Summary
GCN5-typeN-acetyltransferases (GNATs) are enzymes that catalyze the transfer of the acetyl group from acetyl-CoA to a primary amine. GNATs are conserved in all domains of life. Some members of this family of enzymes acetylate the side chain of specific lysine residues in proteins of diverse function. In bacteria, GNAT-catalyzed protein acetylation regulates carbon metabolism, RNA metabolism, and transcriptional regulation. Metabolic regulation in Streptomyces species is of interest due to the role of these organisms in natural product synthesis. Here we identify SlPatA, a GNAT in S. lividans with unique domain organization, and a new acetylation target, namely acetoacetyl-CoA synthetase (SlAacS). The latter has homologues in all domains of life. In vitro and in vivo evidence show that SlAacS is a bona fide acetoacetyl-CoA synthetase. SlPatA acetylates SlAacS more efficiently than it does acetyl-CoA synthetase, an enzyme known to be under acetylation control. SlPatA acetylates SlAacS at the active site residue Lys617 and acetylation inactivates SlAacS. Acetylated SlAacS was deacetylated by a sirtuin-type protein deacetylase. SlAacS acetylation/deacetylation may represent a conserved mechanism for regulation of acetoacetyl-CoA synthetase activity in all domains of life.
Introduction
GCN5-related N-acetyltransferase (GNAT) enzymes catalyze the transfer of an acetyl moiety from acetyl-coenzyme A (Ac-CoA) to a primary amine of small molecules and proteins. GNATs, named for the homology to the yeast GCN5 protein (yGCN5p), are identified by signature sequence motifs and structurally homology (Shaw et al., 1993). GNATs are conserved in all domains of life, and represent one of the largest proteins super families (Vetting et al., 2005). GNATs are involved in acetylation of antibiotics, hormones, tRNA, histones, metabolic enzymes, and transcription factors, implicating GNATs in a wide variety of cellular processes (Vetting et al., 2005, Thao et al., 2010, Ikeuchi et al., 2008, Thao & Escalante-Semerena, 2011b, Spange et al., 2009). Protein lysine acetylation by GNATs occurs on the epsilon amino group of the lysine side chain. Acetylation neutralizes the positive charge of the lysine side chain altering the side-chain chemistry. Protein acetylation has been documented in all domains of life (Soppa, 2010), but recent proteomic approaches performed in eukaryotes (Kim et al., 2006, Choudhary et al., 2009, Weinert et al., 2011, Zhao et al., 2010) and bacteria (Zhang et al., 2009, Yu et al., 2008, Crosby et al., 2012) have expanded our view of the potential role of this posttranslational modification in cell physiology. As new protein acetylation targets and the concomitant protein acetyltransferases are identified, the role of acetylation on protein and cellular functions is broadened.
GNATs are the only class of protein acetyltransferases identified in bacteria. The Gram-negative γ-proteobacterium Salmonella enterica encodes a protein acetyltransferase SePat that contains a large N-terminal domain (~700 residues) with homology to NDP-forming CoA synthetases and a C-terminal GNAT domain (~200 residues) (Starai & Escalante-Semerena, 2004). SePat lacks NDP-forming Ac-CoA synthetase activity, and the role of the domain remains unclear. Biochemical studies indicate that the N-terminal domain of SePat may be required for acetyltransferase activity, subunit interactions, and positive cooperativity (Thao & Escalante-Semerena, 2011a). SePat uses acetyl-CoA to acetylate acetyl-CoA synthetase (SeAcs) and propionyl-CoA to propionylate propionyl-CoA synthetase (PrpE) (Garrity et al., 2007, Takenoya et al., 2010). In each case, acylation occurs at the active site lysine of the CoA synthetase and inactivates the enzyme. Specifically, lysine acylation prevents the first half reaction that activates the fatty acid to the corresponding fatty-acyl-AMP intermediate. In each case, deacylation reactivates the enzyme (Starai et al., 2002, Garrity et al., 2007).
In the photoheterotrophic purple non-sulfur α-proteobacterium Rhodopseudomonas palustris, a SePat homologue (RpPat), and a single-domain GNAT, RpKatA, regulate organic acid degradation by acetylating and inactivating seven acyl-CoA synthetases and three aryl-CoA synthetases (Crosby et al., 2010, Crosby et al., 2012). Acetylation of the acyl- or aryl- CoA synthetases has the same effect as seen in S. enterica. Notably, the protein deacetylation system of R. palustris is more complex than the one in S. enterica. R. palustris encodes a sirtuin-type, NAD+-dependent deacetylase RpSrtN, and a Zn(II)-dependent histone deacetylase homologue RpLdaA. RpLdaA hydrolyzes the acetyl group from acetyllysine releasing free acetate and reactivating acyl-CoA synthetases. At present, the only protein with acetyllysine deacetylase activity in S. enterica is a sirtuin encoded by the cobB gene (Tsang & Escalante-Semerena, 1998, Tucker & Escalante-Semerena, 2010). Protein acetyltransferases have also been described in Gram-positive bacteria. Bacillus subtilis encodes a single domain GNAT, AcuA, which acetylates acetyl-CoA synthetase (BsAcsA) (Gardner et al., 2006). Mycobacterium tuberculosis and M. smegmatis encode protein acetyltransferases, MtPatA and MsPat, respectively, with a cyclic-AMP binding domain fused to the N-terminus of a GNAT domain (Nambi et al., 2010). Binding of cAMP to MtPatA activates the enzyme by exposing the catalytic site (Lee et al., 2012). MsPat acetylates a universal stress protein and Ac-CoA synthetase in M. smegmatis (Nambi et al., 2010, Xu et al., 2011).
Recently, Mikulik et al. demonstrated that Ac-CoA synthetase from the actinomycete Streptomyces coelicolor is acetylated in vivo (Mikulik et al., 2012), but the acetyltransferase responsible for this modification remains unidentified. S. coelicolor encodes 77 putative GNAT acetyltransferases (Pfam00583), whilst the genome of the closely related species S. lividans (Kawamoto & Ochi, 1998) encodes 72 putative GNATs. However, none of the putative GNATs share end-to-end homology with the known bacterial protein acetyltransferases SePat, EcPka, RpPat, RpKatA, BsAcuA, MsPat, or MtPatA. Further understanding of the regulation of metabolism in Streptomyces species is of interest because of the diversity of natural products synthesized by these organisms (Seow et al., 1997, Courtois et al., 2003, McMahon et al., 2012, Chater, 2006).
Here, we identify a GNAT in S. lividans that has protein acetyltransferase activity and contains regions of homology to both the N- and C-terminal domains of SePat. The S. lividans Pat homologue (SlPatA) can acetylate native Ac-CoA synthetase (SlAcs), albeit to a limited extent, but acetylates acetoacetyl-CoA synthetase (SlAacS) substantially better. Prior to this work, the regulation of SlAacS activity by lysine acetylation/deacetylation systems was unknown. Consistent with the effect of acetylation on other AMP-forming acyl-CoA synthetases, acetylation of SlAacS inactivated the enzyme, whilst deacetylation reactivated it. We also report evidence that SlPatA acetylates SlAacS in vivo in S. lividans. Further, we used E. coli as a heterologous host to demonstrate SlAacS regulation by SlPatA in vivo. In summary, we show that a SePat homologue with reversed domain organization has bona fide acetyltransferase activity. Our findings raise the possibility that acetoacetyl-CoA synthetase homologues in all domains of life may be also controlled by lysine acetylation/deacetylation systems.
Results
Actinomycetes and the archaeon Archaeoglobus fulgidus encode a protein acetyltransferase with unique domain organization
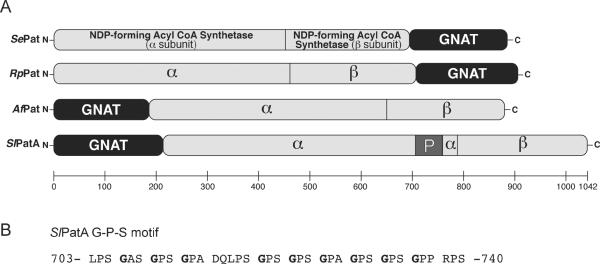
Salmonella enterica and Rhodopseudomonas palustris synthesize a protein acetytransferase Pat enzyme with a large (~700 residues) N-terminal domain with homology to NDP-forming CoA synthetases and a C-terminal (~200 residues) GNAT domain (Starai & Escalante-Semerena, 2004, Crosby et al., 2010). The EFD66247 locus of the genome of the actinomycete Streptomyces lividans encodes a homologue of the SePat and RpPat enzymes in which the GNAT and the NDP-forming CoA synthetase-like domains are reversed (Fig. 1A). We note that the DNA sequence of S. lividans open reading frame (ORF) EFD66247 currently available in databases contains two mistakes, which we discovered upon cloning and sequencing EFD66247. Our sequencing data showed two changes, a transition (T1787C) and a deletion (ΔT1788). BLASTp searches performed with the protein encoded by the corrected DNA sequence identified a full-length NDP-forming CoA synthetase-like domain homologous to the ones found in SePat and RpPat. Further bioinformatics analysis of the corrected sequence of ORF EFD66247 revealed that such reverse domain organization of the putative protein was also present in homologues presumably synthesized by other actinomycetes and the archaeon Archaeoglobus fulgidus. The predicted primary sequence of the EFD66247 protein contained a proline-rich sequence (P663-P753, 26.4% proline) that included a degenerate G-P-S motif (Fig. 1B) (Beck & Brodsky, 1998).
