Abstract
Worsening donor liver quality resulting in decreased organ utilization may be contributing to the recent decline in liver transplants nationally. We sought to examine trends in donor liver utilization and the relationship between donor characteristics and non-use. We used the United Network for Organ Sharing database to review all deceased adult organ donors in the United States who had at least one solid organ transplanted into a recipient. Trends in donor characteristics were examined. Multivariate logistic regression was used to evaluate the association between donor characteristics and liver non-use between 2004 and 2010. Population attributable risk proportions were determined for donor factors associated with non-use. 107,259 organ donors were analyzed. The number of unused livers decreased steadily from 1,958 (66% of donors) in 1988 to 841 (15%) in 2004, but then gradually increased to 1,345 (21%) in 2010. Donor age, body mass index (BMI), and the prevalence of diabetes and donation after cardiac death (DCD) all increased over time, and all four were independently associated with liver non-use. DCD had the highest adjusted odds ratio (OR) for non-use, and the odds increased nearly four-fold between 2004 (OR 5.53; 95% CI, 4.57–6.70) and 2010 (OR 21.31; 95% CI, 18.30–24.81). The proportion of non-use attributable to DCD increased from 9% in 2004 to 28% in 2010. The proportion of donor livers not used has increased since 2004. Older donor age, greater BMI, diabetes, and DCD are all independently associated with non-use and are on the rise nationally. Current trends may lead to significant declines in liver transplant availability.
Keywords: Liver transplantation, Organ donation, Donor selection, Health resources, Donation after cardiac death
INTRODUCTION
Liver transplantation is the treatment of choice for patients with end-stage liver disease, but utilization is limited by inadequate organ availability (1). Efforts to expand the donor pool have included living donation, split liver transplantation, and the use of extended criteria donors, which include older donors, donors with fatty livers, and donation after cardiac death (DCD) donors (1).
Although the intention of extended criteria donation is to increase transplantation, post-transplant outcomes can be inferior when such organs are used (2–8). DCD grafts, procured after donor circulation ceases, are subject to a period of poor perfusion during the asystolic phase, leading to ischemic bile duct damage (9). Consequently, they carry increased risk of recipient morbidity and mortality compared to standard donation after brain death (DBD) grafts, which are procured while circulation is supported (4, 7). DCD transplants are also associated with longer hospital length of stay and increased cost (10), and may not be cost-effective compared to a strategy of remaining on the transplant waiting list until a DBD liver is available (11). Advanced donor age and graft steatosis (fatty liver) are also associated with graft failure (2, 3, 5). For these reasons, transplant centers are more likely to reject liver offers from DCD donors, older donors, and donors with liver steatosis (12, 13).
Following years of liver transplantation growth in the United States, the annual number of deceased donor transplants has fallen since 2006 (14). The reasons for this decline are unclear but are not entirely due to stagnant donation rates since the decline in liver transplants has exceeded the decline in organ donors (14). One potential reason for the decline is that of increasing donor liver non-use due to unacceptable organ quality (15). As the population ages (16) and becomes increasingly obese (17), increasing extended criteria donation could contribute to increasing non-use. It also remains unclear whether the attempt to increase the number of available livers by increasing DCD is being undermined by increased non-use of such livers.
Organ donors who have at least one organ used for transplantation are of particular interest because they lack an absolute contraindication to organ donation, such as an infectious condition that could be transmitted to the recipient. Liver non-use within this group of successful organ donors is therefore likely due to particular issues with liver quality. We sought to evaluate the association between various donor characteristics and liver non-use among this subgroup of organ donors in the United States and to examine temporal trends in characteristics that may explain the decline in liver transplants.
METHODS
Data Source and Study Population
We utilized the United Network for Organ Sharing (UNOS) standard transplant analysis and research files, which contain information on all deceased donors in the United States since October 1, 1987. We limited the sample to donors ≥ 18 years old who had at least one organ (liver, heart, intestine, kidney, lung, or pancreas) transplanted into a patient. Split liver donations were excluded given the likely differences in liver quality required for split livers as compared to whole livers. Donors with a body mass index (BMI) < 14 or > 50 kg/m2 were also excluded, since these were likely the result of data entry errors.
Dependent Variable
The primary outcome was donor liver non-use, defined as a liver that was not procured or a liver that was procured but not transplanted.
Independent Variables
The key variables of interest were age, BMI, diabetes, hypertension, and DCD. BMI, diabetes and hypertension were chosen because of their associations with non-alcoholic fatty liver disease (NAFLD) (18). Liver histology was available for only 30% of donors and therefore was not included in the main analyses. It was included as a secondary analysis as a dichotomized variable (macrosteatosis < 30% versus ≥ 30%) (19). Because the relationship between age and organ non-use was not linear, we specified age categorically (< 30, 30–39, 40–49, 50–59, and ≥ 60 years); similarly, we specified BMI categorically (< 18.5, 18.5–24+, 25–29+, 30–39+, and ≥ 40 kg/m2).
We examined additional donor factors that could be associated with the decision to use a liver (5, 13). We considered year of organ recovery (continuous), sex, race as determined by the organ procurement organization (white, black, or other), cause of death (trauma, cerebrovascular accident [CVA], or other), lifetime smoking (> 20 pack-years), alcohol use (≥ 2 drinks per day), history of illicit drug use, presence of infection, inotrope use at cross-clamp, history of malignancy, alanine aminotransferase (ALT), bilirubin, hepatitis C antibody, hepatitis B core antibody, blood type, and UNOS region. Within the subgroup of DCD donors, prolonged warm ischemia time (WIT) (> 20 minutes) was also evaluated (20). ALT and bilirubin were not normally distributed, and were categorized as ≤ 40, 41–200, 201–400, > 400 U/L, and ≤ 1.2, 1.3–2.5, 2.6–5, > 5 mg/dL, respectively. Aspartate aminotransferase was not included due to collinearity with ALT. Donor height, a component of the donor risk index (5), was not included because it is also a component of BMI.
Time Periods and Missing Data
Trends in donor characteristics and liver utilization were examined from 1988 to 2010. All other descriptive and multivariate analyses were restricted to recoveries performed between June 30, 2004, and December 31, 2010. We selected this time period for our analyses due to completeness of UNOS data and our interest in recent causes of liver non-use. In particular, alcohol intake was not systematically recorded prior to this time. BMI, diabetes, hypertension, DCD, and laboratory values were available since 1995 with varying degrees of completeness. However, from 2004 onward, each individual variable of interest was missing in < 3% of donors. All variables were present in 92% of donors.
