Abstract
Purpose
Sudden unexpected death in epilepsy (SUDEP) is the leading cause of epilepsy-related mortality. Seizure-related respiratory dysfunction (RD), the duration of post-ictal generalized EEG suppression (PGES) and duration of postictal immobility (PI) may be important in the pathophysiology of SUDEP. Peri-ictal interventions may reduce the risk of SUDEP.
Methods
We assessed the impact of peri-ictal nursing interventions on RD, PGES and PI duration in patients with localization-related epilepsy and secondarily generalized convulsions (GC) recorded during video-EEG telemetry in the epilepsy monitoring unit.
Video-EEG data were retrospectively reviewed. Interventions including administration of supplemental oxygen, oropharyngeal suction, and patient repositioning were evaluated. Interventions were performed based on nursing clinical judgment at the bedside and were not randomized. The two-sided Wilcoxon rank-sum test was used to compare GC with and those without intervention. Robust simple linear regression was used to assess the association between timing of intervention and duration of hypoxemia (SaO2 <90%), PGES and PI using data from only the first GC for each patient.
Key Findings
Data from 39 patients with 105 GC were analyzed. PGES >2 seconds occurred following 31 GC in 16 patients. There were 21 GC with no intervention (NOINT) and 84 GC with interventions (INT). In the INT group, the duration of hypoxemia was shorter (P=0.0014) when intervention occurred before hypoxemia onset (mean duration 53.1 sec) than when intervention was delayed (mean duration 132.42 sec). Linear regression indicated that in GC with nursing interventions, earlier intervention was associated with shorter duration of hypoxemia (p<0.0001) and shorter duration of PGES (p=0.0012). Seizure duration (p <0.0001) and convulsion duration (p=0.0457) were shorter with earlier intervention. PI duration was longer for GC with PGES than GC without PGES (p<0.0001). The mean delay to first active non-respiratory movement following GC with PGES was 251.96 sec and for GC without PGES was 66.06 sec. The duration of PI was positively associated with lower SaO2 nadir (p=0.003) and longer duration of oxygen desaturation (p=0.0026). There was no association of between PI duration and seizure duration (p=0.773), between PI duration and PGES duration (p=0.758), or between PI duration and the timing of first intervention relative to seizure onset (p=0.823).
PGES did not occur in the NOINT group. The mean duration of desaturation was longer (110.9 seconds versus 49.9 seconds) (P<0.0001), mean SaO2 nadir was lower (72.8% v 79.7%) (p=0.0086) and mean end-tidal CO2 was higher (58.6 mmHg v 50.3 mmHg) (p=0.0359) in the INT group compared with the NOINT group. The duration of the seizure or of the convulsive component was not significantly different between the INT and NOINT groups.
Significance
Early peri-ictal nursing intervention was associated with reduced duration of RD, and reduced duration of PGES. These findings suggest the possibility that such interventions may be effective in reducing the risk of SUDEP in the outpatient setting. Validation of these preliminary data with a prospective study is needed before definitive conclusions can be reached regarding the efficacy of peri-ictal interventions in reducing the risk of SUDEP.
Keywords: SUDEP, seizure, nursing intervention, hypoxemia, hypercapnia
Sudden unexpected death in epilepsy (SUDEP) occurs with a rate as high as 9.3 per thousand person years in patients with refractory epilepsy (Tomson, et al., 2008). SUDEP is the leading cause of seizure-related mortality in patients with intractable epilepsy (Shorvon & Tomson, 2011). Post-ictal hypoxemia and hypercapnia may be severe and prolonged in patients with seizures of partial onset (Seyal, et al., 2010) and respiratory dysfunction (RD) occurs in about one-third of seizures of partial onset (Bateman, et al., 2008). Seizure-related RD and post-ictal generalized EEG suppression (PGES) may be important in the pathophysiology of SUDEP (Devinsky, 2011). Postictal immobility (PI) is associated with PGES (Semmelroch, et al., 2012). Direct supervision appears to reduce the risk of SUDEP (Langan, et al., 2005), possibly by allowing for prompt intervention after a seizure. Seizure-triggered interventions by personnel in epilepsy monitoring units (EMU) are aimed at reducing morbidity. The extent to which these interventions impact the severity of seizure-related RD, the duration of PGES and PI is unknown. An understanding of the impact of such interventions on post-ictal respiratory and electroencephalographic (EEG) changes in the EMU may guide the use of similar interventions in the outpatient setting and potentially reduce the likelihood of devastating consequences from seizures.
