Abstract
Modafinil may be useful for treating stimulant abuse, but the mechanisms by which it does so are unknown. Indeed, a primary effect of modafinil is to inhibit dopamine transport, which typically promotes rather than inhibits motivated behavior. Therefore, we examined the role of nucleus accumbens extracellular glutamate and the group II metabotropic glutamate receptor (mGluR2/3) in modafinil effects. One group of rats was trained to self-administer cocaine for 10 days and extinguished, then given priming injections of cocaine to elicit reinstatement. Modafinil (300 mg/kg, i.p.) inhibited reinstated cocaine-seeking (but did not alter extinction responding by itself), and this effect was prevented by pretreatment with bilateral microinjections of the mGluR2/3 antagonist LY-341495 (LY) into nucleus accumbens core. No reversal of modafinil effects was seen after unilateral accumbens core LY, or bilateral LY in the rostral pole of accumbens. Next, we sought to explore effects of modafinil on extracellular glutamate levels in accumbens after chronic cocaine. Separate rats were administered non-contingent cocaine, and after 3 weeks of withdrawal underwent accumbens microdialysis. Modafinil increased extracellular accumbens glutamate in chronic cocaine, but not chronic saline pretreated animals. This increase was prevented by reverse dialysis of cystine-glutamate exchange or voltage-dependent calcium channel antagonists. Voltage-dependent sodium channel blockade partly attenuated the increase in glutamate, but mGluR1 blockade did not. We conclude that modafinil increases extracellular glutamate in nucleus accumbens from glial and neuronal sources in cocaine-exposed rats, which may be important for its mGluR2/3-mediated anti-relapse properties.
Keywords: nucleus accumbens, self-administration, microdialysis
INTRODUCTION
Modafinil (2-diphenylmethyl-sulfinyl-2 acetamide, Provigil) is the prototype of a class of cognitive enhancing drugs that is used to treat narcolepsy and other sleep disorders (Minzenberg and Carter, 2008). Both clinical (Anderson et al., 2009; Ballon and Feifel, 2006; Dackis et al., 2005; Dackis et al., 2003; Hart et al., 2008; Martinez-Raga et al., 2008; Myrick and Anton, 2004) and preclinical studies (Reichel and See, 2010; Tahsili-Fahadan et al., 2010) show that modafinil may also have utility in treating psychostimulant addiction. Despite numerous studies examining the use of modafinil to treat narcolepsy, addiction, and other disorders, the cellular mechanisms of action by which this interesting drug exerts its behavioral or clinical effects remain largely unknown.
Modafinil binds to dopamine transporters, and thereby increases extracellular dopamine (Andersen et al., 2010; Madras et al., 2006; Volkow et al., 2009). It has been postulated that this mechanism could permit modafinil to function as a replacement therapy in treating addiction to psychostimulants like cocaine (Karila et al., 2008; Schmitt and Reith, 2011). However, this dopaminergic action of modafinil would be expected to produce reinforcing and motivational effects, yet most (but not all) studies have reported the opposite in humans and animals (Andersen et al., 2010; Nguyen et al., 2011; Reichel and See, 2010; Tahsili-Fahadan et al., 2010; Young and Geyer, 2010). Therefore, the capacity of modafinil to inhibit drug seeking may not entirely arise from inhibiting dopamine transport.
Over a decade ago modafinil was shown in vivo to increase extracellular glutamate in dorsal striatum, thalamus, hypothalamus, and hippocampus (Ferraro et al., 1997; Ferraro et al., 1998; Ferraro et al., 1999), though the mechanisms of these effects are not clear (Perez de la Mora et al., 1999). Many recent studies reveal that extracellular glutamate levels in nucleus accumbens core in particular regulate reinstatement of cocaine- and heroin-seeking, via stimulation of group II metabotropic glutamate receptors (mGluR2/3s) (Baptista et al., 2004; Bossert et al., 2006; Moran et al., 2005; Moussawi et al., 2011; Peters and Kalivas, 2006; Xi et al., 2010). Reinstatement of cocaine seeking is inhibited by increased tone on extrasynaptic mGluR2/3 in accumbens core, but it is not presently known whether modafinil increases extrasynaptic glutamate in this structure. The possibility that modafinil may inhibit drug seeking via this mechanism was recently supported by a report showing that modafinil inhibits reinstatement of extinguished morphine seeking in a conditioned place preference paradigm, and that this effect was prevented by systemic blockade of mGluR2/3 receptors (Tahsili-Fahadan et al., 2010).
Here we used a self-administration/reinstatement model of relapse to test the hypothesis that modafinil attenuates cocaine seeking by acting selectively on mGluR2/3s in nucleus accumbens core. Having validated a role for mGluR2/3s in accumbens core in inhibition of reinstatement, we then employed microdialysis to explore whether modafinil increases extracellular glutamate in accumbens, and if so, if this glutamate increase is of synaptic or non-synaptic origin.
MATERIALS AND METHODS
Subjects
All procedures complied with the NIH guidelines for care of laboratory animals, and were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (250–300 g, Charles River, Wilmington, MA) were used in this study. Rats were housed individually or in pairs on a 12-hour light/dark cycle with food and water ad libitum. All rats were acclimated to the vivarium for 7 days prior to surgery.
Drugs
Modafinil (2-diphenylmethyl-sulfinyl-2 acetamide; 300mg./kg, i.p.; a gift from Cephalon Inc., West Chester, PA) was suspended in 2 ml/kg 0.25% methylcellulose in water. This preparation yielded a suspension of modafinil (unlike DMSO or cyclodextrine vehicles, which dissolve/cage modafinil molecules), so the mixture was stirred constantly until immediately prior to i.p. injection. This dose of modafinil was previously shown to elevate extracellular glutamate levels in the striatum (Ferraro et al., 1998) and to block reinstatement of extinguished morphine place preference (Tahsili-Fahadan et al., 2010). Modafinil was given 90 min prior to reinstatement or self-administration sessions, close to the peak of modafinil-induced glutamate levels in accumbens (based on microdialysis results described below). Cocaine (NIDA, Research Triangle Park, NC) was dissolved in 0.9% sterile saline. The mGluR2/3 antagonist LY-341495 (LY; Tocris; 0.2, 1, and 2μg) was dissolved in sterile artificial cerebrospinal fluid (ACSF; 0.5 μl). Compounds infused through a dialysis probe included tetrodotoxin (TTX, 3 μM, Sigma-Aldrich), w-conotoxin-GVIA (conotoxin, 10 μM, Tocris), (S)-4-carboxyphenylglycine (CPG, 1 μM, Tocris), and (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA, 300 μM, Tocris).