Statistical Analysis
Categorical variables were reported as proportions and counts. Continuous variables were reported as means and standard deviations (SD) when normally distributed and as medians and ranges otherwise. Bivariate comparisons between categorical variables were performed with Pearson’s χ2 test. Comparisons between continuous variables were performed with Student’s t-test or the Wilcoxon rank-sum test, where appropriate. Multivariate logistic regression was used to examine associations between donor characteristics and liver non-use, controlling for all independent variables. Since transplant center practices may change over time, we assessed potential interactive effects between year and all other variables using the likelihood ratio test. For instance, we hypothesized that centers may be more likely to use livers from older donors over time since the population is aging and emphasis has been placed on extended criteria donors nationally (21). Interaction terms for age and DCD with year were statistically significant, improved overall model fit, and were included in the final model. Other interaction terms were omitted from final model estimations.
Subgroup analyses were performed using additional logistic regression models. For the 30% of donors who had histology results available, the association between macrosteatosis and non-use was evaluated. To explore trends in the utilization impact of DCD for individual UNOS regions, a model was constructed including interaction terms for UNOS regions with DCD. To determine if there were differences in odds ratios (ORs) for donor characteristics between the DBD and DCD subgroups, interaction terms between DCD and all other factors were included in another model. Within the subgroup of DCD donors, the association between prolonged WIT and non-use was also determined using logistic regression, adjusting for all other independent variables.
To determine the relative impact of each factor on the overall non-use of livers, population attributable risk proportions (PARP)s were calculated (22). Despite being labeled “proportions”, PARPs of various donor characteristics do not sum to 100% because the characteristics overlap and the PARPs are not independent. Separate PARPs were calculated by year to illustrate temporal trends. Variance-weighted least squares linear regression was used to test trends of PARPs.
All p-values were based on 2-sided tests, and were considered statistically significant when p < 0.05. Analyses were performed using Stata version 12 (StataCorp LP, College Station, TX). The University of North Carolina Institutional Review Board approved this study.
RESULTS
Study Population Characteristics
107,259 individuals meeting inclusion and exclusion criteria donated at least one organ between January 1, 1988, and December 31, 2010. 41,503 (38.7%) donated after June 30, 2004. After this date, the liver was used for transplant in 33,895 (81.7%), and 7,608 (18.3%) were unused. The liver was used in 89.6% of successful heart donors, in 99.7% of intestine donors, in 78.4% of kidney donors, in 90.2% of lung donors, and in 96.9% of pancreas donors. When more than one organ was used, 95% of livers were used. When only one organ was used, 51.2% of these were livers; 46.5% were one or both kidneys; and hearts, intestines, lungs, and pancreas each comprised < 2%. The mean donor age was 43.7 years, 40.9% were female, and 68.4% were white (Table 1). The mean BMI was 27.4 kg/m2, 10.9% had diabetes, and 36.3% were hypertensive. 9.0% were DCD donors. 18.4% had an alcohol use history, 34.0% had a history of drug use, 3.8% were hepatitis C antibody positive, and 42.2% had ALT > 40 U/L. 13.7% of those with available histology had ≥ 30% macrosteatosis.
Table 1.
Characteristics of Organ Donors, Overall and According to DCD Status, 2004–2010
| Characteristic | Overall | DBD, % (N = 37,751) | DCD, % (N = 3,752) | p-valuec | |
|---|---|---|---|---|---|
| % | (Number) | ||||
| Age, yearsa | 43.7 ± 15.6 | 43.9 ± 15.8 | 41.3 ± 13.3 | < 0.001 | |
| Sex | |||||
| Male | 59.1 | (24,540/41,503) | 58.4 | 66.7 | < 0.001 |
| Female | 40.9 | (16,963/41,503) | 41.6 | 33.3 | |
| Race | |||||
| White | 68.4 | (28,403/41,503) | 66.6 | 86.8 | < 0.001 |
| Black | 15.0 | (6,238/41,503) | 15.9 | 5.9 | |
| Other | 16.5 | (6,862/41,503) | 17.4 | 7.3 | |
| BMI, kg/m2,a | 27.4 ± 5.8 | 27.3 ± 5.8 | 27.7 ± 6.0 | < 0.001 | |
| Diabetes | 10.9 | (4,504/41,290) | 11.2 | 7.7 | < 0.001 |
| Hypertension | 36.3 | (14,963/41,209) | 37.3 | 26.1 | < 0.001 |
| Cause of death | |||||
| Trauma | 35.0 | (14,518/41,503) | 34.8 | 36.4 | < 0.001 |
| CVA | 44.3 | (18,400/41,503) | 46.4 | 23.1 | |
| Other | 20.7 | (8,585/41,503) | 18.7 | 40.5 | |
| Smoking | 32.4 | (13,281/40,965) | 32.6 | 30.6 | 0.01 |
| Alcohol use | 18.4 | (7,542/40,931) | 18.2 | 20.7 | < 0.001 |
| Illicit drug use | 34.0 | (13,912/40,961) | 33.6 | 37.2 | < 0.001 |
| Infection | 41.7 | (16,787/40,259) | 41.9 | 39.8 | 0.01 |
| Inotrope support | 55.4 | (22,902/41,317) | 60.3 | 6.5 | < 0.001 |
| Cancer history | 3.7 | (1,545/41,325) | 3.8 | 3.1 | 0.02 |
| ALT, U/Lb | 35 (22–64) | 34 (21–62) | 44 (27–82) | < 0.001 | |
| Bilirubin, mg/dLb | 0.7 (0.5–1.2) | 0.7 (0.5–1.2) | 0.7 (0.4–1.0) | < 0.001 | |
| Hepatitis C antibody | 3.8 | (1,558/41,432) | 4.0 | 1.8 | < 0.001 |
| Hepatitis B core antibody | 5.8 | (2,408/41,413) | 6.1 | 3.2 | < 0.001 |
Abbreviations: DCD, donation after cardiac death; DBD, donation after brain death; BMI, body mass index; CVA, cerebrovascular accident; ALT, alanine aminotransferase.