We assessed the effect of routine nursing interventions triggered by the occurrence of seizures on peri-ictal RD, PGES and PI in the EMU. We limited this analysis to secondarily generalized convulsions (GC) as these most commonly precede SUDEP (Devinsky, 2011).
METHODS
The EMU is embedded in a general neurology ward. Nursing staff are notified of a seizure by at least one of three alarm mechanisms. First, when the patient or sitter presses the event button a designated seizure pager carried by the patient’s nurse is activated. Second, the patients undergo continuous digital pulse oximetry and a drop in oxygen saturation (SaO2) below 85% automatically triggers the nurse’s pager and additionally causes an audible alarm in the patient’s room. Finally, the video stream is monitored remotely by personnel in the cardiac telemetry unit and the patient’s nurse is paged when an unusual patient event is observed. End-tidal CO2 (ETCO2) is recorded with a capnograph and both ETCO2 and SaO2 data are synchronized with the video-EEG (VET) data. Major nursing interventions in the EMU include administration of oxygen via nasal cannula or face mask (OXY), oropharyngeal suction (SUCT) and turning the patient to the lateral recumbent position (REPOS). The time at which each intervention was initiated, when performed, was obtained by review of the video signal. When more than one intervention occurred, the time of the first of any of these interventions was also noted. The necessity for intervening and the type of intervention/s were determined by the nurse at the time of initial clinical assessment of the patient following the seizure alarm. Real-time SaO2, ETCO2 and heart rate data are displayed in the patient’s room and are available to nursing staff. Nursing staff are not trained to interpret EEG tracings.
Data were analyzed in consecutive patients with localization-related epilepsy (LRE) who had GC and available SaO2 and/or ETCO2 data during the peri-ictal period. Prior approval for the study was obtained from the local institutional review board.
The seizure duration (determined from review of EEG data) and onset localization, duration of the convulsive component of the seizure, pre-ictal baseline SaO2 and ETCO2, time of onset of hypoxemia (defined as SaO2 drop below 90%), duration of hypoxemia, SaO2 nadir, peak ETCO2 during the ictal/postictal period, and duration of PGES were recorded. Previously published criteria for determination of PGES, including EEG amplitudes < 10μV, were used (Lhatoo, et al., 2010, Surges, et al., 2011). Details of the methodology for acquiring VET data in patients with medically refractory LRE, including EKG, SaO2 and ETCO2 data, were the same as those published previously (Bateman, et al., 2008, Seyal, et al., 2010). The VET data were analyzed independently by two of the investigators (MS and LMB), both board certified in electroencephalography with >25 and >10 years experience respectively in reviewing VET data and managing patients in the EMU. The investigators were not blinded for this study. Onset of hypoxemia follows seizure onset by a variable interval ranging between 6 and 226 sec (Bateman, et al., 2008). We therefore chose the time of intervention relative to hypoxemia onset, rather than seizure onset, as the point of reference in order to determine the association between intervention and total hypoxemia duration. To determine the association between intervention and PGES duration, the timing of intervention relative to seizure onset was used.
Following our initial analysis of the data, a second review of the video data was necessitated as publication of a recent study (Semmelroch, et al., 2012) indicated that lack of postictal movement was associated with PGES. At this review the time of the arrival of a member of the nursing staff at the patient’s bedside and the time of the first non-respiratory postictal active movement was recorded.