Surgical Procedures
Rats were anesthetized with ketamine (87.5 mg/kg, i.m.), xylazine (5 mg/kg, i.m.), and ketorolac (3 mg/kg, i.p.), placed in a stereotaxic instrument, and bilateral guide cannulae (Plastics One) were implanted over the nucleus accumbens (microdialysis surgical coordinates: AP: +1.8mm, ML: +2.5mm at 6° angle, DV: −5.0mm; behavioral surgical coordinates: AP: +1.8–2.5 mm, ML: +2.5 at 6° angle, and DV: −5.0 mm). Animals trained to self-administer cocaine were also implanted with chronic indwelling jugular catheters that exited the body via a port between the scapulae. All animals undergoing cocaine self-administration received i.v. cefazolin (10 mg) and heparin (10 U) daily starting 3 days after surgery, and continuing throughout self-administration training (administered after sessions). In all studies, rats were allowed 7 days to fully recover post-surgery before self-administration training or non-contingent cocaine administration commenced.
Self-Administration, Extinction and Reinstatement
Behavioral Training
Rats received ten, 2 hr cocaine self-administration sessions (>10 cocaine infusions/day, 0.2 mg/50 μl infusion). Pressing the active lever (fixed ratio 1) yielded a 3.6 sec cocaine infusion, a 2.9 KHz tone, and a light presented above the active lever, followed by a 20 sec timeout period. Presses on the inactive lever had no consequences. For animals used to test modafinil effects on cocaine self-administration, modafinil (300 mg/kg, i.p.) or vehicle was administered 90 min prior to testing on days 10 and 11 (counterbalanced order). An additional day of self-administration testing was conducted 48 hrs after day 10. To test effects of modafinil and intra-accumbens LY on cocaine-primed reinstatement behavior, animals were extinguished to criterion over at least 7 days (<25 active lever presses for two consecutive days).
Reinstatement Testing
LY microinjections were administered using injectors (Plastics One; 28 ga) extending 2 mm beyond the guide cannula. The day before testing, sham injections were made to habituate animals to microinjection procedures. On subsequent test days, microinjections were administered 95 min prior to each reinstatement test in a 0.5 μl volume over 120 sec, and injectors were left in place for 1 min to allow diffusion away from injection sites. Five min after microinjections (90 min prior to reinstatement tests), animals were injected with modafinil (300 mg/kg, i.p.) or vehicle, and returned to their home cages. Immediately prior to reinstatement testing, animals were injected with cocaine (10 mg/kg, i.p.) then placed in the operant chamber for a 2 hr reinstatement test. Active and inactive lever presses did not result in cue presentations or cocaine. Between cocaine-primed reinstatement sessions, animals received extinction training, until they returned to extinction criterion. Reinstatement trials were counterbalanced as follows: vehicle intracranially (i.c.)+vehicle i.p., vehicle i.c.+ modafinil i.p. (300 mg/kg), or LY i.c. (0.2, 1, or 2 μg)+modafinil i.p. Some animals also received LY i.c. (2 μg)+vehicle, i.p. To test whether modafinil itself altered extinction responding or produced reinstatement behavior, separate groups of animals received modafinil (300 mg/kg) or vehicle 90 min prior to saline or cocaine primed reinstatement test sessions. These animals received either saline [with modafinil (n=14) or vehicle (n=8) pretreatment], or cocaine [10 mg/kg; modafinil pretreatment (n=22), vehicle pretreatment (n=12)] injections immediately prior to testing.
Non-Contingent Injections of Cocaine
For microdialysis experiments, rats were administered daily non-contingent injections of cocaine or saline according to two protocols. In the first experiment (Figure 2), cocaine was administered in an ascending daily dosing regimen of 10, 15, 20, 25, and 30 mg/kg, i.p., for 10 days with each dose given on two consecutive days. In the second experiment (Figure 3), rats were administered an injection protocol that produces behavioral sensitization (Pierce et al., 1996). On the first day, animals were injected with saline (0.3 ml i.p.) and the next day with cocaine (15 mg/kg, i.p.) or saline. Rats were then administered cocaine (30 mg/kg i.p.) or saline for 5 consecutive days, followed by a final injection of 15 mg/kg, i.p. cocaine or saline. All microdialysis experiments were conducted a minimum of 21 days after the last daily injection of cocaine.
In Vivo Microdialysis Procedures
In-house probe construction procedures and aCSF content are described elsewhere (Baker et al., 2003). The night prior to collecting samples, the probes were inserted into the accumbens and perfused with aCSF (0.2 μl/min). The following morning, the flow rate was increased to 2.0 μl/min. After two hours, 6 baseline collections were taken in 20 min intervals. All animals were then administered methylcellulose vehicle (2 ml/kg, i.p.). Sixty min later, animals were injected with modafinil (300 mg/kg, i.p.). In the experiment shown in Figure 3, after vehicle administration, the dialysis probe continued perfusion of aCSF, or aCSF containing TTX (3 μM), conotoxin (10 μM), CPG (1 μM) or AIDA (300 μM). Doses were chosen based on previous dialysis studies (Baker et al., 2002; Melendez et al., 2005). Dialysis samples were stored at −80°C before being analyzed for glutamate using HPLC with electrochemical detection, as described elsewhere (Torregrossa and Kalivas, 2008).
