Abstract
Hepatic injury due to cold storage followed by reperfusion remains a major cause of morbidity and mortality after orthotopic liver transplantation (OLT). CD4 T cell TIM-1 signaling co-stimulates a variety of immune responses in allograft recipients. This study analyzes mechanisms by which TIM-1 affects liver ischemia-reperfusion injury (IRI) in a murine model of prolonged cold storage followed by OLT. Livers from C57BL/6 mice, preserved at 4°C in UW solution for 20h, were transplanted to syngeneic recipients. There was an early (1h) increased accumulation of TIM-1+ activated CD4 T cells in the ischemic OLTs. Disruption of TIM-1 signaling with a blocking mAb (RMT1-10) ameliorated liver damage, evidenced by reduced sALT levels and well-preserved architecture. Unlike in controls, TIM-1 blockade diminished OLT expression of Tbet/IFN-γ, but amplified IL-4/IL-10/IL-22; abolished neutrophil and macrophage infiltration/activation; and inhibited NF-κB while enhancing Bcl-2/Bcl-xl. Although adoptive transfer of CD4 T cells triggered liver damage in otherwise IR-resistant RAG−/− mice, adjunctive TIM-1 blockade reduced Tbet transcription, and abolished macrophage activation, restoring homeostasis in IR-stressed livers. Further, transfer of TIM-1HiCD4+, but not TIM-1LoCD4+ T cells, recreated liver IRI in RAG−/− mice. Thus, TIM-1 expressing CD4 T cells are required in the mechanism of innate immune-mediated hepatic IRI in OLTs.
Keywords: Ischemia-Reperfusion Injury, Orthotopic Liver Transplantation, TIM-1, T cell costimulation, innate immunity
Introduction
Hepatic ischemia-reperfusion injury (IRI) represents a major complication in liver transplantation (1). Liver damage resulting from warm ischemia during organ retrieval and prolonged periods of cold preservation prior to the transplant often leads to primary graft nonfunction, predisposes to late chronic rejection, and contributes to the shortage of donor organs. IR-mediated tissue damage combines two phases of ischemia-trigged hypoxic cellular stress, and inflammation-mediated reperfusion injury. Reactive oxygen species (ROS)-inflicted local tissue insult initiates circulatory disturbances, followed by inflammation innate cascades and ultimate hepatocyte death. In agreement with others (2, 3) we have documented the essential role of activated CD4 T cell phenotype in promoting IR-inflammation and hepatocellular damage (4, 5). Indeed, CD4 T cell activation may occur via CD154-CD40 engagement to facilitate liver IRI without de novo Ag-specific stimulation IRI (4). Unlike CD154, stimulating PD-1/B7-H1 signals ameliorated inflammation response, implying that negative co-stimulation pathway can promote homeostasis in IR-stressed liver (6).
T cell Immunoglobulin Mucin (TIM) family members, expressed on certain T cells and APCs, have attracted major attention as mediators of T cell activation and tolerance (7, 8). TIM-1, originally described as a hepatitis A virus receptor (HAVCR1) (9), was then identified as a kidney injury molecule (KIM-1) (10). While absent on naïve CD4 T cells, TIM-1 expression increases following TCR stimulation to provide positive co-stimulatory signal in T cell proliferation and Th1/Th17 cytokine production (11–14). The role of TIM-1 to regulate T cell differentiation was first shown in a Th2-mediated asthma model (12). Monoclonal antibodies against the TIM-1 IgV domain, were generated to block TIM-1 signaling and pathogenic Th1/Th17 responses while enhancing Th2/Treg activity in allergen-induced autoimmune disease models (14, 15).
We have reported that treatment with antagonistic TIM-1 mAb improved the hepatocellular function and diminished TLR4-dependent inflammation in a partial “warm” liver IRI model (16). However, the mechanism of the latter is quite different from IRI cascade in livers subjected to extended periods of cold storage prior to transplantation (1). Hence, to mimic the real-life clinical scenario, we have developed a murine model of prolonged liver cold preservation followed by orthotopic transplantation (OLT) (17). Devoid of confounding Ag-driven rejection, the outcome in this syngeneic liver transplant model depends solely on the function of IR-stressed OLT.
In this study, we examined putative mechanisms by which TIM-1 blockade may contribute to liver homeostasis in IR-stressed OLTs. We first determined the function of endogenous TIM-1 signaling in liver-infiltrating CD4 T cells, and whether targeting CD4-dependent TIM-1 can diminish IR-triggered pro-inflammatory local innate response and promote hepatocyte survival in OLTs. Our findings document that manipulation of TIM-1 costimulation at the CD4 T cell – macrophage interface represents a novel therapeutic concept in the management of liver inflammation and hepatocellular function in transplant recipients.
Materials and Methods
Animals
C57BL/6 (Thy1.2; WT), B6.129S7-Rag1tm1Mom/J (C57BL/6, Thy1.2; RAG−/−) or B6.PL-Thy1a/CyJ (C57BL/6; Thy1.1) mice (male 8–12 weeks old, Jackson Laboratory, Bar Harbor, ME) were housed in the UCLA animal facility under specific pathogen-free conditions and received humane care according to the criteria outlined in Guide for the Care and Use of Laboratory Animals (prepared by the National Academy of Sciences; NIH publication 86-23, revised 1985).