Presented as mean ± standard deviation.
Presented as median (interquartile range).
Student’s t-test used to compare means; Wilcoxon rank-sum test used to compare medians; χ2 test used to compare proportions.
Temporal Trends in Donor Characteristics and Liver Utilization
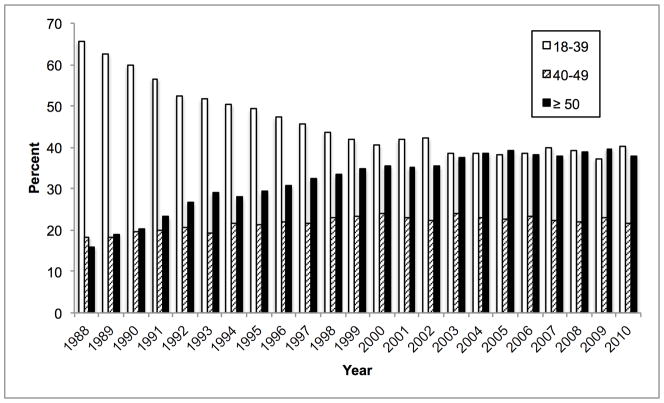
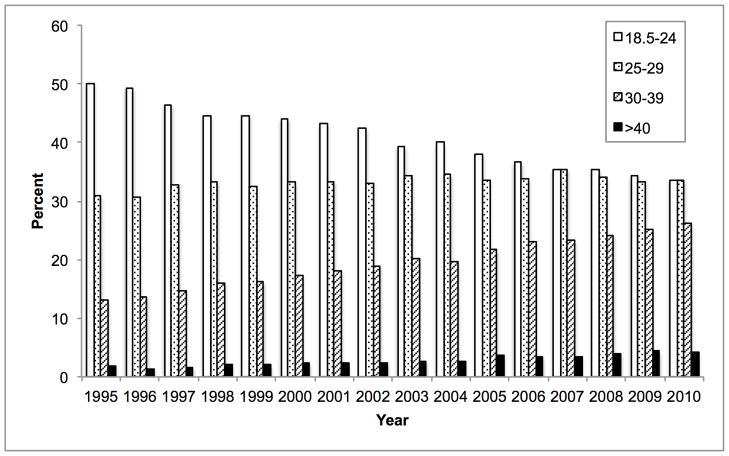
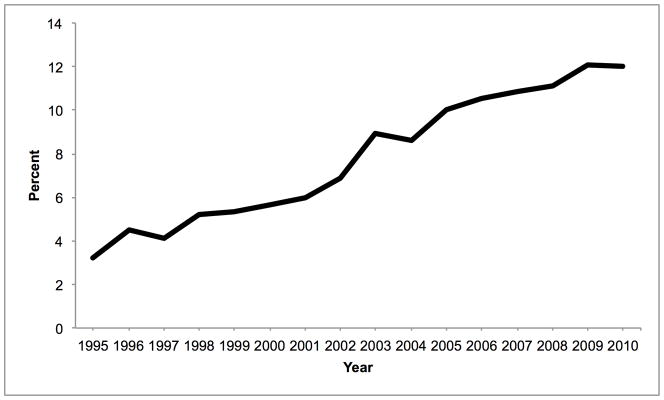
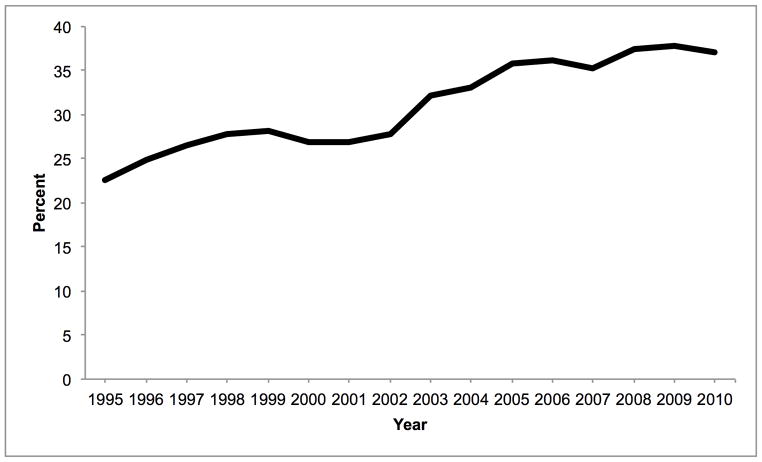
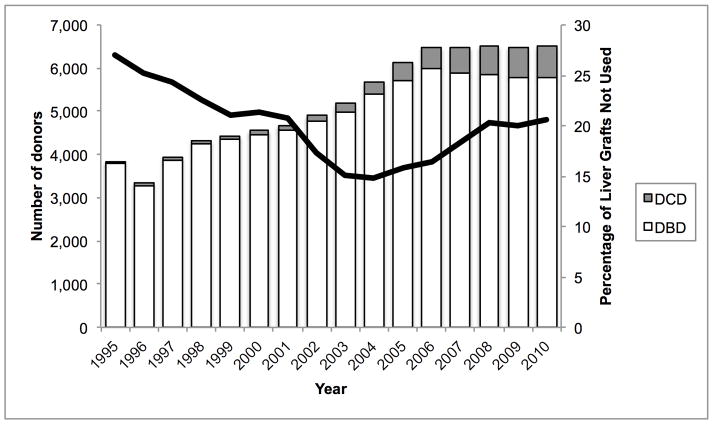
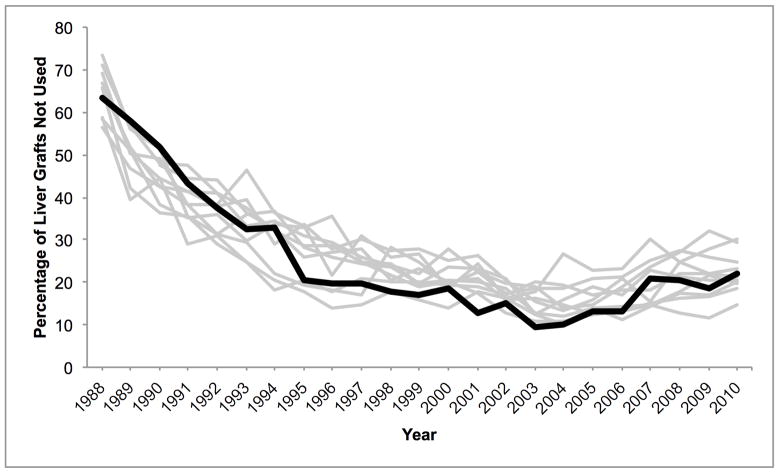
Mean donor age increased from 34.6 to 43.3 between 1988 and 2010. During this time, the percentage aged 18–39 decreased from 65.6% to 40.3% while the percentage ≥ 50 increased from 16.0% to 38.0% (Figure 1A). 84.9% were white in 1988, whereas 67.1% were white in 2010. The prevalence of obesity (BMI ≥ 30 kg/m2) increased from 15.0% in 1995 to 30.3% in 2010 (Figure 1B). The prevalence of diabetes and hypertension both increased from 3.2% and 22.6% in 1995, to 12.0% and 37.2% in 2010, respectively (Figure 1C, D). 65.1% had ALT < 40 U/L in 1995, whereas 56.2% had ALT < 40 U/L in 2010. Bilirubin, however, did not change substantially. DCD increased from 44 (1.1% of donors) in 1995 to 730 (11.2%) in 2010 (Figure 2). Since 2006, the rise in DCD has been accompanied by a proportionate decline in DBD donation. The percentage of livers not used fell between 1988 and 2004 from 66.2% to 14.8%, after which it increased to 20.7% (Figure 2). This utilization pattern was seen across UNOS regions, even in Region 9, which traditionally has been the most aggressive user of expanded criteria livers (Figure 3) (23). Between 1995 and 2010, the proportion of donors who only had one organ used increased slightly from 25.8% to 30.6%. Each year, the median number of organs used was 2. Within the group who only had one organ used, 25.6% were livers in 1995. This figure increased to 59.1% in 2004, and then fell to 45.5% in 2010. Conversely, 67.2% were kidneys in 1995. Kidneys fell to 38.2% in 2004 and then increased to 52.2% in 2010.
Figure 1.
Temporal trends in (A) age, (B) BMI, (C) diabetes, and (D) hypertension expressed as percentages among donors of at least one solid organ.
Figure 2.
Trends in donation after cardiac death and liver non-use, 1995–2010. The bars denote the absolute numbers of donors who had at least one solid organ transplanted, according to donation after cardiac death (DCD) versus donation after brain death (DBD) (left axis). The line denotes the percentage of liver grafts unused among those donors who donated at least one organ (right axis).
Figure 3.
Temporal trends in liver non-use across UNOS regions among donors of at least one solid organ. Region 9 is depicted with a bold black line, compared to the other 10 regions in gray.
Characteristics of DCD Donors
DCD donors were, on average, younger than DBD donors (mean age 41.3 vs. 43.9 years, p < 0.001) (Table 1). Mean BMIs of DCD and DBD donors were 27.7 and 27.3 kg/m2, respectively (p < 0.001). Diabetes and hypertension were more common in DBD donors. DCD donors were more likely to be white, to have a history of drug or alcohol use, and to have ALT > 40 U/L. DBD donors were more likely to be female, to have a CVA cause of death, to have elevated bilirubin, and to have viral hepatitis exposure. DBD donors were also more likely to receive inotropic support. For DCD, the median WIT was 15 minutes (interquartile range 10–24). The WIT was prolonged (> 20 minutes) in 34.3%. The prevalence of prolonged WIT fell from 41.1% in 2002 to 26.7% in 2005, and then returned to 41.1% in 2010.