Histology and Statistics
Rats were deeply anesthetized and perfused intracardially with 0.9% saline and fixed with formalin. Sections (40–60 μm thick) were nissl stained, and injection/dialysis sites were localized using the Paxinos and Watson atlas (2007). Effects of systemic modafinil on cocaine self-administration were determined with repeated measures ANOVAs on lever pressing and cocaine infusions. Effects of systemic modafinil and i.c. LY on cocaine-primed reinstatement were examined with separate repeated measures ANOVAs for each dose of LY in bilateral NAc, bilateral rostral pole, and unilateral NAc cannulae groups, since each animal received (i.c./i.p.): vehicle/vehicle, vehicle/modafinil, and LY (0.2, 1, or 2 μg)/modafinil, or vehicle/vehicle and LY (2 μg)/modafinil. Effects of modafinil on reinstatement of cocaine seeking in the absence of cocaine priming injections was examined with a oneway ANOVA, with modafinil vs. vehicle and cocaine vs. saline priming injection as dependent variables. Microdialysis data were examined using a two-way ANOVA with repeated measures over time. Bonferroni or Tukey post hoc tests were used for multiple comparisons in all cases, as appropriate.
RESULTS
Modafinil Inhibited Reinstatement of Cocaine Seeking but did not Induce Reinstatement
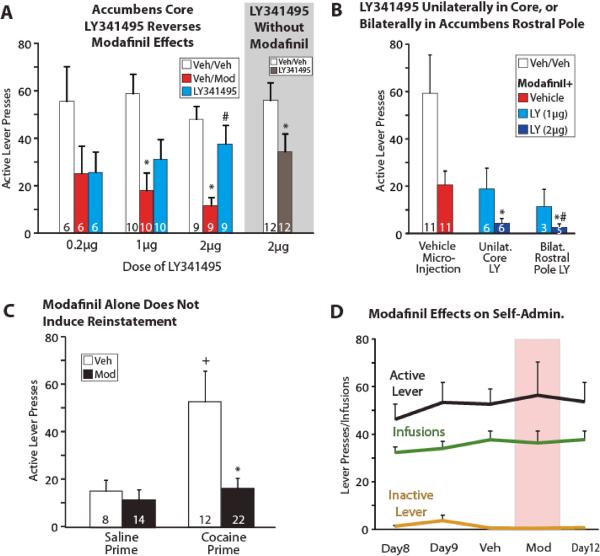
Figure 1A–C shows that vehicle-pretreated rats had robust cocaine-primed reinstatement of active lever pressing, compared to prior extinction training days (animals with bilateral accumbens core cannulae: t13=5.3, p<0.001; animals with cannulae outside accumbens core: t16=3.4, p<0.01). Modafinil (300 mg/kg, i.p., with i.c. vehicle) substantially reduced cocaine-primed reinstatement in all treatment groups (Figure 1A: t13=4.8, p<0.001; Figure 1B: t16=2.3, p<0.05; Figure 1C: t32=3.2, p<0.01). Modafinil pretreatment alone did not significantly alter extinction responding or induce reinstatement of cocaine seeking (Figure 1C; F(3,52)=6.7, p=0.001; Tukey posthocs: veh pretreatment + saline prime vs. modafinil pretreatment + saline prime: n.s.; veh pretreatment + saline prime vs. veh pretreatment + cocaine prime: p=0.015).
Figure 1.
A) Modafinil (Mod; 300 mg/kg, i.p.) reduced cocaine-primed (10 mg/kg, i.p.) reinstatement (vehicle i.c. + vehicle i.p.=white bars; vehicle i.c. + modafinil i.p.=red bars), which was prevented in a dose-dependent manner by bilateral microinjections of the mGluR2/3 antagonist LY-341495 (LY) into nucleus accumbens core (blue bars; 0.2, 1, 2μg). The highest dose of LY-341495, when injected in the absence of modafinil pretreatment (brown bar at right), instead reduced reinstated cocaine seeking. B) Microinjections of LY-341495 (2 μg=dark blue bars, no significant effect of 1 μg=light blue bars) either unilaterally into accumbens core (with contralateral injections into adjacent structures like medial accumbens shell or dorsal striatum; bars at middle), or bilaterally into the far rostral “pole” of accumbens (bars at right) facilitated modafinil's blunting of reinstated cocaine seeking even further (vehicle i.c. + vehicle i.p.=white bar at right; vehicle i.c. + modafinil i.p.=red bar at right). C) As expected, a cocaine prime induced reinstatement relative to a saline prime 90 min after vehicle injections (white bars; +p<0.05). Similar modafinil pretreatment before a saline prime did not alter extinction responding or cause reinstatement (black bars, left). As in panels A and B, modafinil-pretreated animals again reinstated less than vehicle pretreated animals after a cocaine prime (right; *p<0.01). D) Modafinil (300 mg/kg i.p.) during cocaine self-administration did not affect lever pressing or cocaine intake (infusions) compared to i.p. vehicle, prior self-administration days 8&9, or subsequent self-administration day 12. * p<0.05 compared to Veh/Veh; # p<0.05 comparing LY341495 with Veh/Mod. Sample sizes of each group are noted inside bars.
Modafinil Inhibition of Reinstated Cocaine Seeking was Reversed by Intra-Accumbens LY-341495
Microinjections of LY (2μg) into accumbens core reversed modafinil-induced inhibition of cocaine seeking. Figure 1A shows that LY (2μg, i.c.) 5 min prior to systemic modafinil yielded reinstatement equivalent to controls (F(2,16)=13.9, p<0.001, veh/veh vs. veh/mod: t8=7.8, p<0.001; veh/mod vs. 2 μg LY/mod: t8=3.1, p<0.05; veh/veh vs. 2 μg LY+mod: t8=1.3, p=0.2; n=9). The 1 μg dose of LY did not significantly affect modafinil-induced attenuation of reinstatement (F(2,18)=13.2, p<0.001; veh/veh vs. veh/mod: t9=4.3, p<0.01, veh/mod vs. 1 μg LY/mod: t9=1.7, p=0.12, veh/veh vs. 1 μg LY/mod: t9=3.9, p<0.01; n=10), nor did the lowest 0.2 μg dose of LY (F(2,10)=2.3, p=0.15, n=6). When intra-accumbens LY (2μg) was administered in the absence of systemic modafinil (i.p. vehicle), the mGluR2/3 antagonist decreased rather than increased reinstated cocaine seeking (t11=2.6, p<0.05).
