Abstract
Background
Extracellular recordings are used to define gastric slow wave propagation. Signal filtering is a key step in the analysis and interpretation of extracellular slow wave data; however, there is controversy and uncertainty regarding the appropriate filtering settings. This study investigated the effect of various standard filters on the morphology and measurement of extracellular gastric slow waves.
Methods
Experimental extracellular gastric slow waves were recorded from the serosal surface of the stomach from pigs and humans. Four digital filters: finite impulse response filter (0.05–1 Hz); Savitzky-Golay filter (0–1.98 Hz); Bessel filter (2–100 Hz); and Butterworth filter (5–100 Hz); were applied on extracellular gastric slow wave signals to compare the changes temporally (morphology of the signal) and spectrally (signals in the frequency domain).
Key Results
The extracellular slow wave activity/morphology is represented in the frequency domain by a dominant frequency and its associated harmonics in diminishing power. Optimal filters apply cutoff frequencies consistent with the dominant slow wave frequency (3–5cpm) and main harmonics (up to ~2Hz). Applying filters with cutoff frequencies above or below the dominant and harmonic frequencies was found to distort or eliminate slow wave signal content.
Conclusions and Inferences
Investigators must be cognizant of these optimal filtering practices when detecting, analyzing and interpreting extracellular slow wave recordings. The use of frequency domain analysis is important for identifying the dominant and harmonics of the signal of interest. Capturing the dominant frequency and major harmonics of slow wave is crucial for accurate representation of slow wave activity in the time domain. Standardized filter settings should be determined.
Keywords: Gastric electrical activity, signal processing, gastric dysrhythmia, electrogastrography
Introduction
Phasic gastric contractions are coordinated by slow wave activity, which is generated and propagated by the interstitial cells of Cajal (ICC) (1). The gastric slow wave frequency is species dependent, being near 3 cycles per minute (cpm) in humans and pigs (2, 3), and 5 cpm in dogs (4). Extracellular recordings are commonly used for evaluating normal and dysrhythmic patterns of gastric slow wave propagation (2–6).
Gastric slow wave signal content in extracellular recordings is an ensemble of slow transients and faster transients of higher frequency (‘harmonics’1) (7). Sources of noise include motion artifacts due to respiration/ventilation (~12cpm), power-line interference (~50/60 Hz), and other bioelectrical sources, notably cardiac potentials (~1 Hz) (7, 8). Signal filters are used to minimize these sources while optimizing the signal of interest. Furthermore, the use of filters and associated analysis are only as reliable as the quality of the original raw recording.
Very few studies have examined filtering methods for gastric extracellular recordings (9). A wide variety of approaches are currently in use, confounding attempts to compare results and signal quality across studies. In cardiac electrophysiology, by contrast, consensus filtering recommendations exist (10–12). Similarly, defining optimal filtering practices for gastric studies would support the ongoing development of extracellular techniques in basic and clinical motility science (2, 5, 13, 14). Slow wave filtering methods are also a focus of current controversy, following claims by Bayguinov et al that extracellular techniques, in general, cannot record slow waves (15). These authors proposed filtering in the range of 3–5 Hz to 100 Hz, (15), however, others have argued that these parameters would eliminate key signal content, distorting results (7, 8).
This study was performed to address these research questions by comparing digital filtering approaches for gastric extracellular signals. Appropriate filtering strategies are identified.
Material and Methods
Ethical approval was granted by our institutional and national review panels. Digital filters were evaluated on raw unipolar recordings acquired using the ActiveTwo system (Biosemi, The Netherlands), at a sampling frequency of 512 Hz. The data acquisition was performed using a large dynamic range (24 bit delta-sigma analog to digital convertor, resolution 31.2 nV) with no high pass filtering, and a low pass filter by the ADC’s decimation filter due to hardware bandwidth limitations (effective bandwidth from DC (0Hz) to 400Hz at -3dB). Recordings were taken from the gastric serosa of a pig and human using flexible arrays (16) according to our previously published methods (2, 3), and ten representative data segments were analyzed (855 s for pig, 500 s for human).
Four different filters with distinct specifications were identified from recent literature for comparison: Bandpass FIR (Finite impulse response) filter (0.05–1 Hz) (17, 18); SG (Savitzky-Golay) filter (low pass filter with cutoff frequency of 1.98 Hz) (9, 13); Bandpass Bessel filter (2–100 Hz) (15), and a Bandpass Butterworth filter (5–100 Hz) (15). These four filters were applied after the removal of baseline wander (via a moving median window of 20 seconds (9)) and notch filters to remove power line interference for consistent comparison. Data processing and analysis was performed in MATLAB v7.11 (Natick, Massachusetts).