Figure 1. Domain organization of Pat homologues.
(A) Pat homologues encode a GNAT domain (black) and large domain that is homologous to NDP-forming acyl-CoA synthetases (light gray). S. lividans PatA also contains a proline-rich domain in the large domain (dark gray, denoted by “P”). SePat, Salmonella enterica Pat (NP_461586); RpPat, Rhodopseudomonas palustris Pat (NP_949576); AfPat Archaeoglobus fulgidus Pat (NP_070340); SlPatA, Streptomyces lividans PatA (ZP_06527997). (B) The degenerate G-P-S motif Leu703-Ser740 in SlPatA.
Tucker & Escalante-Semerena
The EFD66247 protein (hereafter SlPatA) is a functional protein acetyltransferase
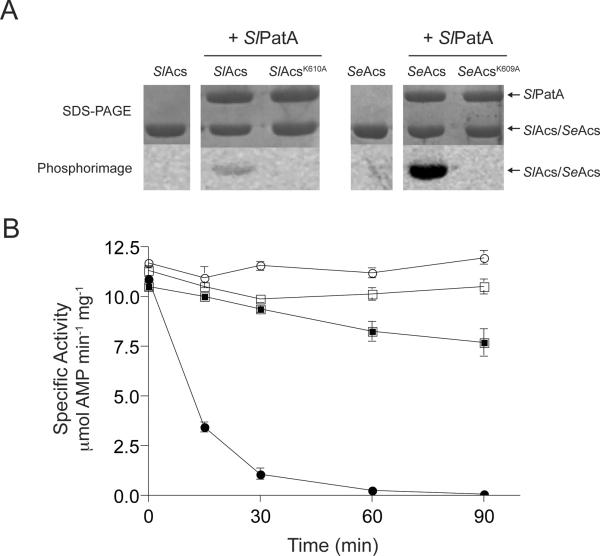
SePat and RpPat have been shown to acetylate the acetyl-CoA synthetase (Acs) enzymes from S. enterica and R. palustris, respectively (Starai & Escalante-Semerena, 2004, Crosby et al., 2010). SePat and RpPat acetylate the epsilon amino group of an active-site lysyl side chain, with the concomitant inactivation of the adenylylation activity of Acs (Crosby et al., 2010, Starai & Escalante-Semerena, 2004). Mikulik et al. recently reported that the Acs homologue from Streptomyces ceolicolor (ScAcs) was acetylated in vivo (Mikulik et al., 2012), suggesting that a protein acetyltransferase was responsible for acetylating Acs in this Streptomyces species. We asked whether recombinant SlPatA could acetylate SlAcs in vitro. For this purpose, SlPatA was purified to homogeneity and incubated with purified SlAcs (encoded by S. lividans locus EFD68454) in the presence of [1-14C]-Ac-CoA. As shown in figure 2A, SlPatA acetylated SlAcs, indicating that SlPatA was a bona fide protein lysine acetyltransferase. To verify the site of acetylation, a SlAcsK610A variant was isolated and tested as a substrate of SlPatA. SlPatA did not modify SlAcsK610A, indicating that the active-site residue K610 was the only site modified by SlPatA. For reference, SlPatA was incubated with SeAcs, a known substrate of SePat (Starai & Escalante-Semerena, 2004), and the active site lysine variant SeAcsK609A. SlPatA modified SeAcs but not SeAcsK609A indicating that SlPatA acetylates only the active-site residue K609 of SeAcs. Relative acetylation of SlAcs and SeAcs was quantified using digital light units. SlPatA modified SeAcs approximately 30-fold more efficiently than SlAcs.
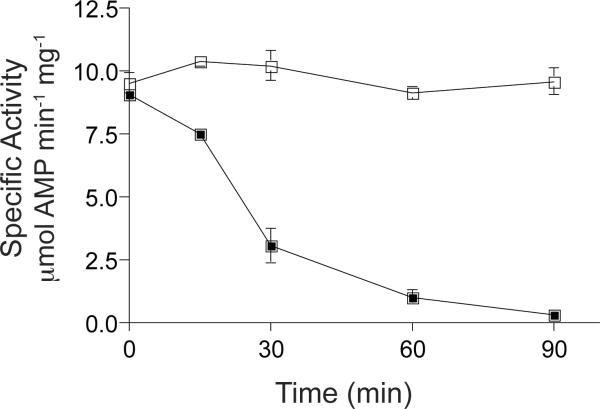
Figure 2. SlPatA acetylates SlAcs from S. lividans.
(A) SlAcs, SlAcsK610A, SeAcs, or SeAcsK609A was incubated with [1-14C]-acetyl-CoA in the presence or absence of SlPatA. Proteins were separated by SDS-PAGE and stained with Coomassie Blue to visualize proteins. Acetylation was visualized by phosphor imaging. SlPatA was incubated with SlAcs or SeAc in the presence or absence of unlabeled acetyl-CoA (B). Reactions were carried out with 1:3 molar ratios of SlPatA to SlAcs/SeAcs. Sample were diluted and assayed to measure SlAcs/SeAcs activity at 0, 15, 30, 60, and 90 min after incubation with SlPatA. SlAcs (squares) and SeAcs (circles) activities were measured in and NADH-consumption assay and activities are reported for the reactions containing acetyl-CoA (closed symbols) and the control reactions lacking acetyl-CoA (open symbols). Reactions were carried out in triplicate. Error bars represent standard deviations.
Tucker & Escalante-Semerena
To compare the effect of SlPatA-mediated acetylation on SlAcs and SeAcs activity, SlAcs or SeAcs was incubated with SlPatA in the presence or absence of acetyl-CoA. When SlAcs was incubated with SlPatA in the presence of Ac-CoA, SlAcs retained approximately 75% of its activity relative to the no acetyl-CoA control (Fig. 2B). This result suggested that SlPatA acetylation of SlAcs was inefficient. SlPatA inactivated SeAcs within 90 minutes of incubation with Ac-CoA, confirming that SeAcs was a better substrate for SlPatA than SlAcs under the conditions tested. Additionally, these data confirm that SlPatA acted catalytically. As a point of reference, under the conditions used SePat fully inactivated SeAcs (Starai & Escalante-Semerena, 2004).
SlPatA acetylates an acetoacetyl-CoA synthetase homologue from S. lividans
Because SlPatA acetylation of SlAcs was inefficient, we looked for additional SlPatA substrates in S. lividans. SePat homologues have been shown to acylate members of the AMP-forming acyl-CoA synthetase family of enzymes (Starai & Escalante-Semerena, 2004, Garrity et al., 2007, Crosby et al., 2012, Crosby et al., 2010). The closest homologue of SlAcs we found in S. lividans was a putative acetoacetyl-CoA synthetase (hereafter SlAacS, encoded by locus EFD70521), whose primary sequence was 39% identical to that of the acetoacetyl-CoA synthetase from rat liver (Ito et al., 1984). SlAacS homologues contained the catalytic residue Lys617 (Table 1), which raised the possibility that SlAacS could be acetylated. To test this possibility, SlPatA and SlAacS were incubated in the presence of [1-14C]-Ac-CoA. As shown in figure 3, SlPatA efficiently used [1-14C]-Ac-CoA to modify SlAacS, and residue K617 was the only site of acetylation in SlAacS. Under the same assay conditions, SlPatA acetylated 15-fold more SlAacS than SlAcs, indicating that SlAacS was a substantially better substrate than SlAcs for SlPatA.
Table 1.
Comparison of SlAacS active site to acetylation substrates and SlAacS homologues
| Species | Gene Name/Locus Tag | Accession Number | Percent amino acid identity to SlAacS | Active site motif |
|---|---|---|---|---|
| S. lividans | aacS | ZP_06532271 | N/A | 610-IPHTLTGKRIEVPVK-624 |
| S. lividans | acs | ZP_06530204 | N/A | 603-LPKTRSGKIMRRLLR-617 |
| S. enterica | acs | NP_463140 | N/A | 602-LPKTRSGKIMRRILR-616 |
| S. enterica | prpE | NP_459366 | N/A | 585-LPKTRSGKMLRRTIQ-599 |
| R. palustris | badA | NP_946014 | N/A | 505-LPKTATGKIQRFKLR-519 |
| R. palustris | aliA | NP_946004 | N/A | 525-MPATPSGKIQKFRLR-539 |
| R. palustris | prpE | NP_949838 | N/A | 591-LPKTRSGKILRGTIK-605 |
| R. palustris | acs | NP_945564 | N/A | 599-LPKTRSGKIMRRILR-613 |
| Pseudomonas aeruginosa PAO1 | PA1997 | NP_250687 | 46 | 606-IPRTLSGKIVELAVR-620 |
| Archaeoglobus fulgidus | acs-1 | NP_069035 | 44 | 608-IPMTLNYKKLEVPIK-622 |
| Caenorhabditis elegans | SUR-5 | NP_509229 | 37 | 658-IPYTSSGKKVEVAVK-672 |
| Rattus norvegicus | AACS | NP_075592 | 39 | 370-IPYTINGKKVEVAVK-384 |
| Homo sapiens | AACS, SUR5 | NP_076417 | 38 | 626-IPYTLNGKKVEVAVK-640 |
Figure 3. SlPatA acetylates SlAacS from S. lividans at the conserved active site lysine.