Prevention of modafinil effects by LY occurred only with bilateral microinjection into the nucleus accumbens core proper, but not when injected into more rostral aspects of accumbens (rostral pole), or unilaterally into accumbens core, with a contralateral injection in the adjacent medial accumbens shell or caudate/putamen. Figure 1B shows that LY (2μg) failed to reverse modafinil's attenuation of cocaine-primed reinstatement when cannulae were i) unilaterally outside the accumbens core [in accumbens shell (n=2) or caudate/putamen (n=4); no differences in effects of LY between these structures were observed], or bilaterally in the rostral pole of accumbens (n=5). In fact, LY into rostral accumbens pole plus systemic modafinil suppressed lever pressing even further than modafinil alone (unilateral NAc cannula: F(2,10)=5.6, p<0.05; rostral pole: F(2,8)=7.3, p<0.05).
Modafinil Did Not Affect Cocaine Self-Administration Behavior
The average cocaine intake during the 10 training sessions (mean±SEM) was 61.7±4.2 mg. Figure 1D shows that modafinil (300 mg/kg) administered 90 min prior to self-administration session 10 or 11 did not affect established cocaine self-administration behavior (n=9). The lack of effect of modafinil on self-administration of cocaine indicated that the reduction in reinstated cocaine seeking is not likely due to nonspecific sedative effects.
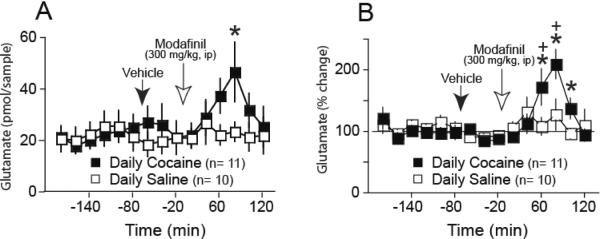
Modafinil Elevated Extracellular Glutamate
In microdialysis experiments, the basal levels of glutamate in each treatment group did not differ significantly, permitting the data to be normalized to percentage of the average of the 6 baseline samples in each animal (Figure 2: Saline=22±5 pmol/sample, n=10; cocaine=21±3, n=11; t(19)=0.14, p=0.889. Figure 3: aCSF=44±7, n=12; CPG=64±12, n=13; AIDA=42±12, n=8; TTX=39±8, n=8; Conotoxin=57±14, n=8; F(4,48)=1.10, p=0.368). Presumably, probe variability prevented us from identifying the reduction in extracellular glutamate in cocaine subjects that has been found previously in no-net-flux dialysis experiments, where interprobe variability does not influence the estimate of glutamate concentrations (Baker et al., 2003). In addition, probes were only partially contained within accumbens core, which is the primary site demonstrating reduced extracellular glutamate after withdrawal from chronic cocaine (Baker et al., 2003; Pierce et al., 1996). Figure 2 shows that 3 weeks after discontinuing daily cocaine injections, modafinil (300 mg/kg, i.p.) increased accumbens levels of extracellular glutamate. Whether evaluated as pmole/sample (Figure 2A) or percent change from baseline (Figure 2B) a two-way ANOVA with repeated measures over time revealed significant effects of Time (F(14,14)=2.55, p=0.002), Figure 2A; F(14,14)=4.5, p<0.001, Figure 2B)), and an interaction between Time and Treatment (F(14,285)=2.07, p=0.014), Figure 1A; F(14,285)=2.4, p=0.003, Figure 2B)]. The differences between saline and cocaine groups were more robust when evaluated as percent change from baseline probably due to inter-probe variability in glutamate recovery. However, both analyses revealed an increase in glutamate that was delayed and showed a peak response at 80+ min after modafinil administration. In contrast, when microdialysis was conducted 3 weeks after discontinuing daily saline injections, modafinil produced only a trend towards elevating extracellular glutamate, and the effect of modafinil in the cocaine group was significantly elevated over the saline group between 80–100 min after injection when the data were analyzed as percent change from baseline. Acute vehicle injection (i.p.) did not alter the levels of glutamate regardless of the pretreatment group.
Figure 2.
Modafinil (300 mg/kg, i.p.) elicited a delayed increase in extracellular glutamate in the accumbens only in rats pretreated 3 weeks earlier with daily cocaine injections. After collecting 6 baseline samples (−180 to −80 min), an injection of vehicle was made, and 60 min later modafinil was injected and samples collected for another 120 min. A) Data presented a mean ± SEM pmol/sample. B) The same data as in panel A shown as percent change from baseline. *p< 0.05 compared to baseline within group, +p <0.05 comparing saline to cocaine group.
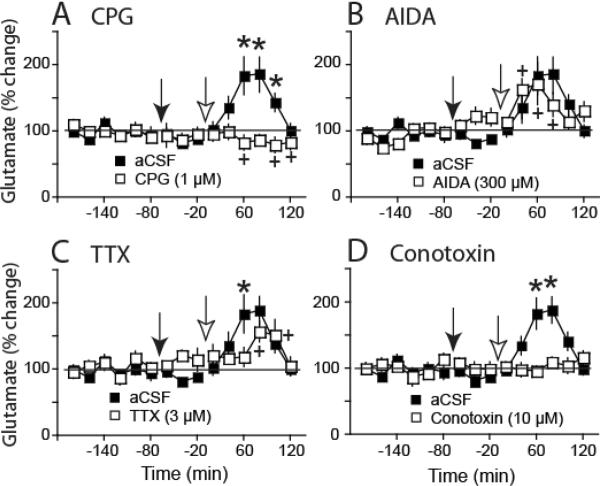
Figure 3.
