Abstract
Bone marrow-derived cells have a potent impact on the formation and progression of tumor metastasis. This study demonstrates that bone marrow directly promotes metastasis to distant sites from tumor cells residing in the bone marrow in multiple types of tumors and multiple tumor cell lines. The bone marrow environment requires less tumor cells for inducing distant metastasis and overcomes the inhibition of metastasis resulting from engineering the tumor cells with reporter genes. This discovery provides an effective approach to generate spontaneous-like metastatic tumor models which will satisfy the urgent need for studying metastasis biology and discovering novel therapeutics.
Keywords: Metastasis, Bone Marrow, Mouse models, Metastatic Cascade
1. Introduction
Metastatic disease remains the major cause of mortality in patients with cancer [1]. Unfortunately, for most tumor types, progress in developing effective therapeutics remains stalled due to the paucity of available bona fide metastatic tumor models [2]. Due to the complex nature of the metastatic cascade, this issue of having adequate preclinical models for the study of metastasis biology is not easily overcome [3]. Recent studies have shown the importance of bone marrow-derived cells, such as hematopoietic progenitor cells [4; 5; 6] and myeloid cells [7; 8], for preparing the pre-metastatic niche and thereby enhancing metastatic progression. Here, we show that directly seeding tumor cells in the bone marrow (BM) promotes distant metastasis in several cell lines in immunocompetent, syngeneic mice, providing a new models for the study of metastasis and therapeutics.
2. Materials and Methods
2.1 Animal Models and Inoculations
Six- to eight-week old A/J (Jackson Laboratory), C57Bl/6, and Balb/c (NCI) mice used in these studies were maintained under NIH guidelines, and all animal protocols have been reviewed and approved by the Institutional Animal Care and Use Committee at the University of Texas MD Anderson Cancer Center. The cancer cell lines CT26, 4T1-Luc and FBL3 (ATCC) cells were maintained in DMEM with 10% FBS and 1% Penn/Strep, and the TBJ (NIH) cells were maintained in RPMI-1640 with 10% FBS and 1% Penn/Strep. Confluent CT26, 4T1-Luc, and TBJ cells were dissociated from the plates with Non-enzymatic PBS-based Dissociation buffer (Gibco) for 5 minutes, and then suspended in 15, 30, or 100 μL PBS for intraosseous (i.o.), subcutaneous (s.c.), or intravenous (i.v.)/intraperitoneal (i.p.), respectively. The cellular concentrations for these inoculations are detailed in the Results and Discussion sections. All animal experiments were performed in syngeneic, immunocompetent mouse models. Intraosseous inoculations were performed by inserting a 27 gauge, ½-inch needle insulin syringe (BD Bioscience) directly into the right tibia, and then suppressing the plunger on the syringe (Fig. 1A). Subcutaneous inoculations were performed by injecting the cell suspension into the flank 1 cm craniodorsal from the tail, intravenous inoculations were performed by injecting the cells suspension into the tail vein, and intraperitoneal inoculations were performed by injecting the cell suspension into the peritoneal cavity.
Fig. 1.
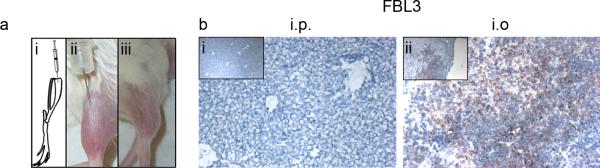
Metastatic development following intraosseous inoculation of FBL3 leukemia cells. a Tumor cell suspensions are inoculated directly into the tibia of syngeneic mice (i and ii), and an initial tumor develops at the inoculation site (iii). b Liver tumors did not develop in C57Bl/6 mice inoculated with 5,000 FBL3 cells via i.p. injections (i) while several tumors developed in the livers inoculated i.o. (ii). All sections were blotted with Ki67 and counterstained with Harris' Hemotoxylin. All field-of-views are representative of sections from all mice in each group (n=3), the larger panels are 10× magnification, and the insets are 4× magnification.