Alignment of SlAacS with known acetylation targets from S. enterica (SeAcs, encoded by locus STM4275; SePrpE, encoded by locus STM0371), R. palustris (RpBadA, encoded by locus RPA0661; RpAliA, encoded by locus RPA0651; RpHbaA, encoded by locus RPA0669; RpPrpE, encoded by locus RPA4504; RpAcs, encoded by locus RPA0211), and S. lividans indicates a conserved active site lysine (A). Acetylation of SlAacS and SlAacSK617A was assessed after incubation of proteins with [1-14C]-acetyl-CoA in the presence or absence of SlPatA. Proteins were separated by SDS-PAGE and stained with Coomassie Blue to visualize proteins. Acetylation was visualized by phosphor imaging (B).
Tucker & Escalante-Semerena
SlAacS is a functional acetoacetyl-CoA synthetase in vivo
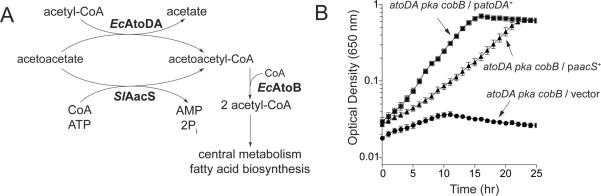
We used strains of Escherichia coli to assess in vivo the ability of SlAacS to convert acetoacetate to acetoacetyl-CoA (Fig. 4A). E. coli encodes a protein acetyltransferase (Pka) homologous to SePat and sirtuin-type protein deacetylase (CobB). Growth experiments were done in a Δpka ΔcobB E. coli strain background to avoid potential SlAacS modification by the E. coli acetylation machinery. E. coli grows on acetoacetate by first converting it to acetoacetyl-CoA using the acetyl-CoA:acetotacetate CoA transferase enzyme (AtoDA, EC 2.8.3.8). In the second step of the pathway, the 3-ketoacyl-CoA thiolase (AtoB, EC 2.3.1.16) enzyme converts acetoacetyl-CoA and CoA to two molecules of acetyl-CoA, which enter central metabolism (Fig. 4A) (Pauli & Overath, 1972). E. coli encodes a second 3-ketoacyl-CoA thiolase, FadA, with a substrate preference for mid-length chain fatty acids that is capable of using acetoacetate as a substrate to generate two molecules of Ac-CoA (Staack et al., 1978). An E. coli atoDA strain cannot grow on acetoacetate because it cannot convert acetoacetate to acetoacetyl-CoA (Pauli & Overath, 1972). An E. coli atoB fadA strain cannot grow on acetoacetate due to the lack of acetoacetyl-CoA thiolase activity (Jenkins & Nunn, 1987). As shown in figure 4B, when SlAacS encoded by the native S. lividans aacS allele was ectopically produced in E. coli, growth of the ΔatoDA ΔcobB Δpka strain on acetoacetate was restored. The final culture density was comparable to that reached when wild type alleles of atoDA were provided in trans. These data suggest that SlAacS converted acetoacetate to acetoacetyl-CoA compensating for the absence of AtoDA in the atoDA strain. The difference in the growth rate of the strain complemented with native S. lividans aacS alleles may be due to codon bias of the S. lividans allele, differences in enzyme kinetic parameters, or enzyme stability in E. coli at 37°C.
Figure 4. SlAacS can substitute for EcAtoDA in E. coli during growth on acetoacetate.
Acetoacetate utilization in E. coli involves a 2-step conversion from acetoacetate to acetoacetyl CoA and from acetoacetyl-CoA to 2 molecules of acetyl-CoA. SlAacS is predicted by homology to catalyze the conversion of acetatoacetate to acetoacetyl-CoA (A). Growth behavior of S. enterica on NCE minimal medium supplemented with acetoacetate (30 mM). Growth experiments were performed at 37°C using a microtiter plate and a microtiter plate reader (Bio-Tek Instruments). Growth experiments were performed in triplicate. Error bars represent standard deviations.
Tucker & Escalante-Semerena
To determine the substrate specificity for SlAacS, the AMP-forming CoA synthetase activity of SlAacS was measured using a continuous spectrophotometric assay with CoA, ATP, and organic acids structurally similar to, and including, acetoacetate (see Experimental procedures for all substrates tested). SlAacS efficiently activated acetoacetate to acetoacetyl-CoA [12 ± 2 μmol AMP min−1 mg−1) and β-hydroxybutyrate to β-hydroxybutyryl-CoA (2 ± 0.2 μmol AMP min−1 mg−1). SlAacS specific activities for all other compounds tested were <0.1 μmol AMP min−1 mg−1. Clearly, acetoacetate was the preferred substrate of SlAacS, indicating that SlAacS was an acetoacetyl-CoA synthetase (EC 6.2.1.16). To confirm the identity of the AacS reaction product, the substrates ATP, CoA, and acetoacetate were incubated in the presence or absence of SlAacS. High performance liquid chromatography (HPLC) was used to separate products from reagents. The enzyme-containing reaction produced a single unique peak with a retention time of 7.25 min. Mass spectral analysis (positive mode) of this sample revealed a molecular ion with a m/z of 852.3, corresponding to the expected mass of acetoacetyl-CoA (851.6 amu).
Lys617 is critical for SlAacS activity and acetylation of Lys617 inactivates SlAacS
In SlAacS, Lys617 is a conserved active site residue, and the site acetylated by SlPatA (Fig. 3A). To assess the role of Lys617 in SlAacS activity, site-directed mutagenesis was used to generate aacS alleles encoding SlAacSK617A and SlAacSK617Q variants. Alanine served as a catalytically inert substitution and glutamine served as a structural mimic for acetyl-lysine. Relative to the activity of wild-type SlAacS (9.6 ± 0.4 μmol AMP min−1 mg−1), both SlAacSK617A and SlAacSK617Q variants were ~20-fold less active (<0.5 μmol AMP min−1 mg−1) indicating that Lys617 was critical for activity, lending support to the hypothesis that acetylation of Lys617 inactivated the enzyme.
To test the effect of SlAacS Lys617 acetylation directly, SlAacS was incubated with SlPatA in the presence or absence of acetyl-CoA. In the presence of both acetyl-CoA and SlPatA, SlAacS activity was reduced >97% indicating that SlPatA acetylation of Lys617 effectively decreases SlAacS activity (Fig. 5).
Figure 5. SlPatA acetylation inactivates SlAacS.
SlAacS was incubated with SlPatA at a 3:1 molar ratio (SlAacS:SlPatA) in the presence (closed squares) or absence (open squares) of acetyl-CoA. Sample were removed, diluted, and assayed to measure SlAcs/SeAcs activity at 0, 15, 30, 60, and 90 min after incubation with SlPatA. SlAacS activity was measured in an NADH-consumption assay. Reactions were carried out in triplicate. Error bars represent standard deviations.
Tucker & Escalante-Semerena
SlAacS is deacetylated and reactivated by sirtuin deacetylase
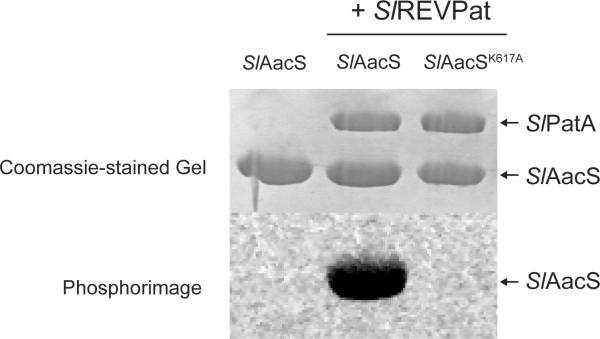
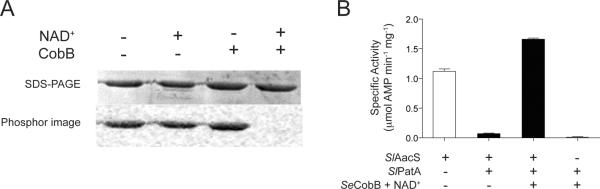
Acetylation of CoA-ligases is reversed by deacetylases (Starai et al., 2002, Garrity et al., 2007, Crosby et al., 2010, Crosby et al., 2012). The S. lividans genome encodes three putative deacetylases, namely two NAD+-dependent sirtuin-type protein deacetylases [EFD65580 (ScCobB2), EFD71509 (ScCobB1)], and one homologue of the R. palustris zinc-dependent protein deacetylase RpLdaA (EFD68590). In S. coelicolor, ScCobB1 (100% identity to EFD71509) deacetylates ScAcs in vitro (Mikulik et al., 2012). For unknown reasons, our attempts to purify or enrich for active S. lividans deacetylases in E. coli extracts were unsuccessful. To circumvent this problem, we used the Salmonella enterica CobB (SeCobB) sirtuin deacetylase enzyme (Starai et al., 2002, Tucker & Escalante-Semerena, 2010), which has been shown to deacetylate heterologous acyl- and aryl-CoA synthetases (Crosby et al., 2010). We used the short form of SeCobB (SeCobBS) sirtuin (Tucker & Escalante-Semerena, 2010) to demonstrate the reversibility of the acetylation of SlAacS Lys617. SlAacS was acetylated with SlPatA and [14C-1]-Ac-CoA, followed by incubation with SeCobBS in the absence and presence of NAD+. In the presence of SeCobBS and NAD+, the amount of radioactivity associated with SlAacS decreased below the limit of detection indicating that SeCobBS deacetylated SlAacS (Fig. 6A), demonstrating that SlAacS acetylation was reversible.