Statistical methods
We compared each of the response variables between interventions preceding onset of hypoxemia and interventions after onset of hypoxemia and between the no intervention (NOINT) and the intervention (INT) groups using the two-sided Wilcoxon rank-sum test.
Interventions were treated as independent variables and the association of these to changes in the duration of hypoxemia and the duration of PGES was determined. Because outliers and leverages were detected, a robust simple linear regression model with MM estimation method was used to study the relationship between a response and independent variable in each case (Yohai, 1987). Multiple GC occurred in some patients, therefore to maintain independence only the first GC in each patient was selected for these analyses. All analyses were performed with SAS v9.2 (SAS Institute Inc., Cary, NC, USA). A p-value < 0.05 was considered statistically significant. The data are presented as mean ± standard deviation (median, range).
RESULTS
There were 104 GC with available SaO2 data recorded in 39 patients (19 female). With one additional seizure, SaO2 data was lost but ETCO2 data was available. The mean age was 35.2 years (range 18–66 years). 42 seizures were of right temporal onset, 32 were of left temporal onset, 3 were of near simultaneous bitemporal onset, 15 were of frontal onset and the onset could not be determined in the remaining seizures. 21 seizures were classified as NOINT and 84 seizures as INT. In the INT group, OXY was administered with 41seizures, REPOS with 47 seizures and SUCT with 65 seizures. With 27 seizures, the patient underwent only one of the three interventions. There were two interventions with 35 seizures and patients underwent all three interventions with 22 seizures. 69 interventions were initiated before the end of the seizure and 15 interventions were initiated postictally.
Video data for 97 GC was available for the second review. The remaining GC that were previously analyzed could not be accessed from the storage media. The nurse was at the bedside, prior to the end of the seizure, during 93 of the 97 seizures. The mean time of nurse arrival at bedside was 92.52 ± 85.76 sec (74, -140–516) before the end of the seizure. Video data was available for 18 GC that had no nursing intervention. With 16 seizures where the nurse chose not to intervene, the nurse was present at bedside 97.13±71.2 sec (78.5, 26–253) prior to the end of the seizure. With the two remaining seizures and no nursing intervention, the nurse was at bedside 3 and 140 seconds after the end of the seizure.
INTERVENTIONS AND RESPIRATORY DYSFUNCTION
Interventions prior to onset of hypoxemia resulted in shorter duration of hypoxemia when compared to interventions occurring after hypoxemia onset (p=0.0014). For interventions prior to hypoxemia onset, the mean duration of hypoxemia was 53.1 ± 45.1 sec (44, 8–158). For interventions occurring after onset of hypoxemia the mean duration of hypoxemia was 132.4 ± 134.9 sec (103, 38–712). The SaO2 nadir was not significantly different for interventions prior to and following onset of hypoxemia (p=0.1013).
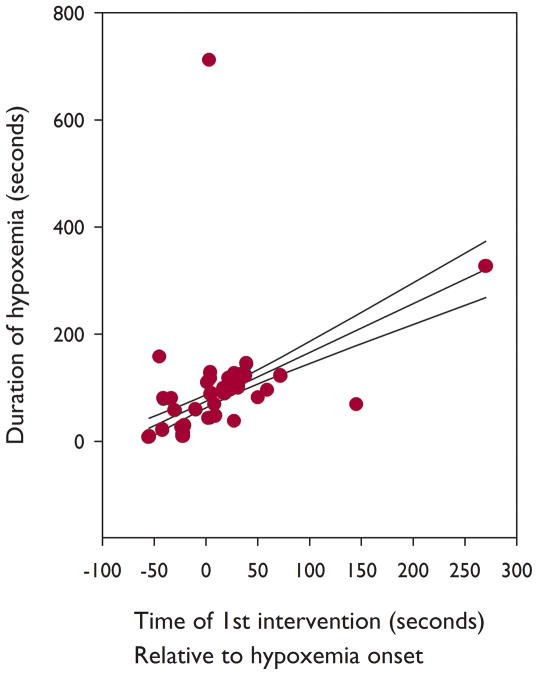
We looked at the relationship between the time of first intervention relative to hypoxemia onset and the duration of hypoxemia. Robust simple linear regression analysis indicated a positive linear relationship between the total duration of hypoxemia and the time of first intervention (p<0.0001) (Duration of hypoxemia = 74.6061+0.9113 × Time of first intervention) (Figure 1). We then assessed the relationship between time of administration of each of the 3 individual interventions and the duration of hypoxemia irrespective of whether the other two interventions occurred. Chi-square analysis indicated that there was no significant difference (p=0.5316) in the slopes of the three interventions versus duration of hypoxemia.