Model of liver “cold” ischemia followed by OLT
Isogeneic OLTs were performed in WT mice, as described (17). Briefly, donor livers were harvested, stored in University of Wisconsin (UW) solution at 4°C for 20 h, and then transplanted orthotopically using the cuff technique. The bile duct was connected by ligation over the stent. Anhepatic time averaged from 15–18 min. Sham-operated animals served as controls. In the treatment groups, animals were infused immediately after completion of OLT with a single dose of antagonistic anti-TIM-1 mAb (RMT1-10; 0.5mg/mouse i.v., Bio X Cell, West Lebanon, NH) or control Ig. Mice were sacrificed at various time-points post-transplant, and OLT/sera samples were collected.
Isolation of CD4 T cell subsets
Enriched (>95%) negatively selected spleen CD4 T cells were separated from syngeneic Thy1.1 mouse donors by using a magnetic cell sorting kit (StemCell Technologies, Vancouver, Canada), according to the manufacturer’s instruction. In separate experiments, TIM-1HiCD4+ and TIM-1LoCD4+ T cells were isolated from ConA-activated (10ug/ml, Sigma-Aldrich, St. Louis, MO) spleen T cells by CD4 negative selection plus PE positive selection Kit (StemCell Technologies). PE rat anti-mouse TIM-1 mAb (RMT1-4, BioLegend, San Diego CA) was used to select TIM-1Hi population (~50% positive by flow cytometry).
Model of liver “warm” ischemia followed by reperfusion
We have developed a new model of liver IRI in RAG−/− mice. These T-/B-cell deficient animals remained untreated or repopulated with normal syngeneic CD4+ T cells (5×106 i.v.), or ConA-activated TIM-1HiCD4+ vs. TIM-1LoCD4+ T cells (2.5×106 i.v.). The animals were anesthetized, injected with heparin (100 U/kg) and an atraumatic clip was used to interrupt the arterial/portal venous blood supply to the cephalad liver lobes (18, 19). After 90 min of warm ischemia, the clip was removed, and recipients were sacrificed at 6 h of reperfusion. Some of normal CD4 T cell-repopulated RAG−/− test recipients were infused with anti-TIM-1 mAb (RMT1-10; 0.5mg/mouse i.v., Bio X Cell) or control Ig at 1 h prior to the hepatic ischemia insult. Sham-controls underwent the same procedure, but without vascular occlusion. All animals were sacrificed at 6 h after reperfusion, and liver/sera samples were collected.
Liver lymphocyte isolation and flow cytometry analysis
Lymphocytes were isolated from OLTs at 1 h post-transplant by using Percoll density gradient (4). Livers were perfused in situ with 10 mL of warm collagenase-PBS, and hepatocytes were removed by low-speed centrifugation. The lymphocyte fraction was separated by 25%/50% discontinuous Percoll density gradient (Pharmacia, New York, NY). After RBC lysis, liver lymphocytes were stained with rat anti mouse TIM-1 (RMT1-10) or control IgG (Bio X Cell), followed with the secondary rabbit anti-rat IgG-PE (eBioscience, San Diego, CA). In some groups, cells were incubated directly with rat anti-mouse CD69-PE (H1.2F3, eBioscience). All cells stained with CD4-FITC (eBioscience) were analyzed on a FACS-Calibur cytometer (BD Biosciences).
The hepatocellular damage
Serum alanine aminotransferase (sALT) levels were measured in sera samples by IDEXX Laboratory (Westbrook, ME).
Histology, immunohistochemistry, and double-immunofluorescence staining
Liver specimens (4μm), stained with hematoxylin and eosin (H&E), were analyzed by modified Suzuki’s criteria (18). Primary mAb against mouse neutrophils Ly-6G (1A8; BD Biosciences, San Jose, CA) and macrophages CD68 (FA-11; AbD Serotec, Raleigh, NC) were used (18). In addition, TIM-4 expressing macrophages were identified by immunofluorescence double staining using rat anti-mouse TIM-4 mAb (RMT4-53; Bio X Cell) with goat anti-rat IgG-Alexa Fluor® 555, followed by rat anti-mouse CD68-Alexa Fluor® 488 (FA-11; AbD Serotec). All slides were mounted with VECTASHIELD medium with DAPI (Vector Labs, Burlingame, CA). Liver sections were evaluated blindly by counting labeled cells in 10 high-power fields (HPF).
Myeloperoxidase activity assay
The presence of myeloperoxidase (MPO) was used as an index of neutrophil accumulation in the liver (18). One absorbance unit (U) of MPO activity was defined as the quantity of enzyme degrading 1mol peroxide/min at 25°C/gram of tissue.
Quantitative RT-PCR
Quantitative PCR was performed with platinum SYBR green quantitative PCR kit (Invitrogen, Carlsbad, CA) by the Chromo 4 detector (MJ Research, Waltham, MA). Primers to amplify specific gene fragments were published (18). Target gene expressions were calculated by their ratios to the housekeeping gene hypoxanthine-guanine phosphoribosyl transferase (HPRT).