Figure 6. SlAacS is deacetylated and reactivated by a heterologous sirtuin.
A. SlAacS previously acetylated by SlPatA with [1-14C]-acetyl-CoA was incubated with the addition of SeCobB and/or NAD+. Proteins were resolved by SDS-PAGE and stained with Coomassie Blue to visualize proteins. Acetylation was visualized by phosphor image. B. SlAacS was incubated in the presence or absence of SlPatA and unlabeled acetyl-CoA and the acetylation reaction was stopped by buffer exchange. SeCobB and NAD+ were added to the reactions and SlAacS activity was measured in triplicate. Error bars represent standard deviations.
Tucker & Escalante-Semerena
We assessed whether deacetylation of SlAacSAc by SeCobBs would restore SlAacS activity. To do this, we incubated SlPatA with SlAacS and acetyl-CoA. After incubation, buffer was exchanged to remove excess acetyl-CoA. SeCobBs and NAD+ were added to the reaction mixture prior to a second incubation period, after which SlAacS activity was restored to levels comparable to the unacetylated SlAacS control (Fig. 6B). Specific activity of unacetylated SlAacS was reduced when compared to the specific activity measurements reported for the substrate specificity determination. This discrepancy was likely due to instability of the enzyme during the acetylation reaction, buffer exchange, and deacetylation reaction.
SlAacS is acetylated in vivo during growth of S. lividans in the presence of acetoacetate
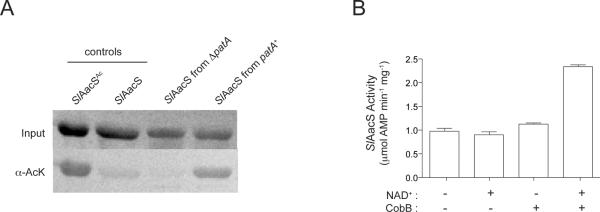
We assessed whether or not SlAacS was acetylated in vivo in S. lividans and whether SlPatA catalyzed the modification of SlAacS. H6-SlAacS was purified from S. lividans patA+ and ΔpatA strains grown in minimal medium supplemented with acetoacetate. We then assessed the acetylation state of SlAacS by two methods. First, we isolated H6-SlAacS enzymes from each strain and determined whether or not they were acetylated. To do this, we used Western blot analysis with polyclonal anti-acetyllysine antibodies. Results of control experiments with non-acetylated and in-vitro acetylated SlAacS (Fig. 7A) showed that acetyllysine was readily detected under the conditions used. A strong signal was detected for acetyllysine in SlAacS isolated from S. lividans patA+, whilst the level of acetyllysine in SlAacS isolated from the S. lividans ΔpatA strain was not significantly above background (Fig. 7A). Based on these data we inferred that SlAacS was acetylated in vivo and that SlPatA was the only protein acetyltransferase that modified SlAacS under the conditions tested. To confirm the modification of SlAacS, H6-SlAacS isolated from S. lividans patA+ was subjected to trypsin digestion and the resulting peptides were analyzed by LC/MS/MS. Sequence determination of the peptides identified K617 as the single site of acetylation of SlAacS (Figure S1).
Figure 7. SlAacS is acetylated in vivo in S. lividans.
(A) H6-SlAacS was isolated from S. lividans pat+ and ΔpatA strains grown on acetoacetate. Acetylation state of the H6-SlAacS proteins was analyzed by anti-acetyllysine Western blot analysis. In vitro acetylated and nonacetylated SlAacS were used as positive and negative controls, respectively. Total protein was visualized by Ponceau S staining prior to Western blot analysis. (B) H6-SlAacS isolated from S. lividans pat+ and ΔpatA strains was incubated with SeCobB and NAD+ to assess the affect of deacetylation on H6-SlAacS activity. For (A) and (B), experiments were conducted on H6-SlAacS isolated in three independent experiments. Error bars represent standard deviations.
Tucker & Escalante-Semerena
Secondly, we quantified the fraction of acetylated SlAacS present in the cell under growth the conditions used. To do this, we isolated H6-SlAacS proteins from S. lividans patA+ and S. lividans δpatA strains and incubated them with SeCobB and NAD+ in vitro. The premise here was that the activity of acetylated H6-SlAacS should increase upon deacetylation by SeCobB, whilst the activity of non-acetylated H6-SlAacS should remain unchanged after incubation with SeCobB and NAD+. In the presence of the co-substrate NAD+, SeCobB deacetylated H6-SlAacS isolated from the S. lividans patA+ strain, resulting in a 2.5-fold increase in H6-SlAacS activity (Figure 7B). In contrast, incubation of H6-SlAacS isolated from the S. lividans ΔpatA strain with SeCobB and NAD+ did not change the activity of H6-SlAacS (data not shown). Collectively, these data indicated that, in S. lividans, SlPatA modulated the activity of H6-SlAacS in vivo.
SlAacS is required for growth of S. lividans on acetoacetate
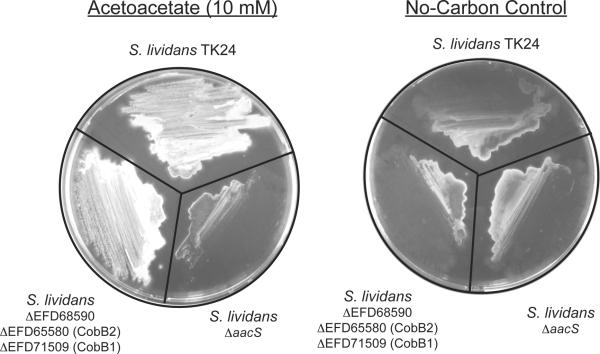
As shown above, SlAacS was acetylated in S. lividans during growth in minimal medium supplemented with acetoacetate. On the basis of these results we made several predictions. First, we posited that S. lividans would not grow if putative genes encoding known protein deacetylases were deleted from the chromosome. This idea assumed that the absence of deacetylase activity would not affect in any way the ability of SlPatA to acetylate SlAacS resulting in a net accumulation of acetylated, inactive SlAacS and should not support growth on acetoacetate. To test this hypothesis, we constructed a strain carrying chromosomal deletions of all three genes encoding putative protein deacetylases, i.e., ΔEFD68590 (Zn(II)-dependent deacetylase), ΔEFD65580 (SlCobB2 sirtuin), and ΔEFD71509 (SlCobB1 sirtuin). Surprisingly, growth of the ΔEFD68590 ΔEFD65580 ΔEFD71509 (JE16752) strain was comparable to that of wild-type S. lividans on acetoacetate (Fig. 8).
Figure 8. SlAacS is required for growth of S. lividans on acetoacetate.
Spores from S. lividans strains TK24 (wild-type), JE16752 (ΔEFD65580 ΔEFD68590 ΔEFD71509), and JE16758 (ΔaacS) were streaked on minimal medium containing acetoacetate (10 mM) or no additional carbon source. Plates were incubated at 30°C for 7 days prior to imaging using a Photodyne digital imaging system.
Tucker & Escalante-Semerena
To assess whether or not SlAacS was required for growth of S. lividans on acetoacetate, we constructed a S. lividans ΔaacS strain. Strains were tested for their ability to grow on minimal medium supplemented with acetoacetate. Unlike the wild-type strain, the ΔaacS (JE16758) strain grew poorly on acetoacetate (Fig. 8), indicating that SlAacS activity was required for growth of S. lividans on this carbon and energy source.
