Abstract
Background
Autoimmune gastrointestinal dysmotility (AGID) is a limited form of dysautonomia. The only proven effector to date is IgG specific for ganglionic nicotinic-acetylcholine receptors containing α3 subunits (α3*-nAChR). Rabbits immunized with recombinant α3-polypeptide produce α3*-nAChR autoantibodies, and profound AGID ensues. Human and rabbit α3*-nAChR-specific-IgGs induce transient hypomotility when injected into mice. Here we describe success and problems encountered inducing gastrointestinal hypomotility in mice by active immunization.
Methods
We repeatedly injected young adult mice of seven different strains susceptible to autoimmunity (spontaneous diabetes or neural antigen immunization-induced myasthenia gravis or encephalomyelitis) with: i) α3-polypeptide, intradermally, or ii) live α3*-nAChR-expressing xenogeneic cells, intraperitoneally. We measured serum α3*-nAChR-IgG twice monthly, and terminally assessed blue dye gastrointestinal transit, total small intestinal α3*-nAChR content (radiochemically) and myenteric plexus neuron numbers (immunohistochemically, ileal-jejunal whole-mount preparations).
Key Results
Standard cutaneous inoculation with α3-polypeptide was minimally immunogenic, regardless of dose. Intraperitoneally-injected live cells were potently immunogenic. Self-reactive α3*-nAChR-IgG was induced only by rodent immunogen; small intestinal transit slowing and enteric α3*-nAChR loss required high serum levels. Ganglionic neurons were not lost.
Conclusions & Inferences
AGID is inducible in mice by active immunization. Accompanying enteric α3*-nAChR reduction without neuronal death is consistent with an IgG-mediated rather than T cell-mediated pathogenesis, as is improvement of symptoms in patients receiving antibody-depleting therapies.
Keywords: autoimmune dysmotility, dysautonomia, ganglionopathy, slow transit
Autoimmune gastrointestinal dysmotility (AGID) is a limited dysautonomia, occurring idiopathically and in paraneoplastic context.1-3 Onset is typically subacute, with early satiety, abdominal pain, nausea, vomiting, weight loss and slow transit. Autoimmune serology aids the diagnosis. Autoantibodies specific for intracellular antigens are surrogate markers of peptide-specific T cell-mediated effector mechanisms. Anti-neuronal nuclear-type 1 autoantibody (ANNA-1/anti-Hu]) and collapsin response-mediator protein antibody ([CRMP]-5) both predict underlying lung carcinoma or thymoma.1, 4 Autoantibodies specific for extracellular domains of plasma membrane antigens have pathogenic potential2, 3, 5. Symptoms improve with antibody-depleting therapies (plasma exchange, B lymphocyte-depleting-IgG or high dose intravenous immune globulin).5-7 Specificities include ganglionic-type (α3*) and muscle-type (α1*) nicotinic acetylcholine receptor (nAChR) and neuronal voltage-gated calcium channel (N-type) or potassium channel-complex.5, 8 Most frequently identified is α3*-nAChR–IgG, the only autoantibody presently proven to cause AGID.5, 9 Rabbits immunized with human α3-polypeptide (extracellular residues 1-205) readily produce autoantibody, and AGID signs parallel serum α3*-nAChR-IgG levels.10 Immune serum IgG (rabbit or human) transfers transient gastrointestinal hypomotility to mice.9 We now report AGID induction by actively immunizing mice.
MATERIALS AND METHODS
Cell lines and α3-nAChR fusion protein
Human neuroblastoma cells IMR-32 (American Type Culture Collection [ATCC]) and SHSY-5Y (Sloan Kettering) were maintained in Gibco minimal essential medium with 10% FBS and 1% nonessential amino acids and Eagle MEM/Ham F12 medium (1:1, supplemented with 15% FBS). Human embryonic kidney cells (HEK-293, ATCC) were maintained in Dulbecco MEM (Gibco) with 10% bovine calf-serum. The stably-transfected HEK-293 line KXα3ß4R2 was derived and maintained as described.11 Recombinant human α3-polypeptide (residues 1-205 fused to GST) was produced from cDNA derived from IMR-3210.
Native α3*-nAChR preparation
Post-nuclear membrane fractions (isolated by differential centrifugation of homogenized IMR-32 cells grown subcutaneously in athymic nude mice and from cultured KXα3ß4R2 cells) were extracted in buffer (10 mM Na3PO4, 1 mM EDTA, 100 mM NaCl, 0.02% NaN3, pH 7.4) containing 2% Triton X-100. Supernates were clarified centrifugally (100,000 g, 30 minutes).