Western blots
Western blots were performed with liver proteins (30μg/sample) and rabbit anti-mouse p-IκBα, Bcl-2, Bcl-xl, and β-actin mAbs (Cell Signaling Technology, Danvers, MA) (18). Relative quantities of proteinswere determined by densitometer and expressed in absorbance units (AU).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
DNA fragments in liver sections, resulting from oncotic necrosis/apoptosis were detected by TUNEL method (Klenow-FragEL DNA Fragmentation Detection Kit, Calbiochem, La Jolla, CA) (18). TUNEL positive cells were counted in 10 HPF/section under light microscopy (x400).
Caspase-3 activity assay
Caspase-3 activity in liver tissue samples was measured by using Caspase-3 Cellular Activity Assay Kit (Calbiochem) according to the manufacturer’s instructions.
Statistical analysis
All values are expressed as the mean±standard deviation (SD). Data were analyzed with an unpaired, two-tailed Student’s-t test. P<0.05 was considered to be statistically significant.
Results
Activated CD4 T cells express TIM-1 in IR-stressed OLTs
We have shown that CD4 T cells accumulate rapidly and in large numbers in mouse OLTs following prolonged periods of cold storage, peaking by 1–6 h post-transplant, and decreasing thereafter (17). In the present study we used OLTs subjected to 20 h of cold ischemia to analyze whether CD4 T cells that sequester in IR-stressed livers do express TIM-1. Indeed, as early as at 1 h post-transplant, we found a massive OLT infiltration with CD4 T cells, which were both activated, as evidenced by CD69 staining (Fig. 1a: 86.2±1.4 vs. 11.0±0.3 isotype control) and expressed TIM-1 (Fig. 1b: 11.0±0.3 vs. 2.3±0.3 isotype control) by flow cytometry.
Figure 1.
B6 livers subjected to ex-vivo cold storage (20 h) were transplanted into syngeneic recipients. By 1 h, CD4 T cells, which infiltrated OLTs were (a) activated, evidenced by CD69 staining; and (b) expressed TIM-1 (representative of 4 experiments). By 6 h after OLT, the hepatocellular function was analyzed by (c) sALT levels, (d) liver histology (representative H&E staining; magnification x100 and x400), and (e) Suzuki’s histological score (*p<0.05, **p<0.01, n=8–10/group).
TIM-1 signaling is required for IRI and its blockade ameliorates OLT damage
We have shown that intrahepatic CD4 T cells can stimulate macrophages via positive CD40–CD154 pathway without de novo activation (4, 20–22), and be regulated by negative PD-1/TIM-3 costimulatory signals during the course of liver IRI (6, 23). To analyze the functional significance of TIM-1 signaling, we collected sera and liver samples at 6 h of reperfusion, the peak of hepatocellular damage in our OLT model (17). Unlike controls given IgG, mice conditioned with anti-TIM-1 mAb were resistant against IRI, as evidenced by sALT levels (Fig. 1c: 7455±1971 vs. 3839±1386 U/L; p<0.05); well-preserved hepatic architecture (Fig. 1d: minimal sinusoidal congestion, no edema, vacuolization or necrosis); and decreased Suzuki’s score of histological liver damage (Fig. 1e: p<0.01).
TIM-1 blockade differentially regulates CD4 T cell cytokine programs in OLTs
We used qRT-PCR to analyze the expression of CD4 T cell cytokines by 6 h of reperfusion in OLTs after 20 h of cold storage. Blocking TIM-1 signaling abolished mRNA levels coding for Th1 transcription factor Tbet and IFN-γ, compared with controls (Fig. 2a: p<0.01). In contrast, the expression of Th2-related IL-4/IL-10 and Treg transcription factor FoxP3 were enhanced after anti-TIM-1 mAb treatment (Fig. 2b, c: p<0.01, p<0.05). Concomitant analysis of Th17-related genes revealed TIM-1 targeted therapy promoted OLT expression of IL-22, and transcription factor RORγt, yet diminished IL-17, as compared with controls (Fig. 2d: p<0.01, p<0.05).
Figure 2.
Quantitative RT-PCR-assisted detection of cytokine/transcription factors in OLTs (6 h post-transplant after 20 h of cold ischemia): (a) IFN-γ, Tbet; (b) IL-4, IL-10; (c) FoxP3, and (d) IL-17, IL-22, RORγt). Data normalized to HPRT gene expression (*p<0.05, **p<0.01, n=4–6/group).