In vivo acetylation control of SlAacS activity in a heterologous system
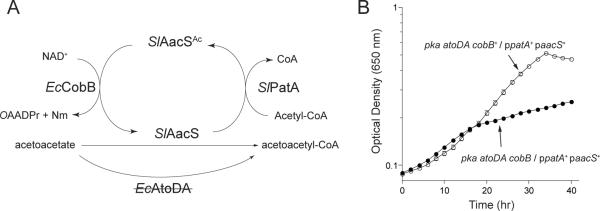
We used E. coli to demonstrate that SlPatA activity controlled SlAacS function in vivo. The E. coli pka allele, encoding a SePat protein acetyltransferase homologue, was disrupted to avoid potential modification of SlAacS by the E. coli protein acetylation machinery. As shown in figure 4B, an atoDA pka strain of E. coli can use SlAacS to activate acetoacetate for subsequent use as a source of carbon and energy. Based on these data we predicted that, although SlPatA would inactivate SlAacS in E. coli, such negative effect on SlAacS activity would be balanced by the activity of the E. coli CobB sirtuin deacetylase enzyme (EcCobB, 92% identical to SeCobB). This prediction is illustrated in figure 9A. For this purpose, the coding sequence of the S. lividans aacS allele was optimized for expression in E. coli. We hypothesized that in the absence of EcCobB, the ΔatoDA ΔcobB Δpka / paacS+ ppatA+ strain (JE16969, the letter `p' preceding a gene denotes that it is plasmid-borne) would not grow on acetoacetate due to the accumulation of inactive SlAacSAc, a prediction that was experimentally confirmed, as shown in figure 9B (solid circles). These data showed that SlPatA acetylation of SlAacS modulated SlAacS activity in vivo in a heterologous host. Synthesis of SlPatA in an E. coli cobB+ strain did not have a deleterious effect during growth on acetoacetate (Fig. 9B, open circles), indicating reversibility of SlAacS acetylation by EcCobB sirtuin deacetylation in vivo. Results of control experiments demonstrated that cobB was not required for growth of E. coli ΔatoDA Δpka / paacS+ in the absence of SlPatA (ppatA+, data not shown).
Figure 9. SlPatA modulates growth of E. coli on acetoacetate using SlAacS.
(A) Schematic of predicted regulation of E. coli atoDA aacS+ growth on acetoacetate. (B) E. coli pka atoDA aacS+ strains encoding or lacking cobB were grown on NCE minimal medium supplemented with acetoacetate (30 mM) in the presence of SlPatA (ppatA+). Growth experiments were performed at 37°C using a microtiter plate and a microtiter plate reader (Bio-Tek Instruments). Experiments were conducted in triplicate. Error bars represent standard deviations.
Tucker & Escalante-Semerena
Discussion
A new GNAT and a new acyl-CoA synthetase in S. lividans reveal a functional lysine acetylation system that may control acetoacetate metabolism in this and other actinomycetes
This work identified two new functions in S. lividans. One is a previously uncharacterized GNAT-type protein acetyltransferase with unique domain organization. This GNAT is conserved in some actinomycetes and in the archaeon Archaeoglobus fulgidus (Fig. 1). The existence of this new GNAT (encoded by EFD66247) was revealed through bioinformatics analysis and DNA sequencing to correct database mistakes in the sequence of the gene. The above mentioned analysis showed a reversal in the domain organization of the protein (Fig. 1) with homology to other protein acetyltransferase (Pat) enzymes previously described. Hence we propose the name SlPatA. The second new function is an acyl-CoA synthetase (encoded by EFD70521) responsible for the activation of acetoacetate to acetoacetyl-CoA, thus we propose the name SlAacS for this protein. The name and functional assignments of these proteins are experimentally supported by in vivo and in vitro data (Figs. 3, 4, 5, 8). Collectively, the data show that SlAacS is used by S. lividans to grow on acetoacetate, and that SlPatA catalyzes the acetyl-CoA-dependent acetylation of SlAacS.
The reversal of the domain organization of SlPatA does not affect its acetyltransferase function
In SlPatA, the large C-terminal domain (NDP-forming CoA ligase homologue) is found at the N-terminus of its homologues in S. enterica, E. coli, and R. palustris. This type of domain inversion has been observed in NDP-forming CoA ligases (Sánchez et al., 2000) and phosphoenolpyruvate-dependent sugar:phosphotransferase systems (Reizer & Saier, 1997). This report expands the phenomenon of domain order reversal to Pat-type GNATs. Further characterization of these multi-domain GNAT enzymes is necessary to understand the how the activity of these enzymes may be regulated.
The role of the large NDP-forming CoA ligase-like domain in SlPatA homologues remains unknown. Recently reported data obtained with SePat suggest that this large domain may be responsible multimerization (Thao & Escalante-Semerena, 2011a). Such information is not yet available for SlPat. The domain inversion in SlPatA may reflect an alternative regulatory mechanism.
The SlPatA C-terminal domain contains a proline-rich region with a degenerate G-P-S motif, a signature of the fibrous protein collagen (Hulmes, 1992). In collagen and bacterial proteins with a G-X-Y motif, these motifs adopt extended fibrillar stretches of amino acids (Hulmes, 1992, Xu et al., 2002). In Klebsiella pneumoniae strain FG9 a G-X-Y motif stabilizes homotrimers of the polysaccharide de-branching enzyme pullulanase (Charalambous et al., 1988). The role of the proline-rich region of SlPatA in enzyme structure, oligomerization, and activity remains under investigation.
Control of SlAacS activity by SlPatA retains features observed in the control of other acyl-CoA synthetases by lysine acetylation
The mechanism of SlAacS control by acetylation shares several features with previously reported enzymes under the same control. These features are: i) the acetylation site is a conserved lysine residue in the active site (in SlAacS it is Lys617, Table 1); ii) activity of the enzyme is abolished upon acetylation; and iii) the modification can be enzymatically removed by a bona fide sirtuin deacetylase (Fig. 6). At the moment, what is unclear is which deacetylase in S. lividans is responsible for activation of SlAacSAc. Our data (Fig. 8) cannot unambiguously identify the protein deacetylase, if any, that reactivates acetylated SlAacS in S. lividans. Further analysis is needed to identify the SlPatA cognate deacetylase.
Pat homologues RpPat and SePat (Fig. 1) were previously shown to acetylate and inactivate the acetyl-CoA synthetases RpAcs and SeAcs, respectively. Notably, SlPatA acetylates SlAacS more efficiently than it does S. lividans acetyl-CoA synthetase, SlAcs (Fig. 2, 5). Mikulik et al. demonstrated that Acs from the closely related S. coelicolor is acetylated in vivo (Mikulik et al., 2012), raising the question of which acetyltransferase acetylates SlAcs in S. lividans. Work is currently underway to identify the role of SlPatA and other protein acetyltransferases in SlAcs regulation in S. lividans.
Physiological roles of acetoacetyl-CoA synthetase acetylation in bacteria, archaea, and eukaryotes
Clearly, S. lividans requires AacS activity for growth on acetoacetate (Fig. 8), however, it is not so clear whether this enzyme is needed to activate internally generated or externally transported acetoacetate. The chemical instability of acetoacetate (Hay & Bond, 1967) makes it likely that the source of substrate for SlAacS is internal.
The S. lividans genome encodes five homologues of E. coli AtoB, the 3-ketoacyl-CoA thiolase that converts acetoacetyl-CoA and free coenzyme A to two molecules of acetyl-CoA (Fig. 4A), which are likely catabolized via the ethylmalonyl-CoA pathway for the degradation of acetate (Erb et al., 2007), since S. lividans lacks the glyoxylate cycle for C2 compound assimilation (Lewis et al., 2010). Since the conversion of acetoacetate to acetyl-CoA by SlAacS requires input of ATP, we propose that acetylation and inactivation of SlAacS prevents excess ATP and CoA consumption for synthesis of acetyl-CoA. This effect has been demonstrated in S. enterica when overproduction of acetyl-CoA synthetase (SeAcs) inhibits growth of S. enterica on acetate due to ATP depletion and loss of energy charge (Chan et al., 2011).
Cells of all domains of life synthesize acetoacetyl-CoA synthetase, including archaea, bacteria, nematodes, and mammals (Table 1). In the Gram-negative, nitrogen-fixing bacterium Sinorhizobium meliloti, the aacS homologue acsA is required for growth on poly-3-hydroxybutyrate (PHB) cycle intermediates acetoacetate and 3-hydroxybutyrate (Cai et al., 2000). Some bacteria use the PHB cycle to accumulate and store carbon in a reduced form. PHB can then be used as a carbon an energy source during times of physiological stress (Anderson & Dawes, 1990). Acetylation of AacS may regulate the PHB degradation in S. meliloti and other organisms that utilize AacS as a step of the PHB cycle, potentially expanding the role of lysine acetylation in prokaryotic cell physiology. The presence of a homologue of SlPat in Archaeoglobus fulgidus raises intriguing questions regarding the physiological role of lysine acylation in this extremely thermophilic, sulfate-reducing archaeon, especially because we did not find SlPat homologues in any other archaeal genomes in the databases.
In Caenorhabditis elegans, the SlAacS homologue SUR-5 negatively regulates a vulval differentiation pathway (Gu et al., 1998). In mammals, acetoacetate-CoA synthetase is important for activation of ketone bodies for cholesterol and fatty acid biosynthesis (Buckley & Williamson, 1973, Geelen et al., 1983). AACS mRNA expression is regulated differentially in adipose tissue of genetically obese and nutritionally obese mice, suggesting that AACS may be important in lipogenesis and ketone body levels during hyper nutritional conditions (Yamasaki et al., 2007).