Liver Non-Use According to Donor Characteristics
In bivariate analyses, non-use was associated with increasing donor age up to 60, above which the liver was more likely to be used. Compared to normal weight donors, those with higher and lower BMI were less likely to be used. Diabetes and hypertension were both associated with non-use. 58.2% of DCD livers were not used as compared to 14.4% of DBD livers. Livers from black donors were more likely to be used. Non-use was also associated with smoking, alcohol use, increasing ALT and bilirubin, and exposure to viral hepatitis. Of those with histology available, 9.4% of livers were not used when macrosteatosis was < 30%, and 52.9% were not used when ≥ 30%. Within the DCD subgroup, 67.9% of livers were not used when the WIT was > 20 minutes, and 52.9% were not used when it was ≤ 20 minutes.
In multivariate analysis, liver non-use was associated with increasing donor age up to 60 (Table 2). As in the bivariate analysis, livers from donors older than 60 were more likely to be used than those from donors aged 50–59. The strength of the association between age and liver non-use increased over time (p for interaction = 0.002). DCD was strongly associated with liver non-use, and this relationship became more pronounced over time. The OR for DCD increased from 5.53 (95% CI 4.57–6.70) in 2004 to 21.31 (95% CI 18.30–24.81) in 2010 (p for interaction < 0.001). High and low BMI and diabetes were associated with non-use, but hypertension was not (Table 3). Black race and illicit drug use were independently associated with organ use, whereas female sex, “other” race, CVA cause of death, alcohol use, increasing ALT and bilirubin, and hepatitis C antibody were associated with non-use. Macrosteatosis ≥ 30% was strongly associated with non-use among those with histology available (adjusted OR 11.16; 95% CI 9.75–12.77). The ORs for most factors were similar in DBD and DCD donors. The strength of the associations between non-use and ages 30–50, alcohol use, non-trauma causes of death, and elevated ALT were greater for DBD than for DCD donors (Table 4). In contrast, age > 50 was more strongly associated with non-use in DCD donors. Among DCD donors, prolonged WIT was associated with non-use (adjusted OR 2.06; 95% CI 1.71–2.47).
Table 2.
Trends in Adjusteda Odds Ratios for Liver Non-Use by Donor Age and Donation After Cardiac Death
| Yearb | 2004 | 2010 |
|---|---|---|
| Age, years | ||
| < 30 | 1 [Ref] | 1 [Ref] |
| 30–39 | 1.43 (1.14–1.79) | 1.68 (1.38–2.04) |
| 40–49 | 1.87 (1.54–2.29) | 2.69 (2.27–3.20) |
| 50–59 | 2.14 (1.75–2.62) | 3.44 (2.89–4.10) |
| ≥ 60 | 1.67 (1.33–2.10) | 3.11 (2.55–3.80) |
| Donation after cardiac death | 5.53 (4.57–6.70) | 21.31 (18.30–24.81) |
Based on the multivariate logistic regression model adjusted for all of the variables in Table 3 as well as UNOS region and ABO blood type.
