are hyperadhesive to T-lymphocytes: kinetics and molecular mechanisms. Microcirculation. 2000;7:13–23. doi: 10.1038/sj.mn.7300088. [DOI] [PubMed] [Google Scholar]
- White CR, Brock TA, Chang LY, Crapo J, Briscoe P, Ku D, Bradley WA, Gianturco SH, Gore J, Freeman BA. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci USA. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCafferty DM, Miampamba M, Sihota E, Sharkey KA, Kubes P. Role of inducible nitric oxide synthase in trinitrobenzene sulphonic acid induced colitis in mice. Gut. 1999;45:864–873. doi: 10.1136/gut.45.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.immunol.10.1.561. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sd USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio SO, Li X, Takeuchi M, Mei C, Francke U, Butcher EC, Briskin MJ. Organization, regulatory sequences, and alternatively spliced transcripts of the mucosal addressin cell adhesion molecule-1 (MAdCAM-1) gene. J Immunol. 1995;155:2477–2486. [PubMed] [Google Scholar]