AacS homologues contain the conserved active site lysine (Table 1). Hence, acetylation of acetoacetyl-CoA synthetase may represent a conserved mechanism of acetoacetyl-CoA synthetase control in these organisms.
Apparent complexity of protein deacetylation in S. lividans
The efficient deacetylation of acetylated SlAacS by a sirtuin deacetylase shows that the posttranslational modification of Lys617 is reversible (Fig. 6). The inability of purified S. lividans sirtuin-type deacetylase homologues, CobB1 and CobB2, or the zinc-dependent protein deacetylase homolog EFD68590 to deacetylate SlAacS in vitro was unexpected because sirtuins from S. enterica and E. coli deacetylated SlAacS in vitro and in vivo, respectively (Fig. 6A, 9B). The identity of the enzyme responsible for SlAacS deacetylation in vivo remains unclear. Frankia sp. CcI3, a related actinomycete, encodes an SlPatA homologue that clusters with an SlAacS homologue and an EFD68590 (Zn(II)-dependent deacetylase) homologue (Fig. S2). This gene clustering suggests that Zn(II)-dependent protein deacetylase EFD68590 may regulate of SlAacS activity.
A triple mutant of the S. lividans protein deacetylase homologues grew on acetoacetate (Fig. 8). This result was unexpected because of the accumulation of acetylated SlAacS (Fig. 7A). We propose that: (i) the level of non-acetylated SlAacS in S. lividans supports growth on acetoacetate as a carbon source, and deacetylation would not be necessary for growth under these conditions; (ii) not all the SlAacS in the cell is acetylated, and what remains provides sufficient activity to support growth of S. lividans on acetoacetate; or (iii) S. lividans may encode an additional class of protein deacetylase capable of reactivating SlAacS.
Experimental procedures
Bacterial strains and growth conditions
All strains and plasmids used in this study are listed in Tables S1 and S2, respectively. Streptomyces strains are derivatives of Streptomyces lividans TK24. ISP-2 medium (Shirling & Gottlieb, 1966) or R2YE medium (Kieser et al., 2000c) was used to culture S. lividans on solid medium. Liquid cultures of S. lividans were grown in either yeast extract-malt extract (YEME) rich medium or NMMP medium supplemented with lithium acetoacetate (10 mM) (Kieser et al., 2000c). S. lividans liquid cultures were grown in baffled flasks (YEME) or with marine-grade stainless steel springs (NMMP) to aid in cell dispersion. Strains were cultured 3 to 5 days at 30°C or 42°C. When necessary, antibiotics were used at the following concentrations: apramycin, 50 μg ml−1 (YEME, ISP-2); thiostrepton, 10 μg ml−1 (YEME), 5 μg ml−1 (ISP2, R2YE, NMMP).
Unless noted otherwise, all E. coli strains used were derivatives of E. coli MG1655. E. coli strains were grown at 37°C in lysogeny broth (LB, Difco) (Bertani, 1951) or no-carbon essential (NCE) minimal medium (Berkowitz et al., 1968) supplemented with lithium acetoacetate (30 mM), MgSO4 (1 mM), and ampicillin (100 μg ml−1). When necessary, antibiototics were used at the following concentrations: ampicillin, 100 μg ml−1; apramycin, 50 μg ml−1; chloramphenicol, 12.5 μg ml−1. L-(+)-arabinose was added at a final concentration of 50 μM to induce the expression of the E. coli codon-optimized S. lividans aacS (EFD70521) from the ParaBAD promoter. Isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to a final concentration of 50 μM IPTG to induce expression of S. lividans patA (EFD66247).
Molecular techniques
DNA manipulations were performed using standard techniques (Elion et al., 2007). Restriction endonucleases were purchased from Fermentas. DNA was amplified using Pfu Ultra II Fusion DNA polymerase (Agilent) or Herculase II Fusion DNA polymerase (Agilent). Site-directed mutagenesis was performed using the Quikchange™ Site Directed Mutagenesis kit (Agilent). Plasmids were isolated using the Wizard Plus SV Miniprep kit (Promega) and PCR products were purified using the Wizard SV Gel and PCR Clean-Up System (Promega). DNA sequencing was performed using BigDye® (ABI PRISM) protocols, and sequencing reactions were resolved at the University of Wisconsin-Madison Biotechnology Center. Oligonucleotide primer sequences are listed in Table S2.
Plasmids used for protein overproduction
The S. lividans patA (EFD66247), aacS (EFD70521), and acs (EFD68454) genes were amplified from purified S. lividans TK24 genomic DNA with the primers listed in Table S2. The first codon of SlAcs (GTG) and SlPatA (TTG) were changed to the more common ATG start codon. The DNA fragments were digested with NheI and EcoRI and ligated into pTEV5 (Rocco et al., 2008) cut with the same enzymes. The resulting plasmids pSlPatA1 and pSlAacS1, and pSlAcs1 direct synthesis of SlPatA, SlAacS, and SlAcs, respectively, with N-terminal H6 tags cleavable by recombinant tobacco etch virus (rTEV) protease prepared as described (Blommel & Fox, 2007). Plasmids directing synthesis of SlAacSK617A and SlAcsK610A variants were generated from the pSlAacS1 and pSlAcs1 plasmids using site-directed mutagenesis.
Construction of other plasmids
E. coli complementation plasmids
The S. lividans aacS (EFD70521) was amplified from purified S. lividans TK24 genomic DNA or from the aacS allele whose codon usage was optimized for E. coli (Genscript) with the primers that included an optimized ribosome-binding site (Table S2). The native S. lividans aacS DNA fragment was cut with HindIII and EcoRI and ligated into pBAD30, cut with the same enzymes. The E. coli codon-optimized S. lividans aacS DNA fragment was cut with EcoRI and KpnI and ligated into pBAD30 (Guzman et al., 1995) cut with the same enzymes. The resulting plasmids pSlAacS4 and pSlAacS6 express the native aacS or optimized aacS genes under the control of the arabinose-inducible ParaBAD.
S. lividans patA (EFD66247), was amplified from purified S. lividans TK24 genomic DNA with the primers listed in Table S2. The patA DNA fragment was digested with NdeI and KpnII and ligated into pSRK-Km (Khan et al., 2008) cut with the same enzymes. The resulting plasmid pSlPatA9 express the S. lividans patA gene under the control of the lacIq-lac promoter-operator system.
S. lividans H6-SlAacS plasmid
The S. lividans aacS gene (EFD70521) was amplified from purified S. lividans TK24 genomic DNA with the primers listed in Table S2. The S. lividans aacS DNA fragment was cut with XbaI and HindIII and ligated into pSE34 (Ward et al., 1986) cut with the same enzymes. The resulting plasmids pSlAacS5 expresses S. lividans aacS with an N-terminal H6 tag under the control of the erm promoter.
Construction of gene deletion in E. coli
An in-frame deletion of atoDA genes in E. coli was constructed as using the phage lambda Red recombinase system as previously described (Datsenko & Wanner, 2000).
Construction of gene deletions in S. lividans
An in-frame deletion of S. lividans patA (EFD66247) was generated using described protocols (Martinez et al., 2004). DNA fragments of 1.5 kb in length were amplified from the DNA upstream and downstream of S. lividans patA using purified S. lividans TK24 genomic DNA. The resulting fragments were cloned into pKC1139 (Bierman et al., 1992) using the In-Fusion ND Cloning Kit (Clontech) and transformed into E. coli Stellar competent cells (Clontech). The resulting plasmid (pKC1139-ΔpatA) was conjugated into S. lividans using the helper strain HB101 harboring pRK2013 (Figurski & Helinski, 1979) on mannitol soya (MS) agar as previously described (Kieser et al., 2000b). Plates were flooded with apramycin (50 μg ml−1 final concentration) 16 h after plating the conjugation mixtures to select for S. lividans carrying pKC1130-ΔpatA. Apramycin-resistant strains were inoculated into 25 ml YEME + apramycin medium and grown in baffled flasks at 30°C for 4 days. Strains were then plated on ISP-2 + apramycin and incubated at 42°C for 3 days to select for a strain in which the plasmid had integrated into the chromosome. Apramycin-resistant strains that grew at 42°C were inoculated into 25 ml YEME in baffled flasks and incubated at 30°C for 4 days and subsequently plated on ISP-2 at 30°C to promote loss of the integrated plasmid. Isolated colonies were screened on ISP-2 for apramycin resistance. Apramycin-sensitive strains were screened by PCR for deletion of the patA gene.