2.2 Metastatic Analyses
In situ images of metastasis were captured with a Panasonic Lumix SMC-ZS3 digital camera. Mice were euthanized on day 35 after inoculation or when any mouse in that group became moribund. Livers were placed in OCT (Sakura Finetek) and frozen in liquid nitrogen, and lungs were inflated with 0.5× OCT/0.5× PBS suspension via an 18 gauge, ½-inch needle on a 5 mL syringe (BD Bioscience), and then placed in OCT and frozen. The frozen tissue sections were either stained with H&E or blotted with anti-mouse Ki67 antibody (eBioscience) at a dilution of 1:800 and stained with a standard DAB protocol. For bioluminescent imaging of firefly luciferase-expressing 4T1 tumors, animals were administered 3.75 mg recombinant D-luciferin (Fisher) in 75 mL saline, and then 10 to 15 min later, the mice were anesthetized with 2.5% IsoFlo. The images were captured with the Xenogen IVIS100 system with the following settings: Bin=M(8), FOV 20, f1, 30s, Em filter=open, camera IVIS13204, and EEV. Bioluminescent data was analyzed using the Living Image Xenogen Software.
2.3 Surgical Removal of Initial Tumor
Under 2.5% IsoFlo anesthesia and aseptic conditions, initial tumors which formed following i.o. inoculation of 4T1-luc cells were removed by surgically amputating the limb below the knee. The lower limb was shaved with clippers and the area was disinfected with betadine. A single quarter-inch incision was made with surgical scissors to expose the femoral artery which was then closed with a single suture halfway up the femur. The lower limb was the removed by a single cut with surgical scissors above the knee joint. The wound was closed with two Autoclip wound clips, and then Buprenorphine [0.05–1 mg/kg SC q 6–12 hr] was administered via i.p. injections. Seven to ten days later, the mice were anesthetized and the wound clips removed.
2.4 Statistical Analyses
The bioluminescence measurements from 4T1-luc metastasis were analyzed via one-way ANOVA with Tukey's multiple comparison post-hoc test to compare all groups (Figs. S3 and S4). The data was first transformed to the natural log for analyses due to significantly unequal variances. All analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software).
3. Results
3.1 Intraosseous inoculation of FBL3 leukemia cells promotes metastatic tumor development
To determine the effect of the BM environment on promoting distant metastasis, different tumor cell lines were injected either via ectopic, i.e., subcutaneous (s.c.), the experimental metastatic method, e.g. intravenous (i.v.) or intraperitoneal (i.p), or into the BM of the tibia, i.e., intraosseous (i.o.). For i.o. injections, the tibia was punctured through the top of the bone (Fig. 1ai), the cell suspension was injected into the BM (Fig. 1aii), and then a primary tumor developed in the tibia (Fig. 1aiii).
The first cell line tested was the Friend virus-induced erythroleukemia FBL3, a cell line used in studies of leukemia [9]. Leukemias arise from hematopoietic cells; therefore, the BM represents an orthotopic site [10]. To induce disseminated growth of FBL3 cells, previous studies used i.p. injections of up to five million cells [11; 12]. Therefore, we compared the metastatic development between i.p. and i.o. injected cells at a reduced cell number—only 5,000 cells per mouse (n=5). At this low dose, none of the i.p. inoculated mice developed metastatic tumors in the livers by day 35 (Fig. 1bi), and all of the i.o. inoculated mice developed metastatic tumors in the livers (Fig. 1bii). After reducing the number of inoculated cells to 1,000, half of the i.o.-inoculated mice (n=4) developed metastasis in the liver by day 35 (Fig. S1). This result demonstrates that the BM microenvironment promotes leukemia metastasis and demonstrates that as few as 1,000 cells in the BM environment can cause leukemia metastasis.