Modafinil increases in glutamate were abolished by CPG and conotoxin, and were partly antagonized by TTX. A) CPG (1μM, filled arrow; a cystine-glutamate exchange inhibitor) was introduced into the dialysis buffer 60 min prior to injecting modafinil (open arrow) and prevented the rise in glutamate, indicating dependence of this effect on cysteine-glutamate exchange. B) AIDA (300 μM; a mGluR1 antagonist) was without effect on modafinil-induced glutamate. C) TTX (3 μM; a voltage-dependent sodium channel inhibitor) produced a partial reduction in modafinil-induced glutamate, indicating partial mediation of this effect by impulse-dependent glutamate release. D) Conotoxin [10 μM; a voltage gated calcium channel (Cav) inhibitor] prevented modafinil-mediated increases in glutamate, suggesting dependence of this effect upon Cav. The aCSF group is repeated in each panel. *p <0.05 compared to aCSF group, + p< 0.05 compared to baseline within group.
Modafinil Increased Extracellular Glutamate via Glial and Neuronal Sources
The fact that modafinil increased glutamate only in the accumbens of animals withdrawn from daily cocaine injections, and not in saline-treated rats, is reminiscent of the glutamate increase produced by N-acetylcysteine injections only in chronic cocaine-treated subjects. N-acetylcysteine increases extracellular glutamate by activating glial cystine-glutamate exchange (Baker et al., 2003). Accordingly, we examined whether reverse dialysis of the cystine-glutamate exchange antagonist CPG inhibited modafinil-induced increase in extracellular glutamate. Figure 3A shows that CPG abolished the modafinil increase in glutamate 3 weeks after the last daily cocaine injection (Time: F(14,14)=3.4, p<0.001, Treatment: F(1,14)=19.3, p<0.001, Interaction: F(14,345)=5.5, p<0.001). The cystine-glutamate exchanger contributes to basal glutamate levels (Baker et al., 2002), and accordingly, glutamate dropped below baseline by 120 min after introducing CPG into the dialysis buffer.
Although the dose of CPG used was likely below that needed to antagonize mGluR1 receptors (Baker et al., 2002), we next determined whether selective blockade of mGluR1 with AIDA affected the modafinil-induced increase in glutamate. Figure 3B shows that AIDA did not prevent modafinil from increasing extracellular glutamate (Time: F(14,14)=3.5, p<0.001, Interaction: F(14,270)=2.3, p=0.006).
To determine if the effect of modafinil on extracellular glutamate may have depended on voltage-dependent sodium or calcium conductances, TTX or conotoxin (respectively) were infused through the dialysis probe. Figure 3C shows that TTX significantly reduced the capacity of modafinil to increase extracellular glutamate (Time: F(14,14)=3.1, p<0.001, Interaction: F(14,270)=2.2, p=0.007). However, TTX inhibited only the rise of glutamate in the sample obtained 60 min after injecting modafinil, and the level of glutamate was elevated by modafinil between 60–100 min after injection. Akin to CPG, Figure 3D shows that conotoxin prevented the modafinil-induced increase in glutamate (Time: F(14,14)=13.1, p<0.001, Interaction: F(14,270)=12.8, p<0.001). However, unlike CPG, conotoxin did not reduce the level of glutamate relative to pre-drug baseline.
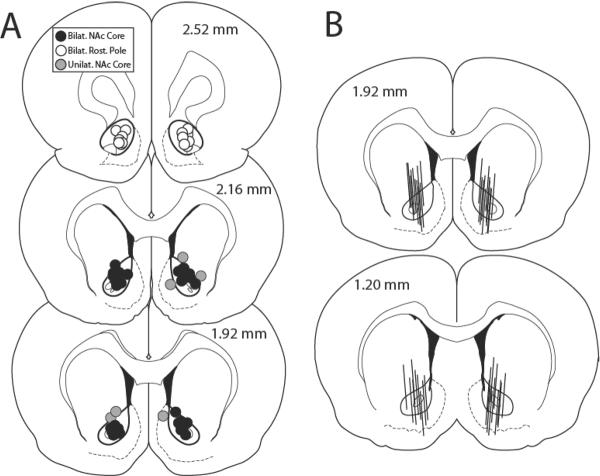
Histology
Figure 4A shows the location of microinjection sites in the nucleus accumbens. Microinjections targeted to the accumbens core proper (black dots) were sites where bilateral LY effectively reversed the inhibitory action of modafinil on cocaine seeking. Injections into the rostral pole of accumbens (white dots) did not reverse modafinil effects. Unilateral accumbens core injections also failed to reverse modafinil-induced attenuation of reinstatement. In these “unilateral accumbens” animals, one injection site was located within accumbens core (black dots), and the contralateral cannula was located outside accumbens core (grey dots in medial accumbens shell and dorsal striatum). Figure 4B shows the location of dialysis probes in the accumbens for animals used in Figure 3. The probes possessed 2 mm of active membrane and all were >50% situated in the accumbens core. However, it is important to note that up to 50% of the active membrane of each probe was also partly in the striatum and/or ventral accumbens shell, unlike in previous reports (Baker et al., 2003; Pierce et al., 1996).
Figure 4.
Location of the dialysis probes and microinjection sites in the accumbens. A) The location of microinjection injection sites were drawn in a coronal view, adapted from Paxinos and Watson (2007) by an individual unaware of the animal's treatment group. Black circles refer to microinjection sites in accumbens core proper, where bilateral injection of LY-341495 prevented modafinil inhibition of reinstated cocaine seeking. White circles indicate sites in rostral pole of accumbens, where bilateral LY-341495 did not decrease modafinil effects. Grey circles indicate injection sites located unilaterally outside accumbens core, in dorsal striatum or medial accumbens shell (contralateral cannulae in these animals were located within accumbens core, represented with black circles). LY-341495 failed to decrease modafinil effects in these animals, indicating that bilateral mGluR2/3 antagonism in accumbens core is required for modafinil effects to be reversed by LY-341495. Numbers refer to mm rostral to Bregma. B). The location of the 2 mm of active membrane in dialysis probes.