Protein purification
SlPatA purification
Plasmid pSlPatA1 was transformed into E. coli strain C41λ(DE3)/pLysSRARE2 (EMD Millipore). The resulting strains were grown overnight and sub-cultured 1:100 (v/v) into 8 liters of superbroth containing ampicillin (100 μg ml−1) and chloramphenicol (12.5 μg ml−1). The cultures were grown shaking at 37 °C to A600 ~ 0.7 and H6-SlPatA synthesis was induced with IPTG (0.5 mM). Upon induction, the cultures were grown overnight at 30°C. Cells were harvested at 6000 × g for 10 min at 4°C in a Avanti J-2 XPI centrifuge fitted with rotor JLA-8.1000 (Beckman Coulter). Cell pellets were resuspended in 30 ml cold His-Bind buffer (buffer A) [tris(hydroxymethyl)aminomethane-HCl (Tris-HCl) buffer (50 mM, pH 8), NaCl (500 mM)], and imidazole (5 mM)) containing phenylmethanesulfonylfluoride (PMSF, 1 mM). Cells were placed on ice and lysed by sonication for 2 min (2-s pulse followed by 4 s of cooling) at level 7 in a model 550 sonic dismembrator (Fisher). The extract was cleared by centrifugation at 4°C for 30 min at 43,367×g. Clarified cell extract was loaded onto a 1 ml HisTrap FF column (GE Healthcare) connected to a computer-controlled ÄKTA fast protein liquid chromatography (FPLC) system. Unbound proteins were eluted off the column by extensive washing with buffer A. A 10-ml wash step with 90% buffer A and 10% buffer B [Tris-HCl buffer (50 mM, pH 8), NaCl (500 mM), and imidazole (250 mM)] was applied to the column a prior to a 10-ml linear gradient 10–100% Buffer B. All fractions containing H6-SlPatA were combined. rTEV protease was added to H6-SlPatA and the SlPatA/rTEV mixture was incubated at room temperature for 3 h. PMSF was added to the protein mixture and incubated 15 min at room temperature. The SlPatA/rTEV mixture was dialyzed at 4°C against buffer C (Tris-HCl (50 mM, pH 8), NaCl (500 mM)) twice for 3 hours and again against buffer C containing imidazole (5 mM) for 12 h. After cleavage and dialysis, protein mixtures were passed over the 1-ml HisTrap column using the buffers described above. Cleaved SlPatA was desorbed from the resin using a 50 ml wash step with 97% buffer A and 3% Buffer B prior to a 25-ml linear gradient 3–100% Buffer B. Purified SlPatA was analyzed by SDS-PAGE. Fractions containing SlPatA were pooled together. SlPatA was stored in Tris-Cl buffer (50 mM, pH 8.0) containing NaCl (100 mM) and glycerol (20%, v/v). SlPatA concentration was determined by measuring absorbance at 280 nm. The molar extinction coefficient used to calculate SlPatA concentration was 57,760 M−1cm−1.
SlAcs and SlAacS purification
Plasmids containing S. lividans acs or aacS were transformed with pRARE2 (EMD Millipore) into a Δpat derivative of C41λ(DE3) (JE9314) to prevent acetylation prior to overproduction. The resulting strains were grown overnight and sub-cultured 1:100 (v/v) into 2 liters of LB containing ampicillin (100 μg ml−1) and chloramphenicol (12.5 μg ml−1). The cultures were grown shaking at 37 °C to A600 ~ 0.7 and protein synthesis was induced with IPTG (0.25 mM). Upon induction, the cultures were grown overnight at 30°C. SlAcs and SlAacS proteins were purified and stored as described above with modifications. During the first purification step, a 10-ml wash step with 94% buffer A and 6% buffer B was applied to the column a prior to a 10-ml linear gradient 6–100% buffer B. SlAcs and SlAacS proteins did not adsorb to the column and were present in the flow-through fractions. The molar extinction coefficient used to calculate protein concentrations were 135,455 cm−1 M−1 for SlAcs and 142,320 cm−1 M−1 for SlAacS.
SeAcs purification
Plasmid pACS10 was transformed into a Δpat derivative of C41λ(DE3) (JE9314). The resulting strain was grown overnight and sub-cultured 1:100 (v/v) into 2 liters of LB containing ampicillin (100 μg ml−1). The culture was grown shaking at 37 °C to A600 ~ 0.7 and protein synthesis was induced with IPTG (0.25 mM). Upon induction, the cultures were grown overnight at 30°C. SeAcs was purified and stored as described (Starai et al., 2002).
In vitro acyl-CoA synthetase assays
SlAacS activity was measured using an NADH-consuming assay (Garrity et al., 2007, Crosby et al., 2010, Fukui et al., 1982, Ito et al., 1984). Reactions (100 μl total volume) contained HEPES buffer (50 mM, pH 7.5), TCEP (1 mM), ATP (2.5 mM) CoA (0.5 mM), MgCl2 (5 mM), KCl (1 mM), phosphoenolpyruvate (3 mM), NADH (0.1 mM), pyruvate kinase (1 U), myokinase (5 U), lactate dehydrogenase (1.5 U) and either acetoacetate, β-hydroxybutyrate, butyrate, isovalarate, malonate, crotonate, isobutyrate, succinate, propionate, or acetate (0.2 mM). Reactions were started by the addition of SlAacS (15 nM). The absorbance at 340 nm was monitored in a 96-well plate using the Spectramax Plus UV-visible spectrophotometer (Molecular Devices). Enzyme activities were calculated as described (Garrity et al., 2007). Specific activity data are presented with standard deviations from triplicate experiments.
HPLC separation and mass spectral analysis of SlAacS reaction product
To determine the molecular mass of the SlAacS product, SlAacS was incubated in the presence of CoA, ATP, and acetoacetate, described above, in a final volume of 1 mL. SlAacS was removed from reactions by filtration using Amicon Ultracel centrifugal filters (3-KDa molecular mass size exclusion). Filtrates containing reaction products and reagents were separated by analytical by reverse-phase ion-pair HPLC using a Beckman Coulter System Gold 126 system equipped with a Phenomenex Synergi Hydro-RP (150 by 4.5 mm, 5 micron particle size, 2.5 ml column volume) column at a flow rate of 1 mL min−1. The solvent system was previously described (Horswill & Escalante-Semerena, 2002, Kawamoto et al., 1998) Briefly, the column was equilibrated with buffer A (100 mM KH2P04, 2 mM tetra-butyl ammonium bromide (TBAB), 15% CH3CN, pH 3.3). Sample was injected onto the column and washed with buffer A for 5 min. Acetoacetyl-CoA was eluted by a 10-min linear gradient from buffer A to buffer B (100 mM KH2P04, 2 mM TBAB, 35% CH3CN, pH 3.3). TCEP was added to each sample to a final concentration of 2 mM before injection. Compounds eluted with the following retention times: CoA, 4.7 min; ATP, 2.6 min; AMP, 2.6 min, Acetoacetyl-CoA, 7.3 min. The sample containing acetoacetyl-CoA that eluted from 7 to 9 min was dried under vacuum using an Eppendorf Vacufuge plus concentrator operating at room temperature. The dried sample was resuspended in dH2O and applied onto a C18 Sep-Pak (Waters) previously conditioned with 10 mL of 100% methanol followed by 10 mL of dH2O. Acetoacetyl-CoA that bound to the column was washed with 10 mL of dH2O before elution with 100% methanol. Acetoacetyl-CoA dissolved in methanol was concentrated under vacuum. The pellet was resuspended in dH2O form mass determination by mass spectrometry. The sample as analyzed on a Bruker Autoflex Matrix Assisted Laser Desorption/Ionization-Time-of-Flight mass spectrometer scanning 80–2275 m/z using 300 shots of nitrogen laser in delayed extraction and positive reflectron mode summed together to form the mass spectrum. Matrix for the analysis was dihydroxybenzoate (15 mg/ml, Sigma) in a 1:1 solution of water: 0.1% TFA, CH3CN (v/v).
In vitro protein acetylation assay
Protein acetylation was observed using radiolabeled Ac-CoA as described (Starai & Escalante-Semerena, 2004, Tucker & Escalante-Semerena, 2010, Crosby et al., 2010). Acetylation reactions contained 2(Bis(2-hydroxyethyl)imino)-2-(hydroxymethyl)-1,3-propanediol (Bis-Tris-HCl) buffer (50 mM, pH 6.0), [1-14C]-Ac-CoA (20 μM), acyl-CoA synthetase (3 μM), glycerol (10%, v/v), and SlPatA (1 μM). Reactions (10 μl total volume) were incubated for min at 30°C. Samples (5 μl) were resolved using SDS-PAGE (Laemmli, 1970) and proteins were visualize by Coomassie Blue staining. Gels were dried and exposed 16 h to a multipurpose phosphor screen (Packard). Labeled proteins were visualized using a Typhoon FLA 9000 Variable Mode Imager (GE Healthcare) equipped with ImageQuant TL software (GE Healthcare).
The effect of acetylation on activity of SlAacS, SlAcs, and SeAcs activity was determined as described (Crosby et al., 2010) with modifications. SlAacS (3μM) was incubated with SlPatA (1 μM) and 50 μM Ac-CoA for 90 minutes at 30°C using the buffer system described above. At 0, 15, 30, 60, and 90 min time points, reactions were diluted 1:20 into 50 mM HEPES buffer pH 7.5 at 4°C. SlAacS, SlAcs, and SeAcs activity were measured as described above using the appropriate organic acid substrate.