3.2 Seeding TBJ neuroblastoma or CT26 colon carcinoma cells into the BM promotes metastatic development
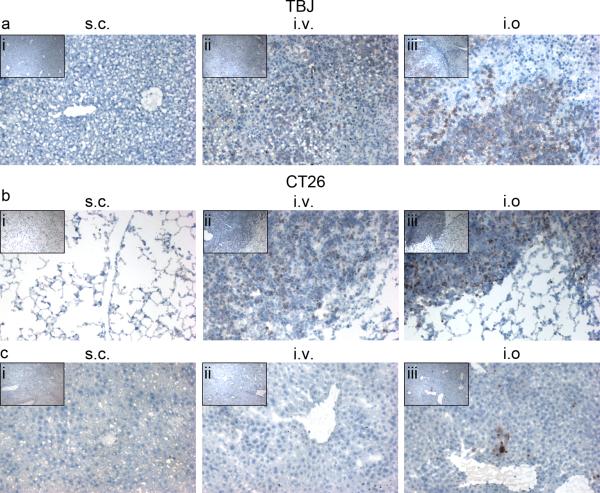
We also compared the metastatic ability of TBJ (neuroblastoma) and CT26 (colon carcinoma) cells inoculated either s.c., i.v., or i.o. (n=5) in syngeneic, immunocompetent mice (A/J and Balb/c, respectively). Although TBJ cells have been reported to spontaneously metastasize from s.c. implanted tumors [13], no metastatic tumors were seen in the livers or any other organs of mice 35 days after inoculation with 200,000 TBJ cells (Figs. 2ai and S2ai); however, i.v. and i.o. inoculation of only 50,000 cells resulted in metastasis to the liver (Figs. 2aii,iii) and ovary (Figure S2aii,iii) by day 18. Surprisingly, liver metastases developed following i.o. inoculation of only 10,000 TBJ cells (Fig. S2b). Likewise in the CT26 model, metastatic tumors did not develop in the lungs or livers of mice 35 days after being inoculated s.c. with 200,000 cells (Figs. 2bi,ci). Interestingly, tumors did not develop in the livers of mice inoculated i.v. with 50,000 cells (Fig. 1cii) although these mice did develop lung metastasis (Fig. 2bii) by day 22. Surprisingly, mice injected i.o. with 50,000 cells developed metastatic tumors in the lungs and livers (Figs. 2biii,ciii). Interestingly, i.o. inoculations of the human cell lines HCT-116, MDA-MB453, and PC3-M (100,000 cells) into athymic nude mice did not result in the development of any metastatic tumors (data not shown), suggesting that an immunocompetent BM environment is required to induce the metastasis. This hypothesis requires further experimental testing.
Fig. 2.
Metastatic development following intraosseous inoculation of TBJ neuroblastoma or CT26 colon carcinoma cells into the BM. a Liver tumors did not develop in A/J mice inoculated s.c. (i) with 200,000 TBJ cells while metastatic liver tumors did develop in mice injected either i.v. (ii) or i.o. (iii) with only 50,000 TBJ cells. b Lung tumors did not develop in mice inoculated s.c. with 200,000 CT26 cells while several tumors developed in the lungs of mice injected either i.v. (ii) or i.o. (iii) with only 50,000 CT26 cells. c In the same CT26-inoculated mice (b), metastatic liver tumors did not develop in either s.c. (i) or i.v. (ii) inoculated mice, but they did develop in i.o. inoculated mice (iii). All sections were blotted with Ki67 and counterstained with Harris' Hemotoxylin. All field-of-views are representative of sections from all mice in each group (n=3), the larger panels are 10× magnification, and the insets are 4× magnification.
3.3 Intraosseous inoculations recapitulate the metastatic ability of 4T1 breast adenocarcinoma cells transfected with luciferase reporter gene
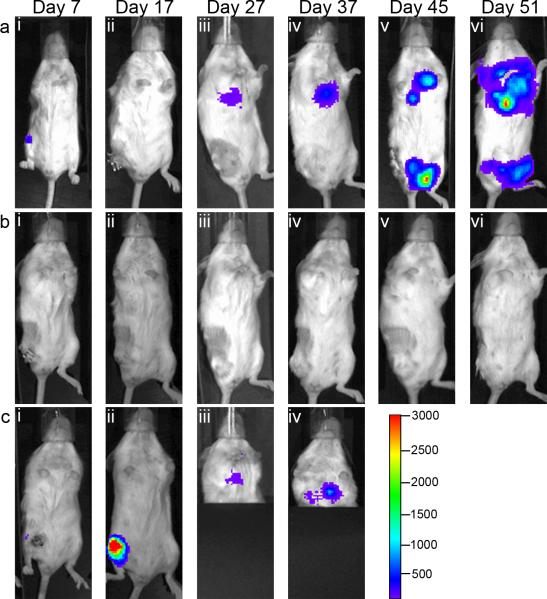
Non-luciferase expressing 4T1 cells have been widely reported to metastasize very aggressively following s.c. and i.v. inoculations [14], but the firefly luciferase gene has been shown to reduce tumorigenicity [15]. To determine whether seeding 4T1-luc in the BM environment reverses this inhibitory effect, this cell line was inoculated s.c., i.v., and i.o. .(100,000, 10,000, and 100,000 cells, respectively), and metastatic lung development was analyzed over a course of 51 days. By day 7, the initial tumors in from i.o. and s.c. inoculated mice were detectable (Figs. 3bi and 3di, respectively). The bioluminescence from these tumors continued to increase; however, ulcerated tumor-burdens mandated euthanasia of s.c.-inoculated mice by day 27. At that point, no bioluminescence was detected in the thoracic cavity (Fig 3cii) and no microscopic metastases were found in the lungs of any s.c.-inoculated mice (Fig 3ciii). By day 27, only i.o.-inoculated mice started to have detectable metastatic tumors in the thoracic cavity (Fig. 3Bii), and this signal increased by day 37 (Fig. 3biii; the lower half of the body was covered to avoid interference from the initial tumor). At this point, i.o.- inoculated mice had to be euthanized due to large tumor burdens (Fig. 3div). The i.v.-inoculated mice were imaged for 51 days and no luminescence was detected (Fig. 3a).