After filtering, the resultant signals were evaluated in both the time and frequency domains. Two measures were used to quantify the filter effects: average slow wave amplitude in the time domain, and maximum spectral component in the frequency domain (computed via the Fourier transform). Amplitude in the time domain was computed by the difference between the minimum and maximum of a running window of two minutes and averaged. In the frequency domain, the spectral component with the highest amplitude was acquired. For statistical analyses, t-tests were performed between the amplitude and frequency of the baseline removed signal, and the filtered signals.
Results
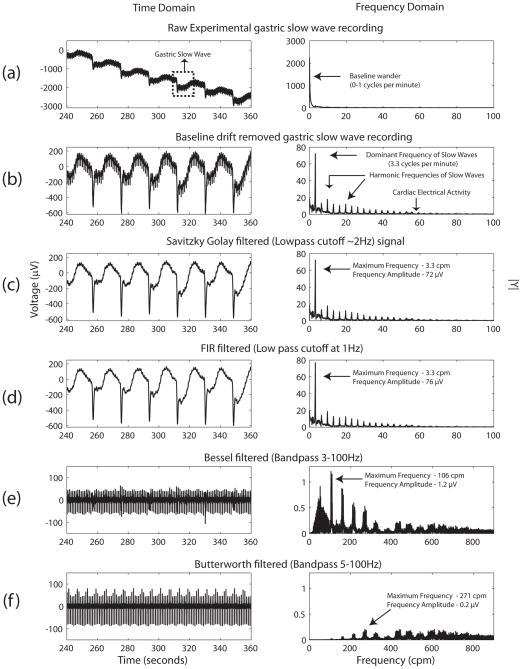
Figure 1 shows a typical human gastric extracellular slow wave recording with the application of the four filters and the subsequent outcomes in signal morphology and spectral components2. Table 1 presents the filtering results from all subjects.
Figure 1.
Application of various filters to a human extracellular in-vivo gastric serosal slow wave recording. The time domain signal (left column) and its corresponding frequency domain (right column, computed via a Fourier transform) are shown. (a) Raw in-vivo gastric slow wave recording. (b) The same signal after removal of baseline wander using a 20 second moving median filter. All of the remaining plots (in the time domain) are filtered from the baseline removed signal. (c) shows the application of a SG (Savitzky-Golay) filter, while (d) the use of a bandpass FIR filter (17, 18). (e) and (f) is the application of bandpass Bessel (3–100 Hz) and Butterworth (5–100 Hz) filter similar to that of Bayguinov et al (15). In the frequency domain, (a)–(d) are displayed in the 0–100 cycles per minute (cpm) range, while (e) and (f) are displayed in the 0–900 cpm range.
Table 1.
Differences in the signal properties in the time and frequency domain with the application of different filters for the representative signal shown in Figure 1. The two measure are: mean signal amplitude in the time domain (to the nearest μV), and mean maximum signal spectral component in the frequency domain (cpm).
| Pig | Human | Average | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Amplitude (μV) | MaxF (cpm) | Amplitude (μV) | MaxF (cpm) | Amplitude (μV) | MaxF (cpm) | Amplitude | MaxF | |
| Baseline removed | 920 | 3.64 | 673 | 3.28 | 796 | 3.46 | - | - |
| SG filtering | 887 | 3.64 | 549 | 3.28 | 719 | 3.46 | 0.571 | 1 |
| FIR filtering | 894 | 3.64 | 561 | 3.28 | 728 | 3.46 | 0.618 | 1 |
| Bessel filtered | 302 | 136.6 | 178 | 108.5 | 240 | 122 | 1×10−4 * | 1×10−5 * |
| Butter filtered | 52 | 300.8 | 183 | 542.5 | 118 | 422 | 3×10−5 * | 6×10−5 * |
MaxF – Maximum Frequency, SG – Savitzky-Golay, FIR – Finite impulse response
shows p values with very strong significance (p-value < 0.001) against the null hypothesis.
In the raw recordings (e.g., Figure 1(a)), the dominant frequency corresponded to the baseline wander, occupying the 0–1 cpm spectrum (0–0.167 Hz). Once baseline wander was removed, the dominant frequency that correlated to the known slow wave frequency became evident in the frequency domain. The pertinent frequencies that are present in the typical slow wave recording, which include the dominant frequency (~3 cpm or ~0.05 Hz) and its faster transients (harmonics), are predominately in the range of 2 Hz and below.
Figure 1(e) and (f) demonstrate that when filter specifications were not in the predominant frequency range of gastric slow waves, the signal integrity in both the time and frequency domain were noticeably impaired compared to the signals in Figure 1(a)–(d). With the SG filter and the FIR filter, where the filter specifications are in range of 0–2 Hz, the signal integrity changed little with the baseline removed signal (average amplitude: 719 μV and 728 μV vs 796 μV, p=0.571 and 0.618) (Table 1). By contrast, when the Bessel filter (3–100 Hz) and the Butterworth filter (5–100 Hz) were applied, the signal integrity was impaired to the baseline signal (average amplitude: 240 μV and 118 μV vs 725 μV; p < 0.001) (Table 1). Furthermore, the maximum frequency components of the Bessel filter and the Butterworth (100–800 cpm) were outside the range of the other filters (0–100 cpm).