Figure 1.
The abscissa is the time of start of an intervention relative to onset of hypoxemia in seconds (see text). Negative numbers indicate that intervention occurred prior to onset of hypoxemia. The ordinate is the duration of hypoxemia in seconds. The circles show the values for the first GC in each patient. The robust simple linear regression line and 95% confidence intervals are shown.
INTERVENTION AND SEIZURE DURATION
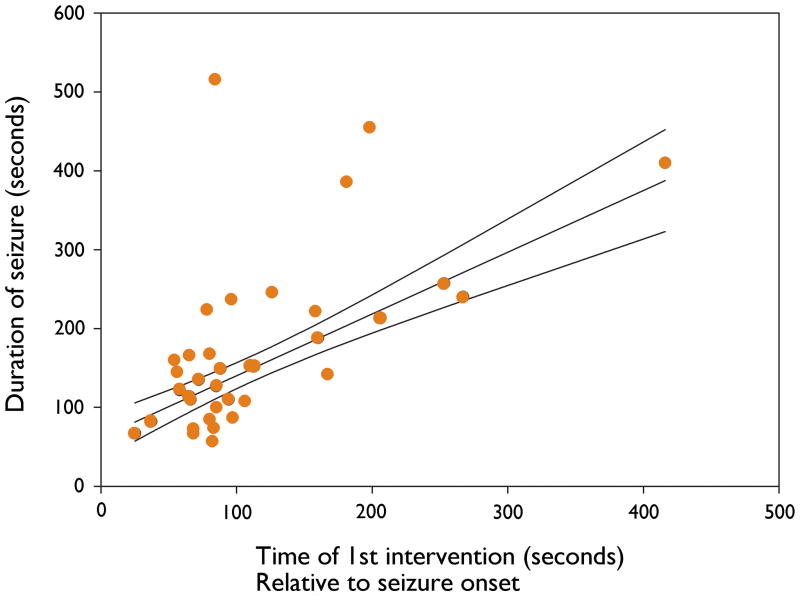
Earlier intervention following seizure onset was associated with a shorter total seizure duration (Robust simple linear regression, p<0.0001) (Seizure duration=61.8524 + 0.7829 × Time of first intervention) (Figure 2). Earlier intervention following seizure onset was also associated with a shorter duration of the convulsive component of the seizure (p=0.0457) (Duration of convulsion=61.8944 +0.10924 × Time of first intervention).
Figure 2.
The abscissa is the time of first intervention relative to seizure onset in seconds. The ordinate is the total duration of the seizure in seconds. Each data point represents the first GC for each patient. The robust simple linear regression line and 95% confidence intervals are shown.
POSTICTAL ABSENCE OF BODY MOVEMENT
Data for the duration of PI was available for 91 GC. In this subset of 91 GC, 26 were followed by PGES and 65 GC had no PGES.