Fig. 3.
Recapitulation of metastatic potential following intraosseous inoculation. Recapitulation of metastatic potential following intraosseous inoculation. Intraosseous inoculations of 10,000 4T1-luc cells results in lung metastasis compared to s.c. and i.v. inoculations with 100,000 cells. On the specified days after inoculation of 4T1-luc cells, bioluminescent images were taken of mice inoculated i.v. (a), i.o. (b), or s.c. (c). A, No metastatic tumors were detectable up to 51 days after inoculation. b, The initial tumor was detectable in the tibia of i.o.-inoculated mice by Day 7 (bi) and metastatic tumors in the thoracic cavity were detectable by day 27 (bii) and the signal increased to Day 37 (biii) when mice had to be euthanized due to tumor burden (diii,iv). c, s.c.-inoculated mice developed detectable subcutaneous tumors by Day 7 (di) bud did not develop detectable lung metastases on Day 27 (ci,ii) when ulceration and ectopic tumor volume mandated euthanasia 27. Ki67 staining of lung tissues from these mice showed that microscopic metastases were not present (ciii). d, The initial tumors that formed from s.c. (di,ii) and i.o. inoculations (diii,iv). The color scale in row c is the scale for all bioluminescent images in this figure. The color range for panels a through c represents from 3,000 to 100 with dashes every 500 luminescent counts, and the range for panel d represents from 30,000 to 1,000 with dashes every 5,000 luminescent counts. All images are representative of mice from each group.
In a separate experiment, mice from each group were sacrificed on day 25 and the thoracic cavity exposed to identify if there were metastatic tumors present. There were very low levels of luciferase activity in both s.c. and i.v. inoculations, yet the i.o. inoculated mice had a significantly higher level of luciferase-expressing metastases in the lungs (Fig S3; the initial tumor was removed prior to imaging to avoid quenching the luminescent signal). Since no microscopic metastases were detected in the s.c.-inoculated mice on day 27 and no i.v.-inoculated mice developed tumors through 51 days, these low levels were most likely artifacts. These data show that i.o. injection of tumor cells is a distinct inoculation type and i.o. inoculations can recapitulate the loss of metastatic potential due to the introduction of plasmids, such as luciferase.
3.4 Intraosseous inoculations are a distinct inoculation route which results in an initial tumor and the development of metastatic tumors
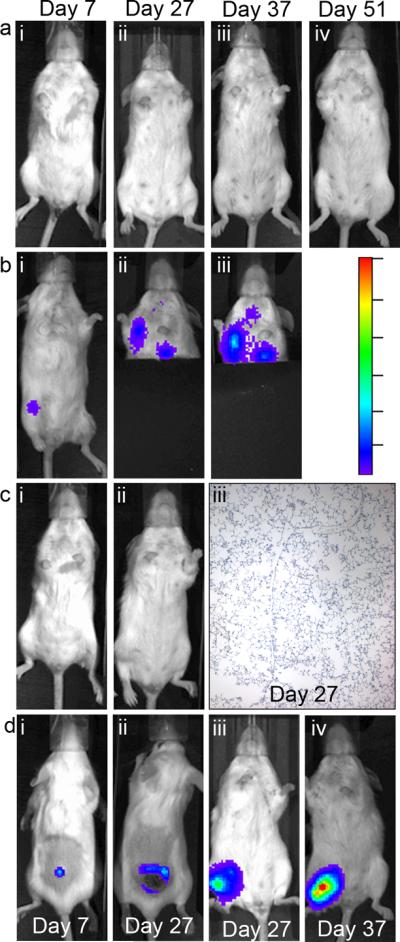
Though the results presented above clearly demonstrate that the BM promotes tumor metastasis, one argument against these data would be that i.o.-induced metastases results from leakage of the cells into the bloodstream during injection, thereby being an i.v. inoculation. To address this concern, we examined whether i.o.-induced metastasis requires an initial tumor to form prior to developing metastatic tumors by surgically removing the inoculated bone 3 or 7 days after inoculation (Fig. 4). As expected, surgically removing tumors after only 3 days and before an initial tumor was detectable completely abolished all metastatic tumor development (Fig. 4b). On the other hand, mice that developed detectable tumors prior to surgery (Day 7) and those mice that only received a sham surgery developed detectable metastatic tumors by day 27 (Figs. 4aiii and 4ciii) and the bioluminescence continued to increase over time (Figs. 4aiv–vi and 4civ; sham mice had to be euthanized on day 37 due to tumor volume). These results show that the metastasis develops as a result of an initial tumor mass and the completion of the metastatic cascade, rather than the cells immediately leaking into the rich vascular network of the BM environment. Furthermore, these data show that this surgical removal model can be used to mimic clinical treatments in that the initial solid tumor is removed yet the metastatic tumors are already established.