Disruption of TIM-1 signaling suppresses neutrophil and macrophage function in OLTs
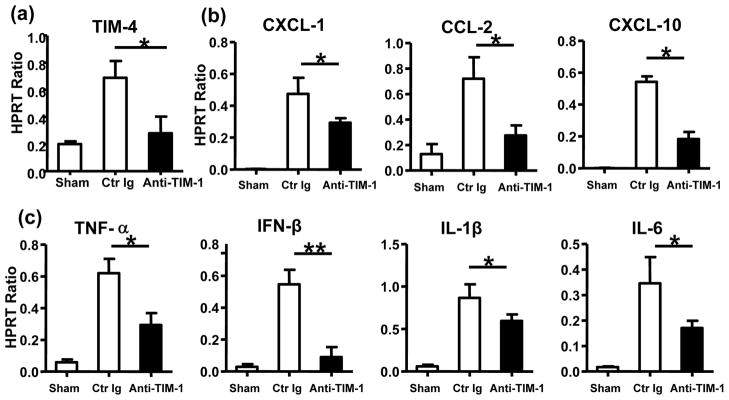
The MPO assay, reflecting intrahepatic neutrophil activity (U/g), was suppressed in anti-TIM-1 mAb treatment group, as compared with controls (Fig. 3a: 0.22±0.18 vs 1.48±0.20; p<0.01). In parallel, we used immunohistochemical stains to analyze migration of neutrophils and macrophages in OLTs. Anti-TIM-1 mAb decreased (p<0.001) the number of Ly-6G neutrophils/HPF, as compared with controls (Fig. 3b, Suppl. Fig. 1a: 4.3±1.7 vs 16.7±3.2; p<0.01). Similarly, the frequency of CD68+ macrophages/HPF in OLTs was reduced (p<0.01) after anti-TIM-1 mAb treatment (Fig. 3c, Suppl. Fig. 1b: 7.1±1.0 vs 20.8±2.4; p<0.01). As TIM-4 represents a key TIM-1 ligand expressed on monocytes (Kupffer cells/macrophages and DCs), we asked whether TIM-1 blockade affected TIM-4 expression in our model. In agreement with reduced frequency of macrophages in IR-stressed livers (Fig. 3c) we found that TIM-1 blockade depressed sequestration of TIM-4-expressing macrophages (CD68) in OLTs, as shown by immunofluorescence double staining (Fig. 3d, Suppl. Fig. 1c: 3.3±1.2 vs 14.7±1.2 in control Ig treated group; p<0.01) and qRT-PCR analysis (Fig. 4a). Consistent with reduced macrophage sequestration in OLTs (Fig. 3c, Suppl. Fig. 1b), TIM-1 blockade depressed neutrophil/monocyte-derived pro-inflammatory chemokine (CXCL-1, CCL-2, and CXCL-10) and cytokine (TNF-α, IFN-β, IL-1β, and IL-6) programs, as compared with controls (Fig. 4b, c; p<0.01 and p<0.05, respectively).
Figure 3.
Neutrophils and macrophages in OLTs following administration of anti-TIM-1 mAb or control Ig (6 h post-transplant after 20 h of cold ischemia). (a) MPO levels (n=4–6/group); immunohistochemical staining of (b) Ly-6G+ neutrophils; and (c) CD68+ macrophages; (d) immunofluorescence double staining of TIM-4-expressing macrophages (TIM-4+CD68+ cells -head arrow). Green – macrophage; red – TIM-4; blue – DAPI nuclear stain. Results scored semi-quantitatively by averaging number of positively-stained cells/field (400x magnification). Representative of 4–6 mice/group (b, c) and 2 mice/group (d).
Figure 4.
Measurement of TIM-4 expression and associated cytokine/chemokine programs in OLTs by quantitative RT-PCR (6 h post-transplant after 20 h of cold ischemia): (a) TIM-4; (b) CXCL-1, CCL-2, CXCL-10; and (c) TNF-α, IFN-β, IL-1β, and IL-6). Data normalized to HPRT gene expression (*p<0.05, **p<0.01, n=4–6/group).
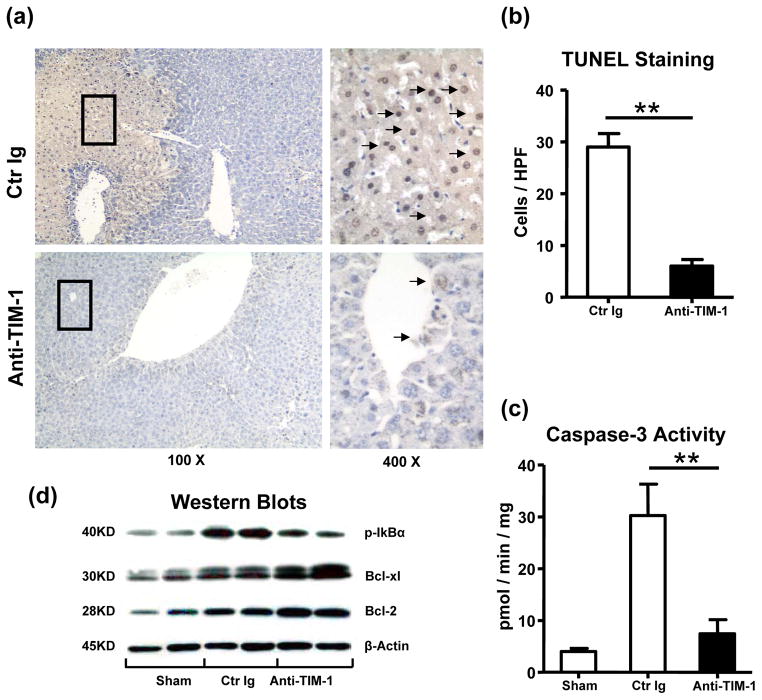
TIM-1 blockade inhibits IR-mediated liver necrosis/apoptosis in OLTs
To determine whether TIM-1 signaling affects hepatic IR-induced apoptotic pathways, we performed TUNEL assay and analyzed the expression of anti- and pro-apoptotic gene products in OLTs by Western blots. Anti-TIM-1 mAb treatment abolished abundant hepatocellular necrosis/apoptosis and caspase-3 activity seen otherwise in control OLTs (TUNELstaining: Fig. 5a, b, 6.5±0.8 vs 28.8±2.4, p<0.01; caspase-3 activity: Fig. 5c, 7.46±3.28 vs 30.26±3.79, p<0.01). In parallel, Western analysis has revealed selectively increased expression of Bcl-2/Bcl-xl, yet suppressed phosphorylation of IκBα proteins after TIM-1 blockade, as compared with controls (Fig. 5d).