PI was significantly longer for GC with PGES than for GC without PGES (p<0.0001). The mean time to first movement following GC with PGES was 251.96 ± 329.05 sec (99.5, 22–1192). The mean time to first movement following GC without PGES was 66.06 ± 150.22 sec (45, 0–1211). There was however no significant association between the duration of postictal immobility and duration of PGES (p=0.7584)
Robust linear regression analysis indicated that the duration of postictal immobility was significantly related to a lower SaO2 nadir (p=0.003) and longer duration of oxygen desaturation (p=0.0026). There was no significant association between the duration of postictal immobility and seizure duration (p=0.7726). There was no significant association between the timing of first intervention relative to seizure onset and the duration of postictal immobility (p=0.823). Only the duration of oxygen desaturation (independent variable) was significantly positively associated with the duration of immobility (dependent variable) (p=0.0379) when a multivariable robust regression model incorporating duration of immobility, SaO2 nadir, duration of desaturation, duration of seizure, duration of PGES, and time to first intervention was used. The multivariable model is limited by the relatively small number of data points and the six variables used.
INTERVENTIONS AND PGES
Robust simple linear regression analysis indicated a significant positive linear relationship between the time of first intervention relative to seizure onset and the duration of PGES (p=0.0012) (Duration of PGES = 21.6385 + 0.1963 × Time of first intervention). None of the seizures in the NOINT group were followed by PGES. 31 of the patients in the INT group had PGES. The mean duration of PGES was 41.6 ± 25.3 sec (36, 2–117).
COMPARISONS BETWEEN INT AND NOINT GROUPS
There was no difference in the mean duration of the seizure (p=0.3514) or mean duration of the convulsive component of the seizure (p=0.3971) between the INT and NOINT groups. There was a significant difference (p<0.0001) in the mean duration of hypoxemia between the two groups. The mean duration of hypoxemia in the NOINT group was 48.9 ± 40.9 sec (37, 10–188) and in the INT group was 110.9 ± 93.9 sec (99, 8–712). The time of onset of hypoxemia from seizure onset was not significantly different between the 2 groups (p=0.3351). The mean interval to onset of hypoxemia from seizure onset for the NOINT group was 101.6 ± 78.3 sec (77, 32–317) and for the INT group was 81.5 ±59.7 sec (63, −12–404). The mean desaturation nadir was 79.8 ± 7.7% (83, 61–91) in the NOINT group and 72.8 ± 11.4% (75, 42–93) in the INT group (p=0.0086). The peak ictal/postictal ETCO2 in the NOINT group was 50.3 ± 7.1 mmHg (51, 40–61) and in the INT group was 58.6 ± 13.1 mmHg (59.5, 38–94). The difference between the two groups was significant (p=0.0359).
DISCUSSION
These data indicate that, when nursing intervention is carried out, earlier nursing intervention following a GC is associated with reduced duration of RD and reduced duration of PGES. Earlier intervention also resulted in reduced duration of the seizure and of its convulsive component. We found that the slopes of the regression lines for timing of individual interventions versus duration of hypoxemia were not significantly different for the three interventions (OXY, SUCT and REPOS). This suggests that any of the major interventions may have been equally effective in improving RD. However, since more than one intervention may have occurred with a given seizure, the relative benefit of a given intervention has not been definitively established.
Severe hypoxemia with oxygen desaturations below 60% occurred with 14 of the 104 seizures with SaO2 data. Prompt resolution of hypoxemia may have relevance in reducing the likelihood of SUDEP. The severity of ictal hypoxemia and hypercapnia is associated with cardiac repolarization abnormalities (Seyal, et al., 2011). Cardiac repolarization abnormalities are associated with increased risk of potentially life-threatening arrhythmias and cardiac sudden death (Elming, et al., 2002, Kannankeril & Roden, 2007, Morita, et al., 2008, Pater, 2005, Schimpf, et al., 2008, Schouten, et al., 1991). Lengthening of the QTc interval has been reported in patients who have subsequently died of SUDEP (Brotherstone, et al., 2010, Kandler, et al., 2005, Surges, et al., 2010).