Interaction terms for age by year and donation after cardiac death by year were significant; odds ratios for the years 2004 and 2010 are presented.
Table 3.
Non-Use of Liver Grafts According to Donor Characteristics, Multivariate Analysis
| Characteristic (N = 38,319) | Adjusted ORa | (95% CI) |
|---|---|---|
| Female | 1.14 | (1.07–1.21) |
| Race | ||
| White | 1 | [Ref] |
| Black | 0.62 | (0.56–0.69) |
| Other | 1.49 | (1.37–1.62) |
| Body mass index, kg/m2 | ||
| < 18.5 | 1.32 | (1.09–1.60) |
| 18.5–24+ | 1 | [Ref] |
| 25–29+ | 1.17 | (1.08–1.26) |
| 30–39+ | 1.97 | (1.82–2.14) |
| ≥ 40 | 4.31 | (3.75–4.95) |
| Diabetes | 1.14 | (1.03–1.25) |
| Hypertension | 0.98 | (0.92–1.06) |
| Cause of death | ||
| Trauma | 1 | [Ref] |
| CVA | 1.16 | (1.06–1.25) |
| Other | 0.89 | (0.81–0.97) |
| Smoking | 0.98 | (0.92–1.05) |
| Alcohol use | 2.57 | (2.39–2.77) |
| Illicit drug use | 0.80 | (0.74–0.86) |
| Infection | 1.03 | (0.96–1.10) |
| Inotrope support | 1.09 | (1.02–1.16) |
| Cancer history | 0.91 | (0.77–1.07) |
| ALT, U/L | ||
| ≤ 40 | 1 | [Ref] |
| 41–200 | 1.70 | (1.59–1.82) |
| 201–400 | 3.16 | (2.72–3.66) |
| > 400 | 10.66 | (9.28–12.24) |
| Bilirubin, mg/dL | ||
| ≤ 1.2 | 1 | [Ref] |
| 1.3–2.5 | 1.53 | (1.42–1.65) |
| 2.6–5 | 2.98 | (2.63–3.39) |
| > 5 | 9.91 | (7.92–12.41) |
| Hepatitis C antibody | 2.78 | (2.42–3.18) |
| Hepatitis B core antibody | 1.13 | (0.99–1.28) |
Abbreviations: OR, odds ratio; CI, confidence interval; CVA, cerebrovascular accident; ALT, alanine aminotransferase.
Based on the multivariate logistic regression model adjusted for all of the other variables in the table as well as age, donation after cardiac death, UNOS region and ABO blood type.
Table 4.
| Characteristic | DBD | DCD |
|---|---|---|
| Age in 2010,c years | ||
| < 30 | 1 [Ref] | 1 [Ref] |
| 30–39 | 1.88 (1.53–2.31) | 1.21 (0.92–1.59) |
| 40–49 | 2.80 (2.32–3.37) | 2.41 (1.88–3.09) |
| 50–59 | 3.36 (2.78–4.05) | 4.20 (3.23–5.45) |
| ≥ 60 | 3.01 (2.44–3.73) | 9.83 (6.38–15.15) |
| Alcohol use | 2.68 (2.47–2.90) | 1.85 (1.51–2.26) |
| Cause of death | ||
| Trauma | 1 [Ref] | 1 [Ref] |
| CVA | 1.21 (1.11–1.32) | 0.88 (0.71–1.09) |
| Other | 0.85 (0.77–0.95) | 0.95 (0.79–1.14) |
| ALT, U/L | ||
| ≤ 40 | 1 [Ref] | 1 [Ref] |
| 41–200 | 1.83 (1.70–1.97) | 1.13 (0.97–1.33) |
| 201–400 | 3.30 (2.81–3.87) | 2.48 (1.64–3.74) |
| > 400 | 11.51 (9.95–13.31) | 5.94 (3.75–9.41) |
Abbreviations: DCD, donation after cardiac death; DBD, donation after brain death; CVA, cerebrovascular accident; ALT, alanine aminotransferase
Based on the multivariate logistic regression model adjusted for all of the variables in Tables 2 & 3 as well as UNOS region and ABO blood type.
Interaction terms for the listed variables by donation after cardiac death were significant; odds ratios for the DBD and DCD subgroups are presented.
Population Attributable Risks for Liver Non-Use
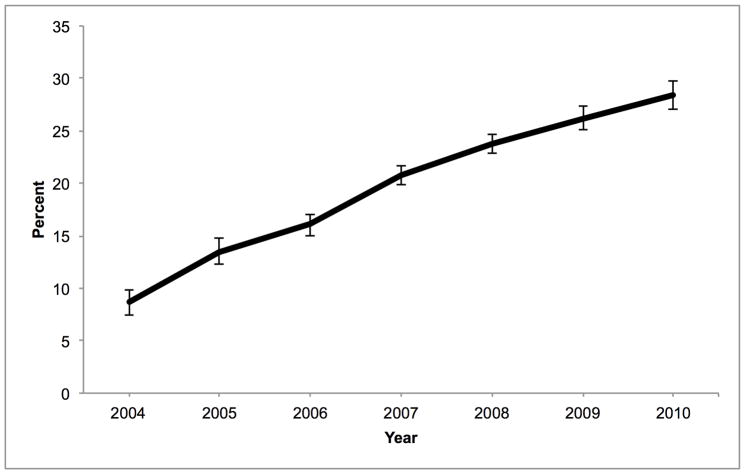
DCD accounted for 8.7% of liver non-use in 2004; this proportion increased to 28.4% in 2010 (Figure 4). PARPs for DCD increased over time in all regions except Region 6 where it peaked at 18.8% in 2006 and fell to 15.3% in 2010. The proportion of non-use due to age ≥ 50 increased from 16.7% in 2004 to 22.9% in 2010. No other factor increased its impact on organ non-use by more than 5% during this time. In 2010, BMI ≥ 25 kg/m2 explained 17.5% of non-use, but diabetes explained only 1.0% (Table 5). White race (compared to black race) was responsible for 16.8% of non-use. Alcohol use accounted for 10.9% of organ non-use, ALT > 40 U/L accounted for 19.9%, bilirubin > 1.2 mg/dL accounted for 9.9%, and hepatitis C antibody accounted for 2.3%. The PARP for prolonged WIT within the DCD subgroup remained unchanged during the study period (8.1%, p for trend = 0.36).