α3*-nAChR-IgG
Mouse serum (5 μL duplicate specimens; serial 10-fold dilutions as necessary) was mixed with solubilized α3*-nAChR (50 fmol complexed with 10-fold excess 125I-epibatidine). Antibodies were detected by radioimmunoprecipitation.10, 12, 13
Animal procedures
The Mayo Institutional Animal Care and Use Committee approved the experiments. Mouse strains susceptible to autoimmunity (spontaneous diabetes mellitus,14 or neural antigen-induced myasthenia gravis15 or encephalomyelitis16) were purchased from Jackson Laboratories (Bar Harbor, ME), acclimated 10 days in a conventional facility, anesthetized with 4% isoflurane/4% O2 and injected with fusion protein emulsified in complete Freund's adjuvant (i.d., six sites;), and with killed B. pertussis organisms (20 billion, s.c., four sites). Live cells were injected i.p., 10 million in 0.25 mL PBS, initially with killed B. pertussis organisms, s.c., as adjuvant. Mice were weighed weekly and bled alternate weekly.
Small intestinal transit
Brilliant Blue FCF (100 μL) was gavaged via 18-gauge needle. Mice were killed 30 min later (CO2 asphyxiation); small intestine was removed (gastroduodenal to ileocecal junction). Transit (distance of dye front from gastroduodenal junction) was expressed as percent total small intestinal length: 100% when dye reached ileocecal junction; 0% when dye remained in stomach.
Neuronal density quantification
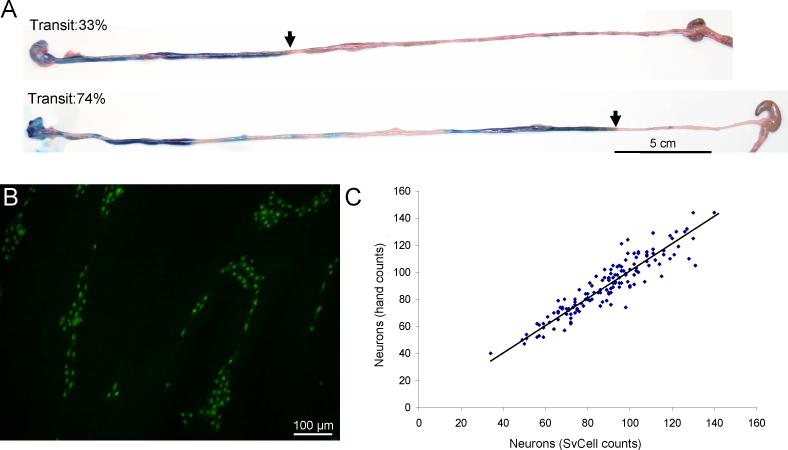
Myenteric plexus neurons (ANNA-1-immunoreactive perikarya)17 were enumerated in coded photomicrographs of muscularis propria whole-mount preparations (1 cm of ileo-jejunal junction tissue; Fig. 1B). Images from four corners and center of each (five randomly-picked fields of 0.59 mm2) were captured by Olympus AX70 fluorescence microscope, 20X objective. Automated object counting (SvCell; Svision LLC, Bellevue WA, employing proprietary soft matching algorithms to segment ANNA-1 positive somata) was validated on a randomly chosen set of 143 images. Numbers obtained from manual counts correlated significantly with numbers obtained by the SvCell program (Fig. 1C). Significance between α3-nAChR-immune and control immune groups was compared by t-test.
Figure 1.
Quantification of small intestinal transit and enteric neurons in mice. (A) Pooling of blue dye in the proximal small intestine of a mouse seropositive for rodent α3*-nAChR-IgG (upper image) contrasts with the segmented distribution in a control mouse immunized with HEK293 cells (lower image). Arrows indicate gavaged dye front. (B) Representative immunohistochemical image of myenteric neuronal cells in whole mount jejuno-ileal junction tissue of a mouse seropositive for rodent α3*-nAChR-IgG. (C) Correlation of neuron numbers obtained by SvCell automated counting with neuron numbers obtained by hand counting from a data set of 143 images (R2 = 0.85, Slope of trend line fitted to data = 1.01).
Small intestinal α3*-nAChR extraction
After luminal flushing with Kreb's solution (mM: 137.4 Na+, 5.9 K+, 2.5 Ca2+, 1.2 Mg2+, 134 C1-, 15.5 HCO3-, 1.2 HPO42-, and 11.5 glucose, saturated with 97% O2 and 3% CO2), tissue was weighed and nAChR was extracted as above.