Figure 5.
Necrosis/apoptosis events in OLTs (6 h post-transplant after 20 h of cold ischemia). (a, b) TUNEL-assisted detection of hepatic necrosis/apoptosis (dark arrows; magnification x100 and x400); (c) caspase-3 activity; (d) Western blot-assisted detection of Bcl-2/Bcl-xl; p-IκBα, and β-actin (**p<0.01, n=4–6/group).
CD4 T cell-dependent TIM-1 signaling is required to facilitate liver IRI
In addition to CD4 T cells, TIM-1 may also serve be expressed by activated CD4 NK1.1 T (NKT) cells (24) and B cells (25), which are known to contribute to liver IR-damage. Hence, to focus on TIM-1+ CD4 T cells only, we have developed a new model of liver IRI in RAG−/− mice. These T-/B/NKT cell-deficient animals were repopulated with purified (>95%) syngeneic spleen CD4+ T cells (5×106 i.v.), and then two days later were subjected to 90 min of liver warm ischemia followed by reperfusion. There was a significant increase in hepatic TIM-1 levels by 6h of reperfusion in RAG−/− mice infused with CD4 T cells, as compared to untreated controls (Fig. 6a, p<0.01). RAG−/− test recipients were then infused with RMT1-10 mAb (0.5 mg i.v. at -1h) to selectively target TIM-1+ transferred CD4 T cells. Indeed, although TIM-1 blockade did not affect the extent of T cell infiltration in the liver, it did prevent the hepatocellular damage in otherwise IR-susceptible RAG−/− infused with CD4 T cells, evidenced by significantly decreased sALT levels (Fig. 6b; 1077.3±206.4 vs. 10337.3±2580.1 U/L; p<0.01), diminished transcription of Tbet (Fig. 7a; p<0.01), and well preserved liver histology architecture, showing minimum sinusoid congestion, edema, and no necrosis (Fig. 6c). Of note, uniformly heightened TNF-α, IL-6, and CXCL-10 levels in RAG−/− mice repopulated with CD4 T cells were largely diminished after adjunctive blockade of TIM-1 signaling (Fig. 7b; p<0.01).
Figure 6.
TIM-1+ CD4 T cells are required to trigger liver IRI in RAG−/− mice. Intrahepatic TIM-1 levels were elevated in livers subjected to 90 min of warm ischemia in RAG−/− mice repopulated with syngeneic CD4 T cells (a). Adjunctive infusion of anti-TIM-1 mAb (day -2) restored IR-liver resistance in RAG−/− mice repopulated with CD4 T cells, as evidenced by (b) decreased sALT levels; and (c) preserved liver histology (representative H&E; magnification x100 and x400) (n=4–6/group). Adoptive transfer of ConA-activated TIM-1Hi but not TIM-1Lo CD4+ T cells (−1 h) mediated IR-liver damage in RAG−/− mice, as shown by (b) sALT levels; and (c) liver histology. (**p<0.01), n=2/group).
Figure 7.
TIM-1+ CD4 T cells mediate IR-triggered (a) Tbet transcription; and promote (b) TNF-α, IL-6, and CXCL-10 expression in RAG−/− recipients adoptively transferred with either syngeneic CD4+ T cells or ConA-activated TIM-1Hi cells. (**p<0.01, *p<0.05, n=4–6/group).
We further analyzed the function of TIM-1 signaling in our model by transferring ex-vivo generated ConA-activated TIM-1Hi or TIM-1Lo CD4+ T cell subsets (2.5×106 i.v.) into RAG−/− mice. At 1 h after cell transfer, both groups of test recipients were subjected to a standard liver IR procedure. By 6 h of reperfusion, pre-activated TIM-1Hi but not TIM-1Lo CD4 T cells triggered significant liver damage in otherwise IR-resistant RAG−/− mice, as shown by increased sALT levels (Fig. 6b; 12598.5±3593.1 vs. 3332.0±377.5 U/L; p<0.01), Tbet transcription (Fig. 7a; p<0.05), and histological liver damage (Fig. 6c). In parallel, hepatic TNF-α, IL-6, and CXCL-10 levels were selectively increased in RAG−/− mice conditioned with TIM-1Hi but not TIM-1Lo CD4+ T cells (Fig. 7b; p<0.01).