Figure 4.
Percentage of overall liver graft non-use among donors of at least one solid organ due to donation after cardiac death, by year. These values are calculated as the population attributable risk proportions, based on the adjusted odds ratios for non-use and annual prevalence of DCD.
Table 5.
Percentagea of overall liver graft non-use due to donor characteristics in 2010
| Characteristic | % of non-useb | (95% CI) |
|---|---|---|
| Age | ||
| < 30 | [Ref] | |
| 30–39 | 3.7 | (2.3–5.1) |
| 40–49 | 11.6 | (9.7–13.6) |
| 50–59 | 15.3 | (13.2–17.2) |
| ≥ 60 | 7.6 | (6.2–9.0) |
| Female | 3.0 | (1.5–4.5) |
| Race | ||
| White | 16.8 | (13.3–20.3) |
| Black | [Ref] | |
| Other | 7.8 | (6.7–8.8) |
| Body mass index | ||
| < 18.5 | 0.3 | (0.1–0.6) |
| 18.5–24+ | [Ref] | |
| 25–29+ | 2.7 | (1.3–4.1) |
| 30–39+ | 10.7 | (9.4–11.9) |
| ≥ 40 | 4.2 | (3.7–4.7) |
| Diabetes | 1.0 | (0.2–1.7) |
| DCD | 28.4 | (27.0–29.8) |
| Cause of death | ||
| Trauma | [Ref] | |
| CVA | 3.5 | (1.5–5.4) |
| Alcohol use | 10.9 | (10.0–11.9) |
| Inotrope support | 2.4 | (0.5–4.2) |
| ALT, U/L | ||
| ≤ 40 | [Ref] | |
| 41–200 | 11.0 | (9.6–12.4) |
| 201–400 | 2.9 | (2.4–3.3) |
| > 400 | 6.0 | (5.5–6.4) |
| Bilirubin, mg/dL | ||
| ≤ 1.2 | [Ref] | |
| 1.3–2.5 | 4.3 | (3.5–5.1) |
| 2.6–5 | 2.9 | (2.5–3.3) |
| > 5 | 2.6 | (2.3–2.9) |
| Hepatitis C antibody | 2.3 | (2.0–2.7) |
Abbreviations: CI, confidence interval; DCD, donation after cardiac death; CVA, cerebrovascular accident; ALT, alanine aminotransferase.
Calculated as the population attributable risk proportion, based on the multivariate logistic regression model.
Percentages do not sum to 100% because population attributable risk proportions are not independent.
DISCUSSION
The recent decline in the number of liver transplants performed in the United States is multifactorial. One potential reason for this decline is a decrement in donor organ quality leading to a decrease in utilization. In this study of all donors in the US who had at least one organ used for transplant, we found that the proportion of livers not used reversed its downward trend in 2004, thereafter rising through 2010 (Figure 2). In 2004, 841 of 5,680 (14.8%) donated livers were not used. Of the 6,506 livers donated in 2010, 1,345 (20.7%) were not used. Had the rate of non-use remained stable at 14.8%, 382 more livers from this group would have been transplanted in 2010.
Older donor age, higher BMI, and diabetes are independently associated with liver non-use. As in the general population (16, 17), all of these factors have become more common in the donor population over a long time period (Figure 1). Therefore, these findings suggest that the quality of donated livers has been declining for many years. Despite this deteriorating quality, non-use continued to decrease before 2004 possibly as a result of the gradual expansion of extended criteria donor liver use while maintaining acceptable post-transplant outcomes (24). However, the tolerance for worse outcomes with extended criteria donor livers has probably reached a limit, thus leading to declining utilization since 2004.
DCD is also independently associated with liver non-use. In contrast to age, BMI, and diabetes, the increase in the prevalence of DCD and the nearly four-fold increase in the odds of having DCD livers not used are particularly remarkable. This increasing reluctance to use DCD livers is undoubtedly the result of growing recognition that post-transplant outcomes are worse, and may be compounded by increased Medicare scrutiny of transplant programs’ outcomes (25). Such reluctance is further justified by recent data demonstrating that patients with low Model for End-Stage Liver Disease scores and those with hepatocellular carcinoma exception points may be better served by declining DCD organs and waiting for a DBD liver (11). Although prolonged WIT is associated with non-use amongst DCD livers, this association did not translate into a rising PARP for prolonged WIT alone, perhaps due to fluctuating rates of prolonged WIT over time. Despite the relatively recent growth of DCD, it appears to be overtaking the other factors as a common reason for liver non-use. By 2010, DCD accounted for more than one quarter of unused livers. This increasing impact of DCD and decreasing utilization was seen across UNOS regions and is not the result of an outlier region influencing national trends. Even Region 9, which historically has been significantly more likely to use marginal quality livers (23), showed a similar increase in non-utilization since 2004 compared to other regions (Figure 3).
Others have raised concerns about increasing reliance on DCD. In the Netherlands, DCD increased while DBD decreased, resulting in no net gain of grafts for kidney transplantation (26). A 2005 conference took note of this trend, and compared it to the United States experience, where increasing DCD was accompanied by increases in DBD. They concluded, “The evolving DCD practice is expected to result in an absolute increase of organ donors, i.e. in addition to DBD” (27). Our results show that DBD has declined while DCD has increased (Figure 2), refuting this prediction.
A worrisome possible explanation for this pattern is that the withdrawal of cardiovascular support preceding DCD is occurring in donors who would have eventually progressed to brain death, thus “converting” potential DBD donors to less desirable DCD. The reasons for possible conversions are unclear. There may be a growing preference for DCD among donor families and intensive care providers as they have become more educated on the DCD option. The Organ Donation Breakthrough Collaborative may have reinforced this preference by emphasizing DCD as a method to expand the donor pool (28), particularly since DCD kidney transplant outcomes are comparable to DBD (29). Indeed, we found that among donors who only had one organ used, livers have been declining while kidneys have been increasing since 2004. Alternatively, aggressive neurological management of donors may prevent the development of brain death (30). Such intensive neurological management has become more common and is associated with DCD (31).