Discussion
Appropriate filtering is critical to the analysis and interpretation of extracellular slow wave recordings. Two key aspects of extracellular signal filtering have been clarified by this study. Firstly, the extracellular slow wave potential is composed of a dominant frequency and its harmonics. Secondly, applying filters (digital or analog) above or below the dominant frequency and/or major harmonics of gastric slow waves will substantially impair the signal quality and integrity.
It is important to note that signal filters in general allow frequencies below or beyond their specified cutoff threshold (e.g., Figure 1). This is because filters do not have characteristics such as infinite roll-off rate and zero attenuation at the cut-off frequency. It is necessary for investigators to consider the balance between the inclusion and exclusion of signal frequencies and the preservation and distortion of signal morphology (9).
In electrocardiology there are established standards for data acquisition, including filter settings, and analysis methods (10–12). Similar standards have been set in the field of cutaneous gastric electrogastrography (19, 20). This standardization promotes best practices and enables consistent comparisons between studies. Similar considerations would benefit the gastric extracellular field, where a variety of filters are in current use. Daniel and Chapman previously commented in 1963 (21), that “Any system with a frequency response from DC to several hundred cycles per second would appear to be adequate to record accurately all of the slow waves …”. Based on the detailed analyses presented in this study of modern signal filters in gastric serosal extracellular recordings, similar conclusions can be drawn. More specifically, to accurately represent slow wave activity in the time domain, the dominant frequency (3–5 cpm) and its major harmonics must be preserved in the frequency domain. In human gastric dsyrhythmias, the slow wave activity is reported to be in the range of 0.5 to 10 cycles per minute (13, 22), and the filter range of 2 Hz and below would still allow for precise slow wave signal representation.
Caution is necessary when interpreting signals filtered with settings outside of these parameters. For example, important morphological features such as the slow wave recovery phase may be eliminated. The findings of this study also disprove recent claims by Sanders et al that “Low pass filtering <1 Hz would attenuate … the signals most likely to be resolved by extracellular recordings” (23). By contrast, high-pass filtering of >1 Hz has the potential to severely distort the underlying signals. Improper filtering may therefore partly explain the results recently presented by Bayguinov et al (using 2–200 and 5–200 Hz filters), who concluded that extracellular slow wave recordings are generally impossible (15). Moreover, applying a low pass filter, in the order of 2 Hz, would likely help to reduce high-frequency motion artifacts of the type presented by Bayguinov et al (15).
There are many challenges in order to prescribe a universal guide for data acquisition and analysis, especially due to differing signals of interest, electrode design, electrode types, type of recording (unipolar or bipolar) and recording hardware. Regardless, a uniform approach to data acquisition and basic analysis should be established. This study has identified that the frequency range of 0–2Hz, in the frequency domain, relates to the majority of extracellular gastric slow wave signal content.
Supplementary Material
Acknowledgments
Funding
This work was funded by the National Institute of Health (R01 DK64775) and the New Zealand Health Research Council.
We thank Linley Nisbet for her expert technical assistance.
Footnotes
refer Appendix A for further explanation of ‘harmonics’; Supporting Information
refer Appendix B for comparisons of filters in other human and pig in-vivo extracellular gastric slow wave recordings; Supporting Information
Disclosures
The authors have no competing interests.
Author contributions
NP designed the study, analyzed the data and drafted the manuscript. GOG, PD and LKC assisted in the design, assisted with experiments, and critically reviewed the manuscript.