DISCUSSION
The present data show for the first time that modafinil attenuates cocaine-primed reinstatement of cocaine seeking. This effect depended on the capacity of modafinil to increase extracellular glutamate in the accumbens of cocaine-experienced animals, as inhibiting mGlu2/3 receptors bilaterally in accumbens core reversed modafinil effects on reinstatement. In chronic cocaine-treated and withdrawn animals, modafinil promoted the release of glutamate from both glial and neuronal release mechanisms, as blocking cystine-glutamate exchange, voltage gated calcium channels (Cav) or action potentials with TTX reduced the rise in glutamate. These results support a potential role for modafinil in treating addictive disorders, and indicate that these therapeutic effects result in part from elevating extrasynaptic glutamate, thereby stimulating accumbens mGluR2/3s.
Previous clinical work has indicated that modafinil might be useful in treating at least some cases of stimulant abuse in humans (Anderson et al., 2009; Dackis et al., 2005; Dackis et al., 2003; Hart et al., 2008). In preclinical models, modafinil also reduced reinstatement of methamphetamine and morphine seeking in self-administration and conditioned place preference paradigms, respectively (Reichel and See, 2010; Tahsili-Fahadan et al., 2010). Modafinil is known to interfere with dopamine transporters, and although this action may not necessarily be similar to the transporter inhibition by cocaine and other rewarding drugs (Loland et al., 2012; Schmitt and Reith, 2011), modafinil does increase dopamine levels in accumbens. Facilitation of accumbens dopamine has been linked to the motivational and rewarding effects of modafinil in preclinical models, including its ability to reinstate drug seeking behaviors in some paradigms (Andersen et al., 2010; Bernardi et al., 2009; Cao et al., 2010; Nguyen et al., 2011; Schmitt and Reith, 2011; Spencer et al., 2010; Young and Geyer, 2010; Zolkowska et al., 2009).
Of course, increased motivation and reward is unlikely to mediate the capacity of modafinil to reduce reinstated drug seeking, and we show here that modafinil inhibition of cocaine seeking instead depends on the stimulation of mGlu2/3s in the accumbens core. mGluR2/3s are located pre-synaptically in accumbens core, and respond to extrasynaptic glutamate by inhibiting synaptic glutamate release there (Moussawi and Kalivas, 2010), reducing reinstatement behavior (Moussawi et al., 2011). Taken together with the literature, our findings support the likelihood that anti-relapse properties of modafinil are due (at least in part) to an increase in extrasynaptic glutamate levels in accumbens, and subsequent stimulation of mGluR2/3 receptors.
Previous reports have shown that modafinil can increase extrasynaptic glutamate in other brain areas (Ferraro et al., 1997; Ferraro et al., 1998; Ferraro et al., 1999). Here, we found increases in nucleus accumbens glutamate in cocaine-experienced, but not naïve animals. This argues that cocaine-induced adaptations interact with modafinil effects on accumbens glutamate. Chronic cocaine down-regulates cystine-glutamate exchange as well as glial glutamate transport via GLT-1 in the accumbens, and this glutamate dysregulation has been strongly linked to reinstatement of cocaine seeking (Kalivas, 2009). Down-regulated GLT-1 mediates the increase in glutamate over-flow measured by microdialysis in the accumbens during cocaine-induced reinstatement (Knackstedt et al., 2010), so it seems probable that down-regulated GLT-1 also contributes to the augmented increases in glutamate by acute modafinil in cocaine-experienced rats.
Although we did not determine the molecular binding site for modafinil in these studies, the rise in extracellular glutamate we observed involves cellular processes akin to what has previously been reported for N-acetylcysteine, which also inhibits cocaine-primed reinstatement (Moran et al., 2005; Xi et al., 2010). Like N-acetylcysteine (Baker et al., 2003), the modafinil-induced increases in extracellular glutamate require activity of cystine-glutamate exchange, as indicated by our finding that CPG blocked modafinil-induced glutamate increases. The cystine-glutamate exchanger is largely glial, and catalyzes the 1:1 stoichiometric exchange of extracellular cystine for intracellular glutamate (McBean, 2002). Whether modafinil acts directly on the exchanger to increase activity, or like N-acetylcysteine indirectly increases its activity remains to be determined (Kupchik et al., 2012). However, indirect regulation appears likely given that the increases in glutamate by modafinil were entirely dependent upon cystine-glutamate exchange (CPG blockade) and Cav conductance (conotoxin blockade), and partly depended on action potential conduction (partial blockade by TTX). It is interesting that the increase in glutamate by modafinil occurred >60 min after injection, which supports the idea that the accumulation of glutamate may not depend directly on synaptic release (since this would be a more rapid event), and therefore may be a function of accumulation due to reduced glutamate uptake (see above). Regardless of the mechanism, the timeframe of glutamate accumulation in the extracellular space corresponds nicely to the blockade of reinstated behavior by modafinil which was administered 90 min prior to initiating cocaine-induced reinstatement, further supporting the contention that increased accumbens glutamate contributes to modafinil's suppression of cocaine seeking.