DCD has become more popular in recent years as a means to capture organs that would not otherwise be available. The challenge is discerning between these situations of net organ gain versus DBD to DCD conversions where the net gain of donated livers is nil. Unfortunately, our findings suggest that conversions could explain the rise in DCD since DBD has declined. Such conversions would be neutral in terms of number of transplanted livers were it not for the increasing reluctance to use DCD livers. The transplant community must consider ways to capture data on any potential conversion problem. If such a problem exists, its harm to liver utilization could be vast.
While the OR for not using a liver because of older age was less than that of DCD, the overall impact of age remains greater because advancing age is more common than DCD, and even a modest increase in age (≥ 30 years) is associated with non-use. The lower OR for those ≥ 60 compared to 50–59 year olds has been reported previously (13), and likely reflects selection bias for only the healthiest older individuals being considered for donation. Like DCD, the impact of age on liver non-use is increasing, but less rapidly. Obesity is also becoming more prevalent among donors, but the overall impact of obesity has not increased, likely because of the relatively modest ORs for overweight and obese donors. Nevertheless, the population-level impact of obesity on liver non-use may increase in the future as its prevalence increases.
Elevated donor ALT is becoming more common, is associated with liver non-use, and has a substantial impact on overall utilization. The greatest increase in ALT prevalence was among those with a mild-to-moderate elevation (41–200 U/L). Whether this finding represents another manifestation of NAFLD is unknown, but if true, would also bode poorly for future organ quality. In contrast, black race was strongly associated with organ use as compared with white race. The reasons for this difference are unclear, but could potentially relate to the lower prevalence of NAFLD in blacks as compared to other races (32). This finding takes on particular importance because of lower rates of organ donation in the black population (15).
DBD and DCD donors represent two distinct populations, and one might expect that the relationships between donor factors and non-use would differ between these groups. However, ORs for non-use were actually similar for most donor factors. When different, most associations were attenuated in the DCD group, likely representing selection bias for DCD donors deemed acceptable for the use of at least one organ.
Additional external factors could impact liver utilization. DonorNet is the electronic UNOS system that has been used to facilitate organ placement since April 30, 2007. Through DonorNet, electronic organ offers can be made simultaneously to multiple recipient centers with detailed donor information available electronically to increase efficiency and improve utilization. Prior to the use of DonorNet, organ offers were made manually, which limited communication and decision making in the donor-recipient matching process. After DonorNet was implemented, there was an increase in the percentage of recovered livers that were discarded, particularly among those with the highest donor risk indices (33). However, our findings show that utilization was already decreasing before DonorNet was implemented, so its effect on utilization remains unclear.
The main limitation of this study is the lack of detail on reasons for the rise in liver non-use. The reasons for organ non-use are recorded broadly in the database as “poor organ function” or “biopsy findings.” The dataset also lacked sufficient biopsy data to directly link donor steatosis to organ non-use, as only 30% of donors had biopsy data. However, obesity and diabetes are strongly associated with NAFLD (18) and have previously been used as surrogates for steatosis (13). Although we did find an association between steatosis and non-use within the subgroup that had available histology, this represents a biased sample, as donors who receive a liver biopsy are highly selected. Therefore, the estimate for this relationship cannot be generalized to the entire donor population, and the overall PARP was not calculated. Finally, this study does not address the stagnation in rates of overall donation that is impacting the national volume of transplants (15). However, utilization of available organs becomes even more critical if we cannot rely on expanding donation.
Despite these shortcomings, the UNOS database is well suited to address our study goal, which was to report national trends in organ utilization. The database has complete coverage of all donors and organ dispositions nationwide. Misclassification of our primary outcome and DCD is probably rare and unlikely to affect our results. Misclassification of other variables such as diabetes may be more likely, but is probably non-differential and would therefore bias the results toward the null.
Among organ donors in the United States who had at least one organ used for transplant, we found that the non-use of livers fell until 2004 and has since increased. Concurrently, the prevalences of advanced donor age, elevated BMI, diabetes, and DCD have all increased. These factors are associated with liver non-use, and the impact of DCD on non-use has grown rapidly. A better understanding of the reasons for the increasing proportion of DCD in particular is critical to understanding this declining utilization. These trends, along with stagnant donation rates, suggest significant declines in liver transplant availability in the coming years.
Acknowledgments
Grants and Financial Support: This work was supported, in part, by the National Institutes of Health, T32 DK07634 (Dr. Orman), 1KL2-RR025746 (Dr. Barritt), 1-K12 HS019468-01 (Dr. Wheeler), and UL1-RR025747 (Drs. Orman, Barritt, and Wheeler), and by Health Resources and Services Administration contract 231-00-0115.
Abbreviations
- ALT
alanine aminotransferase
- BMI
body mass index
- CVA
cerebrovascular accident
- CI
confidence interval
- DBD
donation after brain death
- DCD
donation after cardiac death
- NAFLD
non-alcoholic fatty liver disease
- OR
odds ratio
- PARP
population attributable risk proportion
- SD
standard deviation
- UNOS
United Network for Organ Sharing
- WIT
warm ischemia time
Footnotes
Conflicts of Interest: The authors of this manuscript have no conflicts of interest to disclose.