α3*-nAChR quantification
Cell and tissue extracts were incubated with 125I-epibatidine, with and without non-radioactive epibatidine in 10-fold excess, and quantified using glass fiber filtration.18
RESULTS
Despite repeated intradermal injections of recombinant human α3-polypeptide 1-205 and adjuvants, serum autoantibody detection was infrequent (Table), and levels lower than 3.00 nmol/L (predicts severe AGID in immunized rabbits).10 No gastrointestinal dysmotility signs were evident.
Table.
Antibody responses of mice immunized with α3-nAChR polypeptides or human cells expressing endogenous or recombinant α3*-nAChR
| Serum α3*-nAChR-IgG values (nmol/L) | ||||||
|---|---|---|---|---|---|---|
| Human |
Rodent |
|||||
| Mouse strain | Immunogen† | n | Frequency (%) | Median (range) | Frequency (%) | Median (range) |
| C57BL/6J | Human α3, residues 1 – 205 | 6 | 3 (50) | 0.07 (0.05 – 0.12) | 2 (33) | 0.07 (0.04; 0.10) |
| AKR | Human α3, residues 1 – 205 | 7 | 0 | – | 0 | – |
| SJL | Human α3, residues 1 – 205 | 12 | 1 (8) | 0.05 | 3 (25) | 0.07 (0.05-0.10) |
| 129S/J | Human α3, residues 1 – 205 | 8 | 0 | – | nt | nt |
| NOD | Human α3, residues 1 – 205 | 38 | 5 (13) | 0.13 (0.11 - 0.20) | 9 (24) | 0.09 (0.03-0.16) |
| NOD-DQ8 | Human α3, residues 1 – 205 | 7 | 7 (100) | 0.17 (0.07 - 0.24) | 2 (29) | 0.04 (0.03; 0.06) |
| ABoDQ8 | Human α3, residues 1 – 205 | 11 | 0 | – | 0 | – |
| C57BL/6J | Human neuroblastoma cells (SHSY-5Y) | 18 | 18 (100) | 3.40 (1.20 – 10.0) | 0 | – |
| C57BL/6J | Rat α3β4-transfected HEK-293 cells | 32 | 29 (91) | 0.41 (0.05 – 2.40) | 32 (100) | 14.0 (3.90 – 22.0) |
| C57BL/6J | Non-transfected HEK-293 cells | 24 | 0 | – | 0 | – |
nAChR α3 subunit sequences are identical in rat and mouse; the major extracellular domain differs from the human sequence at 10 of the 205 residues. Polypeptide (150 μg) was injected i.d., 1 to 3 times.
Live cell immunization
We next investigated the immunogenicity of SHSY-5Y neuroblastoma cells expressing endogenous α3*-nAChR. Three weeks after injection, α3*-nAChR-IgG was detected in 50% of C57BL/6J mice; 100% were seropositive after re-inoculating on day 28. Levels peaked after a third inoculation (day 120), far exceeding serum levels of mice injected with α3-polypeptide (Table). Mice receiving human kidney cells (HEK-293) intraperitoneally were seronegative. Despite high α3*-nAChR-IgG levels, no mouse had signs of AGID. Small intestinal transit (80 ± 11%) was not significantly different from control values (67 ± 12%; Fig. 2A). The discontinuous dye distribution pattern in the small intestine of both groups indicated normal peristalsis.
Figure 2.
Intestinal transit, myenteric neuronal counts and small intestinal α3*-nAChR content of mice immunized with live cells. (A) Small intestinal transit in mice immunized with cells expressing rodent α3*-nAChR is significantly slower than in mice immunized with human α3*-nAChR-expressing cells or with control cells. (B) α3*-nAChR protein in lysates of cell lines used as immunogens was quantified by glass fiber filtration assay in terms of macromolecules specifically binding 125I-epibatidine. (C) Myenteric neuron numbers per mm2 of intestinal wall, calculated from images as illustrated in Fig. 1B. (D) Intestinal α3*-nAChR protein (fmol/g) in mice immunized with cells expressing rodent α3*-nAChR was significantly less than in mice immunized with cells expressing human α3*-nAChR or cells lacking α3*-nAChR.