In contrast to bilateral accumbens core, mGluR2/3 antagonism in the rostral accumbens “pole” did not reverse modafinil's inhibition of cocaine seeking, nor did unilateral antagonism in core. Both of these argue for anatomical specificity of LY-341495 (LY) effects. We found that bilateral LY injections in accumbens core were required to reverse modafinil effects reinstatement: No restoration of reinstatement was seen when LY was injected unilaterally in accumbens core, and contralaterally into other nearby structures. As unilateral accumbens mGluR2/3 blockade likely spared effects of modafinil-induced extracellular glutamate at these receptors in accumbens in one hemisphere, no reversal of modafinil reinstatement effects were observed. In addition, LY significantly potentiated the inhibitory effect of modafinil when administered in the rostral pole of accumbens. Rostro-caudal differences in the functional and anatomical organization of the accumbens have been observed previously (Gill and Grace, 2011; Mahler et al., 2007; Pecina and Berridge, 2005; Reynolds and Berridge, 2003; Richard and Berridge, 2011; Vaccarino, 1994). While differences in connectivity offer one possible explanation, it is also possible that cocaine-induced changes in glutamate homeostasis contributing to reinstated cocaine seeking may not be as robust in the far rostral accumbens. For example, some effects of chronic cocaine differ between the core and the shell, and rostral pole of accumbens shares anatomical features with accumbens shell (Pierce et al., 1996; Wolf, 2010; Zahm and Brog, 1992; Zahm and Heimer, 1993). It is not known if similar distinctions in the neuroadaptations produced by repeated cocaine exist between rostral pole and more caudal accumbens core that might contribute to the differential effects of LY observed here.
Interestingly, we found that LY microinjections into accumbens in the absence of modafinil, or into rostral pole even in the presence of modafinil, attenuated reinstatement behavior even below modafinil levels. Crucially, this demonstrates that mGlu2/3 antagonism in accumbens core does not simply increase cocaine seeking, but that it specifically prevents modafinil-induced effects, presumably mediated by extrasynaptic glutamate. Although a previous report did not find similar inhibition of reinstatement by intra-accumbens LY (Moussawi et al., 2011), that earlier study involved microinjections of LY immediately before cue-induced reinstatement sessions, instead of 95min prior to cocaine-primed reinstatement as we did here (to block modafinil effects on glutamate levels that rise post-modafinil, but prior to reinstatement testing; Figure 2). Potentially, this suggests that mGlu2/3 antagonism causes persistent compensatory effects long after acute administration, or suggests a difference between mGluR2/3 roles in cue vs. cocaine primed reinstatement. This issue requires future examination.
It is important to note that the cocaine treatment paradigms used here were different in behavioral and microdialysis experiments. Reinstatement effects were examined in animals allowed to self-administer cocaine, as is required for this model of relapse in addiction. However, microdialysis data were obtained using a non-contingent cocaine/withdrawal model, as this has been shown to produce differences in glutamate between cocaine-exposed and non-exposed animals (Moussawi et al., 2011; Swanson et al., 2001). Although there is extensive overlap between cocaine effects on glutamate homeostasis between the contingent and non-contingent cocaine exposure paradigms (Kalivas, 2009; Steketee and Kalivas, 2011), some distinctions have been noted, especially with regard to the release of glutamate by an acute cocaine challenge. For example, in rats withdrawn from self-administered cocaine, acute cocaine-induced accumbens glutamate increases are entirely TTX dependent (McFarland et al., 2003), but only initial glutamate release was TTX-dependent in rats withdrawn from chronic non-contingent cocaine (Pierce et al., 1996). Additionally, in rats that received chronic non-contingent yoked i.v. cocaine, acute cocaine did not cause accumbens glutamate release (McFarland et al., 2003), though accumbens glutamate release did result after acute cocaine was administered to animals withdrawn from a sensitizing regimen of non-contingent i.p. cocaine (Pierce et al., 1996). Here, we showed that in animals which self-administered cocaine, modafinil reduced reinstatement in an accumbens mGluR2/3- dependent manner, and that in animals exposed to chronic, non-contingent cocaine (but not saline-exposed animals), modafinil increased extrasynaptic accumbens glutamate, which primarily acts upon presynaptic mGluR2/3s (Moussawi and Kalivas, 2010). Though it seems significant that modafinil blocked reinstatement behavior 90 min after injection (a time point where modafinil-induced accumbens glutamate increases were near maximum), we cannot conclude that identical mechanisms exist for modafinil-induced glutamate increases in animals trained to self-administer cocaine. However, it seems probable that similar actions of modafinil underlie both effects.
It is also worth noting that the effects observed here are unlikely to have been caused by generalized states such as sedation or anxiety. First, while modafinil is a stimulant and induces wakefulness, unlike conventional stimulants it does not affect locomotion at 300mg/kg or lower doses in rats; therefore it is unlikely to have reduced reinstatement by suppressing locomotion here (Edgar and Seidel, 1997). Second, modafinil failed to affect cocaine self-administration behavior (Figure 1D), which a drug that non-specifically sedated animals would be expected to have done. Third, modafinil (admittedly at lower doses than used here), failed to affect an elevated plus maze measure of anxiety in mice (Simon et al., 1994). Finally, intra-accumbens injections of LY failed to affect cue-induced reinstatement behavior in previous reports, suggesting a lack of profound locomotor or anxiety effects (Kupchik et al., 2012; Moussawi et al., 2011).
In summary, modafinil inhibits cocaine-primed reinstatement of cocaine seeking, and this behavioral effect likely involves a novel mechanism by which modafinil increases extrasynaptic glutamate in nucleus accumbens core, and thereby activates mGluR2/3s in this structure. Importantly, modafinil-induced suppression of relapse was blocked by accumbens core pretreatment with an mGluR2/3 antagonist. Although dose-and time course-dependency of modafinil effects remain to be fully explored, the present data suggest a causal role for elevated extrasynaptic glutamate, and concomitant activation of mGluR2/3s, in the ability of modafinil to decrease cocaine seeking (among other mechanisms through which this complex drug acts). Together, these results show that modafinil may have potential as a pharmacotherapy for treating relapse to cocaine use, and point to a novel mechanism of action for this poorly understood, but clinically useful drug.
Acknowledgements
This research was supported in part by NIH grants DA015369 (PWK), DA003906 (PWK), DA026692 (SVM) and DA06214 (GAJ).