Fig. 4.
Development of metastasis following surgical removal of initial tumors. Intraosseous-induced metastasis requires the formation of an initial tumor to develop metastasis. Mice inoculated with 10,000 4T1-luc cells underwent either a sham surgery (a) or surgical removal of the initial tumor on days 3 (b) and 7 (c). The luciferase signals from the i.o.-inoculated tumors were detectable by day 7 (ai) and the signal from the initial tumor was completely abolished after surgery. By day 27 after inoculations, both mice that received sham surgeries and those with surgeries on day 7 developed detectable metastases in the thoracic cavity (aiii and ciii) and these signals continued to increase through day 51 (aiv–vi). Mice that receieved surgery after only 3 days did not develop tumors throughout the experiment (c). The color range is in units of luminescent counts. All images are representative of mice from each group.
4. Discussion
These results demonstrate the BM environment of syngeneic, immunocompetent mice promotes the development of distant metastases following inoculation into the BM. This effect was clearly demonstrated in cell lines from four different tumor types in 3 different mouse strains (Figs. 1–4). Importantly, initial tumor development and distant metastases can be induced by seeding as few as 1,000 FBL3 cells into the BM environment (Fig. S1). Furthermore, the BM environment can recapitulate the metastatic potential of tumor cell lines that have lost this potential due to the introduction of reporter genes (Fig. 3). Lastly, we have proven that this is a distinct type of inoculation and not due to leaking of cells into the bloodstream following inoculation into the BM (Fig. 4).
Multiple recent reports have shown that bone marrow derived-cells, specifically mesenchymal stem cells (MSC), have varying effects on different tumor cell lines. Several reports have shown that MSC promote the engraftment and vascularization of primary tumor development [16; 17; 18] and increase the metastatic ability of tumor cells [4; 5; 19; 20]. A recent study showed that co-injection of human tumor grafts with human MSC resulted in increased tumor growth compared to the same grafts injected without human MSC [21]. Further, the MSC did not select the more aggressive tumors, but instead they improved the vascularization of the tumor. When human tumor cells were injected into the bone marrow of a xenogeneic, immundeficient nude mouse model, neither primary nor metastatic tumors developed. These results suggest that that the MSC must be syngeneic or immunocompetent to increase vascularization, improve tumor growth, and promote distant metastases.
Another potential mechanism is through cells that originate in and return to the bone marrow once matured. A suspected player in this role is regulatory T cells (Tregs) [22], and the bone marrow is a known reservoir for CD4+/CD25+ Tregs. Current reports demonstrate that infiltration of Tregs into tumors is a poor prognostic marker, and depletion of CD4+/CD25+ Tregs can suppress the development of pulmonary metastases in the 4T1 model [23]. Tregs can promote the progression of tumor growth by suppressing the native immune response [22], but recent studies have shown that Tregs can promote metastasis through RANKL-mediated repression of maspin, a metastatic inhibibitor [24]. Since the bone marrow acts as a reservoir for mature CD4+/CD25+ Tregs [25], intraosseous inoculations into the bone marrow may stimulate the growth of the initial tumor through immunosuppression and promote metastasis through RANKL-induced maspin repression.
Therapies that reduce the tumor volume of implanted primary tumors, either orthotopic or ectopic, do not correlate to the effects of these therapies on tumor growth at metastatic sites as is seen in the clinic [2]. Also, genetically engineered models only focus on a single genetic background while most cancers harbor mutations in a multitude of genes [3]. Additionally, experimental metastatic models are initiated with a single bolus intravenous injection of a large amount of tumor cells which deviates from actual metastatic development in that a large single dose of tumor cells is released into the bloodstream [2] and the first four steps of the metastatic cascade (primary neoplasm, vascularization, invasion, and embolism) are omitted [3]. While experimental metastatic models do hold value for preliminary therapeutic and mechanistic studies, the most valuable models will be those that begin with an initial neoplasm with the subsequent development of metastasis, as occurs in human tumors. Although, surgical removal of a primary orthotopic tumor is the ideal model for preclinical studies, this intraosseous model is a valuable alternative for those tumor cell lines that do not spontaneously metastasize from the orthotopic site. Likewise, intraosseous inoculation models may be a helpful tool for further clarification of the effects of bone marrow-residing cells.