Discussion
This report complements our recent findings on the role of TIM-1 – TIM-4 costimulation in the mechanism of liver warm IRI (16). Here, we used a clinically-relevant mouse model of prolonged (20 h) liver cold storage followed by syngeneic OLT, to demonstrate that: i/graft-infiltrating activated CD4 T cells readily expressed TIM-1; ii/TIM-1 blockade ameliorated the hepatocellular damage, implying a crucial role of TIM-1+ CD4 T cells in the mechanism of IRI in OLTs; iii/disruption of TIM-1 pathway inhibited neutrophil and macrophage infiltration/function, resulting in OLT hepatoprotection. Finally, by employing a newly developed RAG−/− adoptive transfer system, we document the pathogenic function of CD4 T cell-dependent TIM-1 signaling in macrophage TIM-4 innate immune activation in IR-stressed OLTs.
Expressed selectively on Ag-activated T cell subsets, TIM-1 may affect a diverse range of pathophysiological functions. Indeed, cross-linking of TIM-1 on CD4 T cells with an agonist mAb provides a costimulatory signal for T cell activation/proliferation, Th1/Th17 polarization, as well as deprogramming of Th2/Treg expansion (14, 26). Enhanced TIM-1 signaling exacerbates experimental autoimmune encephalomyelitis (EAE) (14) and accelerates allogeneic islet rejection (26). Consequently, disruption of TIM-1 inhibits T cell generation and Th1/Th17 cytokine programs, enhances Th2/Treg polarization (14, 27), ameliorates the severity of EAE, and promotes cardiac allograft survival (27). These studies have focused on T cell-dependent TIM-1 activation per se, with little reference to the innate immune component. Our group was the first to report on the efficacy of TIM-1 blockade in liver “warm” IRI, evidenced by improved hepatocellular function and diminished TLR4-dependent local inflammation (16), data confirmed by others in a mouse model of “warm” renal IRI (28). We have also documented that activated CD4 T cells co-stimulate macrophages in IR-stressed livers via CD40–CD154 signals and without de novo activation (4, 20–22), a process tightly controlled by negative PD-1/TIM-3 pathways (6, 23). Based on these data, we reasoned that by serving as an innate immune response stimulator that can transfer the inflammatory innate immune signal to liver macrophages, TIM-1 expressing hepatic CD4 T cells may be critical players in the mechanism of “cold” ischemia-mediated OLT damage.
First, we found that prolonged cold storage did increase intra-hepatic sequestration of activated TIM-1+ CD4 T cells, consistent with pro-inflammatory transcription/cytokine gene expression profile in IR-stressed OLTs. Second, TIM-1 blockade ameliorated the hepatocellular damage, evidenced by decreased sALT levels and mitigation of cardinal histological features of liver IRI, i.e., edema, vacuolization and necrosis. As TIM-1 disruption suppressed Th1/Th17 functions (14, 26, 27), the paramount importance of this co-stimulation pathway has been recognized in auto-/allo-immune disease and transplant rejection models (14, 26, 27). In our present study, treatment with anti-TIM-1 mAb diminished Tbet, IFN-γ and IL-17 yet significantly increased IL-4, IL-10, IL-22, FoxP3, and RORγt expression levels in OLTs. TIM-1 may directly regulate Tbet and FoxP3, the key Th1 and Treg transcription factors in CD4 Th cell differentiation, respectively (14, 26, 27) to facilitate downstream signature biomarkers of liver damage (i.e., IFN-γ) or cytoprotection (i.e., IL-10). Indeed, we have shown that IL-22, an IL-10 gene family cytokine member, produced by Th17 cells (29), can function as an important hepatocyte survival factor needed for homeostasis in IR-stressed livers (30).
APC-dependent TIM-4 signaling is known to stimulate TIM-1 on CD4 T cells to trigger Th cell activation/polarization in adaptive immune response. Consequently, a specific TIM-4 blockade on DCs arrested both T cell activation and proliferation, with resultant prolongation of islet allograft survival (Dr. Najafian, personal communication). Hence, it was important for us to determine as to whether or not CD4 T cell-dependent TIM-1 signaling can indeed trigger macrophage TIM-4 to augment TLR4-driven innate inflammation in OLTs subjected to cold ischemia. Indeed, TIM-1 blockade diminished liver TIM-4 levels and depressed macrophage activation, consistent with decreased expression of liver IRI signature biomarkers, i.e., TNF-α, IL-1β, IL-6, CXCL-10 and CCL-2 (MCP-1) in OLTs. Of note, TIM-1 blockade not only suppressed total macrophage recruitment into OLTs, but it also decreased local infiltration by activated macrophages that specifically express TIM-4 (CD68+TIM-1+ cells). These findings, supported by our pilot data on the efficacy of selective TIM-4 targeted therapy to ameliorate liver IRI (H. Ji, unpublished) highlight the essential role of CD4 T cell – macrophage cross talk and of an intact TIM-1-TIM-4 signaling pathway in our model.