Footnotes
Author Contributions: SVM: conceived experiments, conducted experiments, wrote paper, analyzed data, MH-S: conducted experiments, wrote paper, RL: conceived experiments, conducted experiments, PTF: conceived experiments, conducted experiments, CT: conducted experiments, RVF: conducted experiments, PWK: conceived experiments, analyzed data, wrote paper, GA-J: conceived experiments, wrote paper.
REFERENCES
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology. 2010;210:439–448. doi: 10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug and alcohol dependence. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature neuroscience. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. The Journal of clinical psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Lewis JR, Lattal KM, Berger SP. Modafinil reinstates a cocaine conditioned place preference following extinction in rats. Behav Brain Res. 2009;204:250–253. doi: 10.1016/j.bbr.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Prisinzano TE, Okunola OM, Kopajtic T, Shook M, Katz JL, Newman AH. Structure-Activity Relationships at the Monoamine Transporters for a Novel Series of Modafinil (2-[(diphenylmethyl)sulfinyl]acetamide) Analogues. ACS medicinal chemistry letters. 2010;2:48–52. doi: 10.1021/ml1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O'Brien CP. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug and alcohol dependence. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Seidel WF. Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. The Journal of pharmacology and experimental therapeutics. 1997;283:757–769. [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O'Connor WT, Tanganelli S, Rambert F, Fuxe K. The antinarcoleptic drug modafinil increases glutamate release in thalamic areas and hippocampus. Neuroreport. 1997;8:2883–2887. doi: 10.1097/00001756-199709080-00016. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O'Connor WT, Tanganelli S, Rambert FA, Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neuroscience letters. 1998;253:135–138. doi: 10.1016/s0304-3940(98)00629-6. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, Tanganelli S, O'Connor WT, Perez de la Mora M, Mendez-Franco J, Rambert FA, Fuxe K. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1999;20:346–356. doi: 10.1016/S0893-133X(98)00085-2. [DOI] [PubMed] [Google Scholar]
- Gill KM, Grace AA. Heterogeneous processing of amygdala and hippocampal inputs in the rostral and caudal subregions of the nucleus accumbens. Int J Neuropsychopharmacol. 2011;14:1301–1314. doi: 10.1017/S1461145710001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature reviews Neuroscience. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lepine JP. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW. The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biological psychiatry. 2012;71:978–986. doi: 10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland CJ, Mereu M, Okunola OM, Cao J, Prisinzano TE, Mazier S, Kopajtic T, Shi L, Katz JL, Tanda G, Newman AH. R-Modafinil (Armodafinil): A Unique Dopamine Uptake Inhibitor and Potential Medication for Psychostimulant Abuse. Biological psychiatry. 2012 doi: 10.1016/j.biopsych.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. The Journal of pharmacology and experimental therapeutics. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances 'liking' of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Martinez-Raga J, Knecht C, Cepeda S. Modafinil: a useful medication for cocaine addiction? Review of the evidence from neuropharmacological, experimental and clinical studies. Current drug abuse reviews. 2008;1:213–221. doi: 10.2174/1874473710801020213. [DOI] [PubMed] [Google Scholar]
- McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends in pharmacological sciences. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. The Journal of pharmacology and experimental therapeutics. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. European journal of pharmacology. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton R. Recent advances in the pharmacotherapy of alcoholism. Current psychiatry reports. 2004;6:332–338. doi: 10.1007/s11920-004-0019-7. [DOI] [PubMed] [Google Scholar]
- Nguyen TL, Tian YH, You IJ, Lee SY, Jang CG. Modafinil-induced conditioned place preference via dopaminergic system in mice. Synapse. 2011;65:733–741. doi: 10.1002/syn.20892. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th Edition ed. Academic Press/Elsevier; Boston, MA: 2007. [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de la Mora M, Aguilar-Garcia A, Ramon-Frias T, Ramirez-Ramirez R, Mendez-Franco J, Rambert F, Fuxe K. Effects of the vigilance promoting drug modafinil on the synthesis of GABA and glutamate in slices of rat hypothalamus. Neuroscience letters. 1999;259:181–185. doi: 10.1016/s0304-3940(98)00905-7. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology. 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. The European journal of neuroscience. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors. PloS one. 2011;6:e25790. doi: 10.1371/journal.pone.0025790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Panissaud C, Costentin J. The stimulant effect of modafinil on wakefulness is not associated with an increase in anxiety in mice. A comparison with dexamphetamine. Psychopharmacology. 1994;114:597–600. doi: 10.1007/BF02244990. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Madras BK, Bonab AA, Dougherty DD, Clarke A, Mirto T, Martin J, Fischman AJ. A positron emission tomography study examining the dopaminergic activity of armodafinil in adults using [(1)(1)C]altropane and [(1)(1)C]raclopride. Biological psychiatry. 2010;68:964–970. doi: 10.1016/j.biopsych.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahsili-Fahadan P, Carr GV, Harris GC, Aston-Jones G. Modafinil blocks reinstatement of extinguished opiate-seeking in rats: mediation by a glutamate mechanism. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2203–2210. doi: 10.1038/npp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Kalivas PW. Neurotensin in the ventral pallidum increases extracellular gamma-aminobutyric acid and differentially affects cue- and cocaine-primed reinstatement. The Journal of pharmacology and experimental therapeutics. 2008;325:556–566. doi: 10.1124/jpet.107.130310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FJ. Nucleus accumbens dopamine-CCK interactions in psychostimulant reward and related behaviors. Neuroscience and biobehavioral reviews. 1994;18:207–214. doi: 10.1016/0149-7634(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA : the journal of the American Medical Association. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends in neurosciences. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Peng XQ, Li J, Chun L, Gardner EL, Thomas AG, Slusher BS, Ashby CR., Jr. Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. Journal of neurochemistry. 2010;112:564–576. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Action of modafinil--increased motivation via the dopamine transporter inhibition and D1 receptors? Biological psychiatry. 2010;67:784–787. doi: 10.1016/j.biopsych.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. The Journal of comparative neurology. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. The Journal of pharmacology and experimental therapeutics. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]