A recent report (Semmelroch, et al., 2012) indicates that PGES is associated with postictal lack of movement. Our data confirm that the presence of postictal immobility is associated with the occurrence of PGES but the duration of postictal immobility is not associated with the duration of PGES. These findings suggest that the pathophysiological mechanisms underlying PGES may also trigger PI but that the subsequent course of PGES and PI is not directly linked. It is possible that a lack of patient movement in the postictal period could influence a nursing decision to intervene. However most (69 of 84) nursing interventions were initiated prior to the end of the seizure rather than in the postictal period. Postictal immobility may be a factor predisposing to SUDEP conceivably by hindering repositioning of the face and increasing the likelihood of suffocation. The duration of postictal immobility is associated with the severity and duration of ictal/postictal oxygen desaturation. However there was no significant association between the timing of first intervention and duration of postictal immobility.
The finding indicating that early intervention was associated with shorter seizure duration was unexpected. There is some evidence that sensory stimulation may inhibit seizure activity. In kindled rats, novel and repeated sensory stimuli during partial seizures reduced seizure severity (Valentine, et al., 2005). Olfactory stimulation suppresses seizures in kindled rats (Ebert & Loscher, 2000). Somatosensory stimulation may suppress seizures (Symonds, 1961). It is possible that interventions may indirectly reduce the duration of RD by reducing seizure duration. Additionally, it is also possible that there is a more direct effect with interventions activating brainstem respiratory centers, reversing upper airway obstruction, and improving oxygen delivery to the pulmonary alveoli.
The group of seizures in which a major nursing intervention was not performed has characteristics that distinguish it from the group of seizures in which at least one major nursing intervention was carried out. The mean durations of the seizures and the convulsive components were not different from seizures that resulted in an intervention. The mean duration of desaturation was however significantly shorter, the mean SaO2 nadir was higher and the mean peak ETCO2 was lower in the NOINT group relative to the INT group. Finally, PGES only occurred in the groups in which an intervention was performed. Nursing staff are not continuously observing patients in this EMU and therefore would not always have immediate knowledge of the actual duration of the seizure or of the convulsive component when assessing the patient in the ictal/post-ictal period. Nursing personnel do however have immediate access to real-time heart rate, SaO2 and ETCO2 information and this information may have been used in the decision-making process. Nursing staff are not EEG trained and would not be able to incorporate EEG data, including the presence of PGES, in the decision to intervene. Our results are in line with conclusions from another recent retrospective study (Semmelroch, et al., 2012) showing that nursing interventions were more likely in the group of seizures with PGES. These observations suggest that nursing staff may be more concerned about the clinical state of patients with seizures followed by PGES than those without PGES. PGES may therefore be a marker for the severity of seizure-related clinical change.
The retrospective nature of this study is a limitation. Nursing staff in this EMU may have varying levels of expertise in the management of a patient during and after a generalized convulsion. This may have impacted the degree of effectiveness with which the various interventions occurred. We were unable to discern a difference in the three major interventions on hypoxemia or PGES duration. However given the design of this study we cannot exclude the possibility that any of these interventions may have been more robustly associated with the duration of hypoxemia or PGES. Nonetheless the data have demonstrated an association between the time of first intervention and duration of hypoxemia and PGES. A prospective study, controlled for equivalent level of nursing expertise and randomized to the various interventions, may be warranted to determine which of these interventions may be most effective.
We have shown that in the group of seizures where one or more interventions were performed, early intervention was associated with a shorter duration of PGES. The association between PGES and SUDEP remains uncertain and requires further investigation. One study demonstrated that there was an association between PGES of greater than 20 seconds following generalized convulsive seizures and an increased risk of SUDEP (Lhatoo, et al., 2010). Another study, while confirming the association between convulsions and PGES, failed to demonstrate an association between PGES and SUDEP (Surges, et al., 2011).