TLR4 activation stimulates immune activation via MyD88- and TRIF-dependent pathways (31). We have shown that IRF3 but not MyD88 signaling promotes downstream NF-κB activation and inflammation in the mechanism of liver IRI (1, 32). We have also reported that PD-1 activation may directly inhibit hepatocyte apoptosis by modulating caspase-3 activity, as well as by enhancing anti-apoptotic Bcl-2 and Bcl-xl (6). Here, TIM-1 blockade decreased macrophage activation and suppressed phosphorylation/proteolytic degradation of IκB subunit and caspase-3, while promoting Bcl-2/Bcl-xl. Moreover, disruption of TIM-1 indirectly inhibited downstream TLR4-NF-κB pro-inflammatory programs, and abolished TNFR/IL-1R de novo activation, all of which are known to enhance the hepatocyte survival.
Neutrophil-derived ROS contribute to IR-hepatocellular damage (1). Indeed, unlike in sham-controls, neutrophil infiltration and MPO activity increased sharply in Ig-treated OLTs. In contrast, liver transplants subjected to cold storage in anti-TIM-1 mAb-conditioned mice were characterized by decreased neutrophil infiltration/MPO activity and depressed CXCL-1 (KC) levels, the key neutrophil chemoattractant. As macrophage-derived inflammatory cytokines enhance neutrophil activation and target tissue sequestration, disruption of TIM-1 during organ IRI can exert its regulatory function indirectly through cytokine/chemokine networks.
The parenchyma damage in liver IRI has been characterized by both necrosis and apoptosis (33). Cold IR-induced hepatocyte swelling, plasma membrane rupture, and cytochrome C release, combined with ROS promoting mitochondrial permeability transition, result in ATP depletion-dependent oncotic necrosis and caspase-dependent apoptosis (1). Hepatocyte oncotic necrosis and apoptosis, which render parenchymal cytodestruction, proceed via DNA degradation detected by TUNEL assay (33). TIM-1 blockade inhibited local necrosis/apoptosis, evidenced by decreased frequency of TUNEL+ cells and caspase-3 activity in IR-OLTs. It is plausible that disruption of costimulation suppressed macrophage activation, and prevented hepatocellular damage by modifying pro-/anti-apoptotic ratio, decreasing the release of apoptogenic factors, such as cytochrome c from mitochondria into the cytosol, maintaining mitochondria integrity, or promoting ATP generation (34).
In addition to CD4 T cells, TIM-1 may serve as an endogenous ligand for CD4 NK1.1 T (NKT) cells (23) some B cells (24), and LMIR5/CD300b (leukocyte mono-Ig-like receptor 5) myeloid cells (35). Hence, to focus on CD4 T cell-dependent TIM-1 function, we have developed a new model of liver IRI in RAG−/− mice. Adoptive transfer of spleen CD4+ T cells from syngeneic donors triggered IR-mediated innate immune response that culminated in hepatocellular damage in otherwise resistant T-/B/NKT cell-deficient hosts. This was accompanied by elevated levels of hepatic TIM-1+ cells in RAG−/− test recipients. Although adjunctive TIM-1 blockade did not diminish the extent of T cell infiltration in OLTs, it did prevent hepatic IRI and restored homeostasis, evidenced by reduced sALT levels and preserved hepatic architecture. Consistently, TIM-1 disruption diminished otherwise heightened pro-inflammatory cytokine/chemokine levels (TNF-α, IL-6, and CXCL-10). Furthermore, adoptive transfer of ex vivo generated ConA-activated TIM-1HiCD4+ but not of TIM-1LoCD4+ T cells resulted in IR-triggered hepatocellular damage, accompanied by increased expression of pro-inflammatory cytokine/chemokine programs in otherwise IR-resistant RAG−/− test recipients. These findings are important, as they directly document the pathogenic role of TIM-1+ CD4 T cells in the mechanism of liver IRI. In addition, our results document the ability of TIM-1 ablation to modulate CD4 T cell functions and innate immune activation via its macrophage TIM-4 binding partner, with resultant recreation of hepatic homeostasis in IR-stressed livers.
In conclusion, this study is the first to document the essential role of intrinsic CD4 T cell-dependent TIM-1 signaling in cold hepatic ischemia-mediated OLT inflammation/damage. In addition, our results show that targeting TIM-1 polarizes CD4 T cell phenotype and overcomes resistance to liver IRI mediated by macrophage innate immune functions. Hence, harnessing immune regulatory mechanisms after disruption of TIM-1 costimulation represents a novel therapeutic means to manage T cell activation and inflammation in IR-stressed liver transplants.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants RO1 DK062357; DK 062357-06S1 (JWKW); The Diann Kim and The Dumont Research Foundations. YZ received a grant from Science and Technology Department of Zhejiang Province (China). HJ is a recipient of American Society of Transplant Surgeons Scientist Scholarship.