Disclaimer: The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011;11(9):1773–84. doi: 10.1111/j.1600-6143.2011.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spitzer AL, Lao OB, Dick AA, Bakthavatsalam R, Halldorson JB, Yeh MM, et al. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl. 2010;16(7):874–84. doi: 10.1002/lt.22085. [DOI] [PubMed] [Google Scholar]
- 3.McCormack L, Petrowsky H, Jochum W, Mullhaupt B, Weber M, Clavien PA. Use of severely steatotic grafts in liver transplantation: a matched case-control study. Ann Surg. 2007;246(6):940–6. doi: 10.1097/SLA.0b013e31815c2a3f. discussion 6–8. [DOI] [PubMed] [Google Scholar]
- 4.Foley DP, Fernandez LA, Leverson G, Chin LT, Krieger N, Cooper JT, et al. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242(5):724–31. doi: 10.1097/01.sla.0000186178.07110.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 6.Merion RM, Pelletier SJ, Goodrich N, Englesbe MJ, Delmonico FL. Donation after cardiac death as a strategy to increase deceased donor liver availability. Ann Surg. 2006;244(4):555–62. doi: 10.1097/01.sla.0000239006.33633.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jay C, Ladner D, Wang E, Lyuksemburg V, Kang R, Chang Y, et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant - an analysis of the national registry. J Hepatol. 2011;55(4):808–13. doi: 10.1016/j.jhep.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 2010;10(11):2512–9. doi: 10.1111/j.1600-6143.2010.03293.x. [DOI] [PubMed] [Google Scholar]
- 9.Taner CB, Bulatao IG, Willingham DL, Perry DK, Sibulesky L, Pungpapong S, et al. Events in procurement as risk factors for ischemic cholangiopathy in liver transplantation using donation after cardiac death donors. Liver Transpl. 2012;18(1):100–11. doi: 10.1002/lt.22404. [DOI] [PubMed] [Google Scholar]
- 10.Broomhead RH, Patel S, Fernando B, O’Beirne J, Mallett S. Resource implications of expanding the use of donation after circulatory determination of death in liver transplantation. Liver Transpl. 2012;18(7):771–8. doi: 10.1002/lt.23406. [DOI] [PubMed] [Google Scholar]
- 11.Jay CL, Skaro AI, Ladner DP, Wang E, Lyuksemburg V, Chang Y, et al. Comparative effectiveness of donation after cardiac death versus donation after brain death liver transplantation: Recognizing who can benefit. Liver Transpl. 2012;18(6):630–40. doi: 10.1002/lt.23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imber CJ, St Peter SD, Lopez I, Guiver L, Friend PJ. Current practice regarding the use of fatty livers: a trans-Atlantic survey. Liver Transpl. 2002;8(6):545–9. doi: 10.1053/jlts.2002.31747. [DOI] [PubMed] [Google Scholar]
- 13.Sayuk GS, Leet TL, Schnitzler MA, Hayashi PH. Nontransplantation of livers from deceased donors who are able to donate another solid organ: how often and why it happens. Am J Transplant. 2007;7(1):151–60. doi: 10.1111/j.1600-6143.2006.01600.x. [DOI] [PubMed] [Google Scholar]
- 14.Organ Procurement and Transplantation Network. [cited 2011 December 13]; Available from: http://optn.transplant.hrsa.gov/data/
- 15.Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):973–86. doi: 10.1111/j.1600-6143.2009.03008.x. [DOI] [PubMed] [Google Scholar]
- 16.From the Centers for Disease Control and Prevention. Public health and aging: trends in aging-- United States and worldwide. JAMA. 2003;289(11):1371–3. [PubMed] [Google Scholar]
- 17.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 18.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 19.McCormack L, Dutkowski P, El-Badry AM, Clavien PA. Liver transplantation using fatty livers: always feasible? J Hepatol. 2011;54(5):1055–62. doi: 10.1016/j.jhep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 20.de Vera ME, Lopez-Solis R, Dvorchik I, Campos S, Morris W, Demetris AJ, et al. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center. Am J Transplant. 2009;9(4):773–81. doi: 10.1111/j.1600-6143.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine. Non-Heart-Beating Organ Transplantation: Practice and Protocols. The National Academies Press; 2000. [PubMed] [Google Scholar]
- 22.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49(3):865–72. [PubMed] [Google Scholar]
- 23.Hayashi PH, Axelrod DA, Galanko J, Salvalaggio PR, Schnitzler M. Regional differences in deceased donor liver transplantation and their implications for organ utilization and allocation. Clin Transplant. 2011;25(1):156–63. doi: 10.1111/j.1399-0012.2010.01214.x. [DOI] [PubMed] [Google Scholar]
- 24.Shiffman ML, Saab S, Feng S, Abecassis MI, Tzakis AG, Goodrich NP, et al. Liver and intestine transplantation in the United States, 1995–2004. Am J Transplant. 2006;6(5 Pt 2):1170–87. doi: 10.1111/j.1600-6143.2006.01273.x. [DOI] [PubMed] [Google Scholar]
- 25.Howard RJ, Cornell DL, Schold JD. CMS oversight, OPOs and transplant centers and the law of unintended consequences. Clin Transplant. 2009;23(6):778–83. doi: 10.1111/j.1399-0012.2009.01157.x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen B, Smits JM, Haase B, Persijn G, Vanrenterghem Y, Frei U. Expanding the donor pool to increase renal transplantation. Nephrol Dial Transplant. 2005;20(1):34–41. doi: 10.1093/ndt/gfh506. [DOI] [PubMed] [Google Scholar]
- 27.Bernat JL, D’Alessandro AM, Port FK, Bleck TP, Heard SO, Medina J, et al. Report of a National Conference on Donation after cardiac death. Am J Transplant. 2006;6(2):281–91. doi: 10.1111/j.1600-6143.2005.01194.x. [DOI] [PubMed] [Google Scholar]
- 28.Shafer TJ, Wagner D, Chessare J, Zampiello FA, McBride V, Perdue J. Organ donation breakthrough collaborative: increasing organ donation through system redesign. Crit Care Nurse. 2006;26(2):33–42. 4–8. quiz 9. [PubMed] [Google Scholar]
- 29.Snoeijs MG, Winkens B, Heemskerk MB, Hoitsma AJ, Christiaans MH, Buurman WA, et al. Kidney transplantation from donors after cardiac death: a 25-year experience. Transplantation. 2010;90(10):1106–12. doi: 10.1097/TP.0b013e3181f83b0b. [DOI] [PubMed] [Google Scholar]
- 30.Arrich J, Holzer M, Herkner H, Mullner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2009;(4):CD004128. doi: 10.1002/14651858.CD004128.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Saidi RF, Bradley J, Greer D, Luskin R, O’Connor K, Delmonico F, et al. Changing pattern of organ donation at a single center: are potential brain dead donors being lost to donation after cardiac death? Am J Transplant. 2010;10(11):2536–40. doi: 10.1111/j.1600-6143.2010.03215.x. [DOI] [PubMed] [Google Scholar]
- 32.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 33.Gerber DA, Arrington CJ, Taranto SE, Baker T, Sung RS. DonorNet and the potential effects on organ utilization. Am J Transplant. 2010;10(4 Pt 2):1081–9. doi: 10.1111/j.1600-6143.2010.03036.x. [DOI] [PubMed] [Google Scholar]