The mechanisms underlying PGES are unknown. Suppression of EEG activity without preceding or following slowing of background frequency may be directly related to anoxia (Aminoff, et al., 1988), a consequence of acute hypercapnia (Mises, et al., 1982, Schindler & Betz, 1976), or related to seizure-associated cortical spreading depression (Koroleva & Bures, 1983, Van Harreveld & Stamm, 1953). The duration of cortical spreading depression is a direct function of oxygen concentration in inspired air and increasing oxygen availability shortens the duration of CSD (Takano, et al., 2007). Any of these mechanisms would potentially be amenable to measures that improve respiratory function.
Prompt peri-ictal nursing interventions may be effective in ameliorating respiratory dysfunction and in reducing the duration of PGES. Our findings appear to demonstrate the efficacy of these interventions in the EMU. These data, pending confirmation by prospective studies, provide a foundation to guide development of measures in the outpatient setting that may reduce the risk of SUDEP.
Acknowledgments
Study funding
Statistical support for this publication was made possible by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Footnotes
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
DISCLOSURES
Masud Seyal has received funding from Citizens United for Research in Epilepsy (CURE). Chin-Shang Li has NIH funding for statistical support. Grant Number UL1 RR024146.
Lisa M. Bateman has received funding from Citizens United for Research in Epilepsy (CURE) and from Forest Laboratories.
Contributor Information
Lisa M. Bateman, Email: lmbateman@ucdavis.edu.
Chin-Shang Li, Email: cssli@ucdavis.edu.
References
- Aminoff MJ, Scheinman MM, Griffin JC, Herre JM. Electrocerebral accompaniments of syncope associated with malignant ventricular arrhythmias. Ann Intern Med. 1988;108:791–796. doi: 10.7326/0003-4819-108-6-791. [DOI] [PubMed] [Google Scholar]
- Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131:3239–3245. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherstone R, Blackhall B, McLellan A. Lengthening of corrected QT during epileptic seizures. Epilepsia. 2010;51:221–232. doi: 10.1111/j.1528-1167.2009.02281.x. [DOI] [PubMed] [Google Scholar]
- Devinsky O. Sudden, unexpected death in epilepsy. N Engl J Med. 2011;365:1801–1811. doi: 10.1056/NEJMra1010481. [DOI] [PubMed] [Google Scholar]
- Ebert U, Loscher W. Strong olfactory stimulation reduces seizure susceptibility in amygdala-kindled rats. Neurosci Lett. 2000;287:199–202. doi: 10.1016/s0304-3940(00)01161-7. [DOI] [PubMed] [Google Scholar]
- Elming H, Brendorp B, Kober L, Sahebzadah N, Torp-Petersen C. QTc interval in the assessment of cardiac risk. Card Electrophysiol Rev. 2002;6:289–294. doi: 10.1023/a:1016345412555. [DOI] [PubMed] [Google Scholar]
- Kandler L, Fiedler A, Scheer K, Wild F, Frick U, Schneider P. Early post-convulsive prolongation of QT time in children. Acta Paediatr. 2005;94:1243–1247. doi: 10.1111/j.1651-2227.2005.tb02083.x. [DOI] [PubMed] [Google Scholar]
- Kannankeril PJ, Roden DM. Drug-induced long QT and torsade de pointes: recent advances. Curr Opin Cardiol. 2007;22:39–43. doi: 10.1097/HCO.0b013e32801129eb. [DOI] [PubMed] [Google Scholar]
- Koroleva VI, Bures J. Cortical penicillin focus as a generator of repetitive spike-triggered waves of spreading depression in rats. Exp Brain Res. 1983;51:291–297. doi: 10.1007/BF00237205. [DOI] [PubMed] [Google Scholar]
- Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology. 2005;64:1131–1133. doi: 10.1212/01.WNL.0000156352.61328.CB. [DOI] [PubMed] [Google Scholar]
- Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010;68:787–796. doi: 10.1002/ana.22101. [DOI] [PubMed] [Google Scholar]