Next, we investigated the immunogenicity of KXα3ß4R2 cells (stably expressing rat α3 and ß4 nAChR subunits, and 5-fold more α3*-nAChR protein than neuroblastoma cells; Fig. 2B). After three intraperitoneal inoculations, 29 of 32 mice (91%) were α3*-nAChR-IgG-seropositive; levels were lower than in mice receiving neuroblastoma cells (Table). However, terminal tissue harvest revealed pooling of gavaged dye in the proximal intestine of 14 mice (45%), suggesting loss of coordinated peristalsis (Fig. 1A). Furthermore, despite lower frequency and levels of α3*-nAChR-IgG, small intestinal transit was significantly slowed (49 ± 16%; Fig. 2A).
Severe hypomotility despite relatively modest α3*-nAChR-IgG levels raised the possibility that antibody induced by rodent α3*-nAChR immunization was more reactive with rodent antigen than with human. Specific testing revealed serum IgG reactive with rodent α3*-nAChR in 100% of mice immunized with rat α3*-nAChR-expressing cells, but in no mouse immunized with human α3*-nAChR–expressing cells (Table). Rat and mouse α3-nAChR subunit amino acid sequences are identical, but human α3-nAChR subunit differs in 10 of 205 extracellular domain residues accessible to circulating IgG. These data implicate autoantibody as the cause of slowed small intestinal transit and loss of coordinated peristalsis.
To evaluate histometric evidence of neuronal cytotoxicity, we counted neurons in whole-mount preparations of jejuno-ileal junction tissue (Fig. 1B). Neuron numbers did not differ significantly in enteric ganglia of mice immunized with human α3*-nAChR, rodent α3*-nAChR or non-transfected HEK-293 cells (Fig. 2C; respectively, 100 ± 19 neurons/mm2, 102 ± 14 neurons/mm2 and 113 ± 16 neurons/mm2). Thus, neuronal cytotoxicity did not explain the hypomotility.
Total small intestinal α3*-nAChR content (γ-emission from Triton-X100-solubilized membrane macromolecules ligated with 125I-epibatidine and captured on glass fiber filters) was 85% less in tissues of mice immunized with rodent α3*-nAChR-expressing cells than in tissues of mice immunized with human α3*-nAChR-expressing-cells or with non-transfected cells (respectively, 61 ± 41 fmol/g, 409 ± 173 fmol/g and 384 ± 91 fmol/g; Fig. 2D).
DISCUSSION
“Self”-α3*-nAChR-reactive autoantibody production is required to develop AGID in actively immunized mice. Profound intestinal α3*-nAChR loss without loss of enteric neurons indicates that hypomotility was not attributable to neuronal death. The most plausible mechanism is α3*-nAChR internalization and degradation following its cross-linking in neuronal plasma membranes by circulating IgG. This mechanism underlies α1*-nAChR loss from muscle cells exposed to serum from rodents with experimental myasthenia gravis19 and partial aquaporin-4 water channel loss from astrocytes exposed to serum from patients with neuromyelitis optica.20 α3*-nAChR internalization is compatible with: i) reduction of agonist-induced depolarization of α3*-nAChR-expressing cells following application of human autoantibody,21 ii) transient gastrointestinal hypomotility in mice injected with α3*-nAChR-IgG9 and iii) rapid patient recovery from AGID symptoms following antibody-depleting therapies.7, 22 Reproducible induction of a mouse model of AGID by active immunization will enable detailed analysis of genetic susceptibility determinants, pathogenic and immunoregulatory mechanisms, and implementation of novel preclinical therapeutic trials.
ACKNOWLEDGMENTS
We thank Tara Adams, Stacy Hall, Christine Hachfeld and James Thoreson for excellent technical assistance.
FUNDING
This work was supported by the National Institutes of Health (DK71209; DK68055).
Footnotes
AUTHOR CONTRIBUTIONS
JWM: Study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript.
KEH: Acquisition of data, analysis and interpretation of data.
JPF: Study design and technical support.
TJK: Study concept and design, analysis and interpretation of data.
SJG: Study design and technical support.
YX: Material support and critical revision of the manuscript.
VAL: Study concept and design, analysis and interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content, obtained funding and study supervision.
DISCLOSURES
The authors disclose no conflicts. Serological testing for neural autoantibodies is offered on a service basis by Mayo Collaborative Service, Inc., an agency of Mayo Foundation. Neither Dr. Lennon nor her laboratory benefit financially from this testing.