Abbreviations
- IRI
ischemia and reperfusion injury
- mAb
monoclonal antibody
- MPO
myeloperoxidase
- ROS
reactive oxygen species
- sALT
serum alanine aminotransferase
- TIM-1
T cell immunoglobulin mucin-1
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- WT
wide type
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zwacka RM, Zhang Y, Halldorson J, et al. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldwell CC, Tschoep J, Lentsch AB. Lymphocyte function during hepatic ischemia/reperfusion injury. J Leukoc Biol. 2007;82:457–464. doi: 10.1189/jlb.0107062. [DOI] [PubMed] [Google Scholar]
- 4.Shen X, Wang Y, Gao F, et al. CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology. 2009;50:1537–1546. doi: 10.1002/hep.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen XD, Ke B, Zhai Y, et al. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:296–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- 6.Ji H, Shen X, Gao F, et al. Programmed death-1/B7-H1 negative costimulation protects mouse liver against ischemia and reperfusion injury. Hepatology. 2010;52:1380–1389. doi: 10.1002/hep.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuchroo VK, Umetsu DT, DeKruyff RH, et al. The TIM gene family: emerging roles in immunity and disease. Nat Rev Immunol. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 8.Freeman GJ, Casasnovas JM, Umetsu DT, et al. TIM genes: A family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan G, Totsuka A, Thompson P, et al. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 1996;15:4282–4296. [PMC free article] [PubMed] [Google Scholar]
- 10.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 11.Umetsu SE, Lee WL, McIntire JJ, et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 12.McIntire JJ, Umetsu SE, Akbari O, et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 13.de Souza AJ, Oriss TB, O’malley KJ, et al. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci U S A. 2005;102:17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao S, Najafian N, Reddy J, et al. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204:1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sizing ID, Bailly V, McCoon P, et al. Epitope-dependent effect of anti-murine TIM-1 monoclonal antibodies on T cell activity and lung immune responses. J Immunol. 2007;178:2249–2261. doi: 10.4049/jimmunol.178.4.2249. [DOI] [PubMed] [Google Scholar]
- 16.Uchida Y, Ke B, Freitas MC, et al. The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology. 2010;51:1363–1372. doi: 10.1002/hep.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen XD, Gao F, Ke B, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver Transpl. 2005;11:1273–1281. doi: 10.1002/lt.20489. [DOI] [PubMed] [Google Scholar]
- 18.Ji H, Shen XD, Zhang Y, et al. Activation of cyclic adenosine monophosphate-dependent protein kinase a signaling prevents liver ischemia/reperfusion injury in mice. Liver Transpl. 2012;18:659–670. doi: 10.1002/lt.23399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji H, Zhang Y, Shen X, et al. Neuropeptide PACAP in mouse liver ischemia and reperfusion injury: immunomodulation via cAMP-PKA pathway. Hepatology. 2012 doi: 10.1002/hep.25802. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen XD, Ke B, Zhai Y, et al. CD154-CD40 T-cell co-stimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74:315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- 21.Ke B, Shen XD, Gao F, et al. Gene therapy for liver transplantation using adenoviral vectors: CD40-CD154 blockade by gene transfer of CD40Ig protects rat livers from cold ischemia and reperfusion injury. Mol Ther. 2004;9:38–45. doi: 10.1016/j.ymthe.2003.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke B, Shen XD, Gao F, et al. The CD154-CD40 T-cell co-stimulation pathway in liver ischemia and reperfusion inflammatory responses. Transplantation. 2005;79:1078–1083. doi: 10.1097/01.tp.0000161248.43481.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida Y, Ke B, Freitas MC, et al. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology. 2010;139:2195–2206. doi: 10.1053/j.gastro.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HS, Kim HS, Lee CW, et al. T cell Ig domain and mucin domain 1 engagement on invariant NKT cells in the presence of TCR stimulation enhances IL-4 production but inhibits IFN-gamma production. J Immunol. 2010;184:4095–4106. doi: 10.4049/jimmunol.0901991. [DOI] [PubMed] [Google Scholar]
- 25.Ding Q, Yeung M, Camirand G, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degauque N, Mariat C, Kenny J, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueno T, Habicht A, Clarkson MR, et al. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J Clin Invest. 2008;118:742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rong S, Park JK, Kirsch T, et al. The TIM-1:TIM-4 pathway enhances renal ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:484–495. doi: 10.1681/ASN.2010030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma HL, Liang S, Li J, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chestovich PJ, Uchida Y, Chang W, et al. Interleukin-22: implications for liver ischemia-reperfusion injury. Transplantation. 2012;93:485–492. doi: 10.1097/TP.0b013e3182449136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 32.Zhai Y, Qiao B, Gao F, et al. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47:199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
- 33.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 34.Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011;278:403–413. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- 35.Yamanishi Y, Kitaura J, Izawa K, et al. TIM1 is an endogenous ligand for LMIR5/CD300b: LMIR5 deficiency ameliorates mouse kidney ischemia/reperfusion injury. J Exp Med. 2010;207:1501–1511. doi: 10.1084/jem.20090581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.