Supplementary Material
Acknowledgments
Role of the Funding Source This research was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672 and NIH grant RO1CA120895. The funding sources did not have any roles in designing, implementing, or preparing these studies or manuscript.
Financial Support: This research is supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672 and NIH grant RO1CA120895.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement The authors have no conflicts of interest to disclose.
References
- [1].Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ellis LM, Fidler IJ. Finding the tumor copycat. Therapy fails, patients don't. Nat Med. 2010;16:974–975. doi: 10.1038/nm0910-974. [DOI] [PubMed] [Google Scholar]
- [3].Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11:135–141. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol Med. 2007;13:72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- [5].Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Taylor M, Rossler J, Geoerger B, Laplanche A, Hartmann O, Vassal G, Farace F. High levels of circulating VEGFR2+ Bone marrow-derived progenitor cells correlate with metastatic disease in patients with pediatric solid malignancies. Clin Cancer Res. 2009;15:4561–4571. doi: 10.1158/1078-0432.CCR-08-2363. [DOI] [PubMed] [Google Scholar]
- [7].Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, Bou-Reslan H, Kallop D, Weimer R, Ludlam MJ, Kaminker JS, Modrusan Z, van Bruggen N, Peale FV, Carano R, Meng YG, Ferrara N. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107:21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- [9].Rosenfeld C, Cheever MA, Gaiger A. WT1 in acute leukemia, chronic myelogenous leukemia and myelodysplastic syndrome: therapeutic potential of WT1 targeted therapies. Leukemia. 2003;17:1301–1312. doi: 10.1038/sj.leu.2402988. [DOI] [PubMed] [Google Scholar]
- [10].Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22:941–950. doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Messer RJ, Dittmer U, Peterson KE, Hasenkrug KJ. Essential role for virus-neutralizing antibodies in sterilizing immunity against Friend retrovirus infection. Proc Natl Acad Sci U S A. 2004;101:12260–12265. doi: 10.1073/pnas.0404769101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlen C, Greenberg PD. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- [13].Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cutrera J, Dibra D, Xia X, Hasan A, Reed S, Li S. Discovery of a linear peptide for improving tumor targeting of gene products and treatment of distal tumors by IL-12 gene therapy. Mol Ther. 2011;19:1468–1477. doi: 10.1038/mt.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brutkiewicz S, Mendonca M, Stantz K, Comerford K, Bigsby R, Hutchins G, Goebl M, Harrington M. The expression level of luciferase within tumour cells can alter tumour growth upon in vivo bioluminescence imaging. Luminescence. 2007;22:221–228. doi: 10.1002/bio.953. [DOI] [PubMed] [Google Scholar]
- [16].Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- [17].Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- [18].Ame-Thomas P, Maby-El Hajjami H, Monvoisin C, Jean R, Monnier D, Caulet-Maugendre S, Guillaudeux T, Lamy T, Fest T, Tarte K. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007;109:693–702. doi: 10.1182/blood-2006-05-020800. [DOI] [PubMed] [Google Scholar]
- [19].Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the `pre-metastatic niche': within bone and beyond. Cancer Metastasis Rev. 2006;25:521–529. doi: 10.1007/s10555-006-9036-9. [DOI] [PubMed] [Google Scholar]
- [20].Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- [21].Derose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, Factor R, Matsen C, Milash BA, Nelson E, Neumayer L, Randall RL, Stijleman IJ, Welm BE, Welm AL. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17:1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Koh BI, Kang Y. The pro-metastatic role of bone marrow-derived cells: a focus on MSCs and regulatory T cells. EMBO reports. 2012;13:412–422. doi: 10.1038/embor.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hong H, Gu Y, Zhang H, Simon AK, Chen X, Wu C, Xu XN, Jiang S. Depletion of CD4+CD25+ regulatory T cells enhances natural killer T cell-mediated anti-tumour immunity in a murine mammary breast cancer model. Clinical and experimental immunology. 2010;159:93–99. doi: 10.1111/j.1365-2249.2009.04018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.