REFERENCES
- 1.Lennon VA, Sas DF, Busk MF, et al. Enteric neuronal autoantibodies in pseudoobstruction with small-cell lung carcinoma. Gastroenterology. 1991;100:137–42. doi: 10.1016/0016-5085(91)90593-a. [DOI] [PubMed] [Google Scholar]
- 2.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–55. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- 3.Pasha SF, Lunsford TN, Lennon VA. Autoimmune gastrointestinal dysmotility treated successfully with pyridostigmine. Gastroenterology. 2006;131:1592–96. doi: 10.1053/j.gastro.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol. 2001;49:146–54. [PubMed] [Google Scholar]
- 5.Dhamija R, Tan KM, Pittock SJ, Foxx-Orenstein A, Benarroch E, Lennon VA. Serologic profiles aiding the diagnosis of autoimmune gastrointestinal dysmotility. Clin Gastroenterol Hepatol. 2008;6:988–92. doi: 10.1016/j.cgh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. N Engl J Med. 1999;340:227–8. doi: 10.1056/NEJM199901213400311. [DOI] [PubMed] [Google Scholar]
- 7.Iodice V, Kimpinski K, Vernino S, Sandroni P, Fealey RD, Low PA. Efficacy of immunotherapy in seropositive and seronegative putative autoimmune autonomic ganglionopathy. Neurology. 2009;72:2002–08. doi: 10.1212/WNL.0b013e3181a92b52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraichely RE, Farrugia G, Pittock SJ, Castell DO, Lennon VA. Neural autoantibody profile of primary achalasia. Dig Dis Sci. 2010;55:307–11. doi: 10.1007/s10620-009-0838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vernino S, Ermilov LG, Sha L, Szurszewski JH, Low PA, Lennon VA. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004;24:7037–42. doi: 10.1523/JNEUROSCI.1485-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lennon VA, Ermilov LG, Szurszewski JH, Vernino S. Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease. J Clin Invest. 2003;111:907–13. doi: 10.1172/JCI17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Molecular Pharmacol. 1998;54:322–33. doi: 10.1124/mol.54.2.322. [DOI] [PubMed] [Google Scholar]
- 12.McKeon A, Lennon VA, Lachance DH, Fealey RD, Pittock SJ. Ganglionic acetylcholine receptor autoantibody: oncological, neurological, and serological accompaniments. Arch Neurol. 2009;66:735–41. doi: 10.1001/archneurol.2009.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vernino S, Kryzer TJ, Lennon VA. Autoimmune autonomic neuropathy and neuromuscular hyperexcitability disorders. In: Rose NR, Hamilton RG, Detrick B, editors. Manual of Clinical and Laboratory Immunology. 6 edn. ASM Press; Washington, DC: 2002. pp. 1013–7. [Google Scholar]
- 14.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol. 2008;20:111–18. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christadoss P, Lennon VA, Krco CJ, Lambert EH, David CS. Genetic control of autoimmunity to acetylcholine receptors: role of Ia molecules. Ann NY Acad Sci. 1981;377:258–77. doi: 10.1111/j.1749-6632.1981.tb33737.x. [DOI] [PubMed] [Google Scholar]
- 16.Miller SD, Karpus WJ. Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol. 2007 doi: 10.1002/0471142735.im1501s77. Chapter 15:Unit 15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairman CL, Clagett-Dame M, Lennon VA, Epstein ML. Appearance of neurons in the developing chick gut. Dev Dyn. 1995;204:192–201. doi: 10.1002/aja.1002040210. [DOI] [PubMed] [Google Scholar]
- 18.Vernino S, Adamski J, Kryzer TJ, Fealey RD, Lennon VA. Neuronal nicotinic ACh receptor antibody in subacute autonomic neuropathy and cancer-related syndromes. Neurology. 1998;50:1806–13. doi: 10.1212/wnl.50.6.1806. [DOI] [PubMed] [Google Scholar]
- 19.Lennon VA. Immunofluorescence analysis of surface acetylcholine receptors on muscle: modulation by auto-antibodies. In: Jenden DJ, editor. Cholinergic Mechanisms and Psychopharmacology: Advances in Behavioral Biology. Plenum Publishing Co; New York: 1977. pp. 77–92. [Google Scholar]
- 20.Hinson SR, Romero MF, Popescu BF, et al. Molecular outcomes of NMO-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci USA. 2012;109:1245–50. doi: 10.1073/pnas.1109980108. Epub 2011 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Low PA, Jordan J, et al. Autoimmune autonomic ganglionopathy: IgG effects on ganglionic acetylcholine receptor current. Neurology. 2007;68:1917–21. doi: 10.1212/01.wnl.0000263185.30294.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder C, Vernino S, Birkenfeld AL, et al. Plasma exchange for primary autoimmune autonomic failure. N Engl J Med. 2005;353:1585–90. doi: 10.1056/NEJMoa051719. [DOI] [PubMed] [Google Scholar]