- Mises J, Ghnassia MD, Delegue L, Pellerin D, Chassevent J. [EEG recordings in prolonged hypercapnia during surgery of a cervico-mediastinal tumor in a 3-year-old child] Rev Electroencephalogr Neurophysiol Clin. 1982;12:219–226. doi: 10.1016/s0370-4475(82)80047-6. [DOI] [PubMed] [Google Scholar]
- Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet. 2008;372:750–763. doi: 10.1016/S0140-6736(08)61307-0. [DOI] [PubMed] [Google Scholar]
- Pater C. Methodological considerations in the design of trials for safety assessment of new drugs and chemical entities. Curr Control Trials Cardiovasc Med. 2005;6:1. doi: 10.1186/1468-6708-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpf R, Borggrefe M, Wolpert C. Clinical and molecular genetics of the short QT syndrome. Curr Opin Cardiol. 2008;23:192–198. doi: 10.1097/HCO.0b013e3282fbf756. [DOI] [PubMed] [Google Scholar]
- Schindler U, Betz E. Influence of severe hypercapnia upon cerebral cortical metabolism, CSF electrolyte concentrations and EEG in the cat. Bull Eur Physiopathol Respir. 1976;12:277–284. [PubMed] [Google Scholar]
- Schouten EG, Dekker JM, Meppelink P, Kok FJ, Vandenbroucke JP, Pool J. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84:1516–1523. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- Semmelroch M, Elwes RD, Lozsadi DA, Nashef L. Retrospective audit of postictal generalized EEG suppression in telemetry. Epilepsia. 2012;53:e21–24. doi: 10.1111/j.1528-1167.2011.03296.x. [DOI] [PubMed] [Google Scholar]
- Seyal M, Bateman LM, Albertson TE, Lin TC, Li CS. Respiratory changes with seizures in localization-related epilepsy: analysis of periictal hypercapnia and airflow patterns. Epilepsia. 2010;51:1359–1364. doi: 10.1111/j.1528-1167.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- Seyal M, Pascual F, Lee CY, Li CS, Bateman LM. Seizure-related cardiac repolarization abnormalities are associated with ictal hypoxemia. Epilepsia. 2011;52:2105–2111. doi: 10.1111/j.1528-1167.2011.03262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet. 2011;378:2028–2038. doi: 10.1016/S0140-6736(11)60176-1. [DOI] [PubMed] [Google Scholar]
- Surges R, Adjei P, Kallis C, Erhuero J, Scott CA, Bell GS, Sander JW, Walker MC. Pathologic cardiac repolarization in pharmacoresistant epilepsy and its potential role in sudden unexpected death in epilepsy: a case-control study. Epilepsia. 2010;51:233–242. doi: 10.1111/j.1528-1167.2009.02330.x. [DOI] [PubMed] [Google Scholar]
- Surges R, Strzelczyk A, Scott CA, Walker MC, Sander JW. Postictal generalized electroencephalographic suppression is associated with generalized seizures. Epilepsy Behav. 2011;21:271–274. doi: 10.1016/j.yebeh.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Symonds C. Some observations on the facilitation or arrest of epileptic seizures. In: Garland H, editor. Scientific aspects of neurology; Leeds neurological sciences colloquium, 1959–60. Livingstone, Edinburgh: 1961. pp. 142–152. [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, Kasischke KA, Nedergaard M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol. 2008;7:1021–1031. doi: 10.1016/S1474-4422(08)70202-3. [DOI] [PubMed] [Google Scholar]
- Valentine PA, Fremit SL, Teskey GC. Sensory stimulation reduces seizure severity but not afterdischarge duration of partial seizures kindled in the hippocampus at threshold intensities. Neurosci Lett. 2005;388:33–38. doi: 10.1016/j.neulet.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A, Stamm JS. Spreading cortical convulsions and depressions. J Neurophysiol. 1953;16:352–366. doi: 10.1152/jn.1953.16.4.352. [DOI] [PubMed] [Google Scholar]
- Yohai vj. High breakdown point and high efficiency robust estimates for regression. Annals of Statistics. 1987;15:642–656. [Google Scholar]