References
- 1.Huizinga JD, Lammers WJEP. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1–G8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 2.O’Grady G, Du P, Cheng LK, et al. Origin and propagation of human gastric slow-wave activity defined by high-resolution mapping. Am J Physiol Gastrointest Liver Physiol. 2010;299:G585–G592. doi: 10.1152/ajpgi.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egbuji JU, O’grady G, Du P, et al. Origin, propagation and regional characteristics of porcine gastric slow wave activity determined by high-resolution mapping. Neurogastroenterol Motil. 2010;22:e292–e300. doi: 10.1111/j.1365-2982.2010.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lammers WJEP, Ver Donck L, Stephen B, Smets D, Schuurkes JAJ. Origin and propagation of the slow wave in the canine stomach: the outlines of a gastric conduction system. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1200–G1210. doi: 10.1152/ajpgi.90581.2008. [DOI] [PubMed] [Google Scholar]
- 5.Lammers WJEP, Ver Donck L, Stephen B, Smets D, Schuurkes JAJ. Focal Activities and ReEntrant Propagations as Mechanisms of Gastric Tachyarrhythmias. Gastroenterology. 2008;135:1601–1611. doi: 10.1053/j.gastro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez WC. The Electrogastrogram and what is shows. J Am Med Assoc. 1922;78:1116–1119. [Google Scholar]
- 7.O’Grady G. Gastrointestinal extracellular electrical recordings: fact or artifact? Neurogastroenterol Motil. 2012;24:1–6. doi: 10.1111/j.1365-2982.2011.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakayama S. Frequency analysis may distinguish the effects of calcium antagonists on mechanical and electrical activity. Neurogastroenterol Motil. 2012;24:397–397. doi: 10.1111/j.1365-2982.2012.01882.x. [DOI] [PubMed] [Google Scholar]
- 9.Paskaranandavadivel N, Cheng LK, Peng D, O’Grady G, Pullan AJ. Improved signal processing techniques for the analysis of high resolution serosal slow wave activity in the stomach. Conf Proc IEEE Eng Med Biol Soc. 2011:1737–1740. doi: 10.1109/IEMBS.2011.6090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breithart G, Cain ME, El-Sherif N, et al. Standards for analysis of ventricular late potentials using high resolution or signal-averaged electrocardiography. Eur Heart J. 1991;12:473–480. doi: 10.1093/oxfordjournals.eurheartj.a059926. [DOI] [PubMed] [Google Scholar]
- 11.Kligfield P, Gettes LS, Bailey JJ, et al. Recommendations for the Standardization and Interpretation of the Electrocardiogram. Circulation. 2007;115:1306–1324. doi: 10.1161/CIRCULATIONAHA.106.180200. [DOI] [PubMed] [Google Scholar]
- 12.Kossmann CE, Brody DA, Burch GE, et al. Recommendations for Standardization of Leads and of Specifications for Instruments in Electrocardiography and Vectorcardiography. Circulation. 1967;35:583–602. doi: 10.1161/01.cir.35.3.583. [DOI] [PubMed] [Google Scholar]
- 13.O’Grady G, Angeli TR, Du P, et al. Anormal initiation and conduction of slow wave activity in gastroparesis defined by high-resolution electrical mapping. Gastroenterology. 2012;143(3):589–98. doi: 10.1053/j.gastro.2012.05.036. e1-3 http://www.ncbi.nlm.nih.gov/pubmed/22643349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakayama S, Ohishi R, Sawamura K, Watanabe K, Hirose K. Microelectrode array evaluation of gut pacemaker activity in wild-type and mice. Biosens Bioelectron. 2009;25:61–67. doi: 10.1016/j.bios.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Bayguinov O, Hennig GW, Sanders KM. Movement based artifacts may contaminate extracellular electrical recordings from GI muscles. Neurogastroenterol Motil. 2011;23:1029–e1498. doi: 10.1111/j.1365-2982.2011.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du P, O’Grady G, Egbuji J, et al. High-resolution mapping of in-vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: methodology and validation. Ann Biomed Eng. 2009;37:839–846. doi: 10.1007/s10439-009-9654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JDZ, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401–409. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- 18.Wang ZS, Elsenbruch S, Orr WC, Chen JDZ. Detection of gastric slow wave uncoupling from multi-channel electrogastrogram: validations and applications. Neurogastroenterol Motil. 2003;15:457–465. doi: 10.1046/j.1365-2982.2003.00430.x. [DOI] [PubMed] [Google Scholar]
- 19.Koch KL, Stern RM. Handbook of electrogastrography. Oxford University Press; USA: 2004. [Google Scholar]
- 20.Verhagen MAMT, Van Schelven LJ, Samsom M, Smout AJPM. Pitfalls in the analysis of electrogastrographic recordings. Gastroenterology. 1999;117:453–460. doi: 10.1053/gast.1999.0029900453. [DOI] [PubMed] [Google Scholar]
- 21.Daniel E, Chapman K. Electrical activity of the gastrointestinal tract as an indication of mechanical activity. Dig Dis Sci. 1963;8:54–102. doi: 10.1007/BF02233560. [DOI] [PubMed] [Google Scholar]
- 22.Koch KL. The electrifying stomach. Neurogastroenterol Motil. 2011;23:815–818. doi: 10.1111/j.1365-2982.2011.01756.x. [DOI] [PubMed] [Google Scholar]
- 23.Sanders KM, Hennig GW, Bayguinov O. Response to Dr Nakayama letter. Neurogastroenterol Motil. 2012;24:398–398. [Google Scholar]
- 24.Ng J, Goldberger JJ. Understanding and interpreting dominant frequency analysis of AF electrograms. J Cardiovasc Electrophysiol. 2007;18:680–685. doi: 10.1111/j.1540-8167.2007.00832.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.