In vitro deacetylation assays
Acetylated SlAacS was deacetylated with Salmonella enterica CobBS as described (Tucker & Escalante-Semerena, 2010). In vitro acetylated SlAacS (3 μM, radiolabeled or non-radiolabeled) was incubated with SeCobBS (3 μM) in deacetylation buffer containing 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, 50 mM, pH 7.0), NAD+ (1 mM) for 60 min at 37°C (25 μl reaction volume). Reaction mixture samples (5 μl) were resolved by SDS-PAGE, and subjected to phosphor imaging analysis to assess the acetylation state of SlAacS after incubation with SeCobBS. SlAacS activity of the non-radiolabeled reactions was measured using the CoA synthetase assay described above.
Purification or SlAacS from S. lividans
Plasmid pSlAacS5 was introduced into S. lividans TK24 and S. lividans ΔpatA (JE16707) by polyethyleneglycol (PEG)-assisted protoplast transformation as described (Kieser et al., 2000b, Kieser et al., 2000a) to generate JE16731 and JE16821 (Table S1), respectively. Cells were plated on R2YE and grown at 30°C for 16 hours. Plates were flooded with thiostrepton (10 μg ml−1 final concentration) and incubated 3 days at 30°C to select for strains harboring pSlAacS5. S. lividans strains harboring the pSlAacS5 plasmid encoding H6-SlAacS were grown in 30 ml YEME + thiostrepton for 5 days. Cells were harvested by centrifugation at 2000 × g for 10 min at 4°C. Cell mass was measured and cells were resuspended in an equal volume of NMMP. Approximately 0.1 g of cells were inoculated into 250 ml NMMP + thiostrepton supplemented with acetoacetate (10 mM). Strains were grown for 24 h at 30°C with shaking. Cells were harvested as described above and washed twice with 50 ml wash buffer containing Tris-HCl buffer (50 mM, pH 8.0) and NaCl (500 mM). Cells pellets were resuspended in 30 ml buffer A (described above) supplemented with PMSF (1 mM), Sigma protease inhibitor cocktail for histidine-tagged proteins (100 μl), and lysozyme (1 mg ml−1). Cells were lysed by sonication and cell debris was removed by centrifugation. H6-SlAacS was purified using 250 μl HisPur Ni-NTA resin (Pierce). Using the same buffer system described for SlAacS above. SlAacS-containing fractions were combined and dialyzed overnight into HEPES buffer (50 mM, pH 7.5) containing NaCl (150 mM) and glycerol (20%, v/v).
SlAacS of SlAacS activity was assessed using the deacetylation reaction described above. SlAacS (1 μM) purified from JE16731 and JE16821 (Table S1) were incubated with or without SeCobBS as described in the above section for 1 h at 37°C. A sample for the deacetylation reaction mixtures was used to assess SlAacS activity. The concentration of SlAacS in the CoA synthetase activity assay was 25 nM.
Western blot analysis of H6-SlAacS purified from S. lividans
Purified SlAacS or in vitro acetylated SlAacS were resolved along with H6-SlAacS purified from S. lividans strains JE16731 and JE16821 (Table 1) (2 ug each) using SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore). Total protein was visualized by staining 15 seconds in Ponceau-S staining solution (0.1% (w/v) Ponceau S in 5% (v/v) acetic acid) and de-staining with distilled water. After extensive de-staining, the PVDF membranes were probed with polyclonal rabbit α-acetylated lysine antibodies (1:1,700; Calbiochem) as primary antibody and goat α-rabbit immunoglobulin G conjugated to calf intestinal alkaline phosphatase (Pierce) (1:10,000) as secondary antibody to detect SlAacSAc. Signal was detected using nitro-blue tetrazolium chloride and 5-bromo-4-chloro-3′-indolylphosphate p-toluidine salt (NBT-BCIP) 1-Step Solution according to the manufacturer's instructions (Pierce).
Determination of the SlAacS acetylation site by mass spectrometry
To determine the identity of the in vivo modification of SlAacS, H6-SlAacS purified from S. lividans was resolved by SDS-PAGE and the band corresponding to SlAacS was excised. “In Gel” digestion and mass spectrometric analysis was done at the Mass Spectrometry Facility [Biotechnology Center, University of Wisconsin-Madison]. The digestion was performed as outlined on the website: http://www.biotech.wisc.edu/facilities/massspec/protocols/ingelprotocol.
Peptides were analyzed by nanoLC-MS/MS using the Agilent 1100 nanoflow system (Agilent, Palo Alto, CA) connected to a hybrid linear ion trap-orbitrap mass spectrometer (LTQ-Orbitrap, Thermo Fisher Scientific, Bremen, Germany) equipped with a nanoelectrospray ion source (Proxeon Biosystems, Odense, Denmark). Chromatography of peptides prior to mass spectral analysis was accomplished using C18 reverse phase HPLC trap column (Zorbax 300SB-C18, 5μM, 5×0.3mm, Agilent) and capillary emitter column (in-house packed with MAGIC C18, 3 μM, 15×0.075mm, Michrom Bioresources, Inc.) onto which 8μl of extracted peptides were automatically loaded. NanoHPLC system delivered solvents A: 0.1% (v/v) formic acid in water, and B: 95% (v/v) acetonitrile, 0.1% (v/v) formic acid at either 10 μL/min, to load sample, or 0.20 μL/min, to elute peptides directly into the nano-electrospray over a 60 minutes 1% (v/v) B to 60% (v/v) B followed by 10 minute 60% (v/v) B to 100% (v/v) B gradient. As peptides eluted from the HPLC-column/electrospray source survey MS scans were acquired in the orbitrap with a resolution of 100 000 and up to 5 most intense peptides per scan were fragmented and detected in the ion trap over the 400 to 2000 m/z; redundancy was limited by dynamic exclusion. Raw MS/MS data were converted to mgf file format using Trans Proteomic Pipeline (Seattle Proteome Center, Seattle, WA). Resulting mgf files were used to search user defined amino acid sequence database using in-house Mascot search engine 2.2.07 (Matrix Science, London, UK) with Cysteine carbamidomethylation as fixed modification and Lysine acetylation, methionine oxidation, and Asparigine/Glutamine deamidation as variable modifications. Peptide mass tolerance was set at 20 ppm and fragment mass at 0.8 Da. Protein annotations and significance of identification was done with help of Scaffold software (version 3.6.1, Proteome Software Inc., Portland, OR). Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm (Keller et al., 2002). Protein identifications were accepted if they could be established at greater than 95.0% probability and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (Nesvizhskii et al., 2003). Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Supplementary Material
Acknowledgments
This work was supported by USPHS grant R01-GM62203 to J.C.E.-S. A.C.T. was supported by the NIH Molecular Biosciences Training Grant T32-GM07215.
We thank Michael Thomas (UW – Madison) for providing Streptomyces lividans TK24 and plasmids pKC1139 and pSE34. We also thank Michael Thomas, John Barkei, and Matt McMahon for valuable advice about working with S. lividans.
Footnotes
The authors have no conflict of interest to declare.
References
- Anderson AJ, Dawes EA. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K, Brodsky B. Supercoiled protein motifs: the collagen triple-helix and the alpha-helical coiled coil. J. Struct. Biol. 1998;122:17–29. doi: 10.1006/jsbi.1998.3965. [DOI] [PubMed] [Google Scholar]
- Berkowitz D, Hushon JM, Whitfield HJ, Jr., Roth J, Ames BN. Procedure for identifying nonsense mutations. J. Bacteriol. 1968;96:215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman M, Logan R, O'Brien K, Seno ET, Rao RN, Schoner BE. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- Blommel PG, Fox BG. A combined approach to improving large-scale production of tobacco etch virus protease. Protein Expr. Purif. 2007;55:53–68. doi: 10.1016/j.pep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley BM, Williamson DH. Acetoacetate and brain lipogenesis: developmental pattern of acetoacetyl-coenzyme A synthetase in the soluble fraction of rat brain. Biochem. J. 1973;132:653–656. doi: 10.1042/bj1320653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai GQ, Driscoll BT, Charles TC. Requirement for the enzymes acetoacetyl coenzyme A synthetase and poly-3-hydroxybutyrate (PHB) synthase for growth of Sinorhizobium meliloti on PHB cycle intermediates. J. Bacteriol. 2000;182:2113–2118. doi: 10.1128/jb.182.8.2113-2118.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CH, Garrity J, Crosby HA, Escalante-Semerena JC. In Salmonella enterica, the sirtuin-dependent protein acylation/deacylation system (SDPADS) maintains energy homeostasis during growth on low concentrations of acetate. Mol. Microbiol. 2011;80:168–183. doi: 10.1111/j.1365-2958.2011.07566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charalambous BM, Keen JN, McPherson MJ. Collagen-like sequences stabilize homotrimers of a bacterial hydrolase. EMBO J. 1988;7:2903–2909. doi: 10.1002/j.1460-2075.1988.tb03148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater KF. Streptomyces inside-out: a new perspective on the bacteria that provide us with antibiotics. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361:761–768. doi: 10.1098/rstb.2005.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther T, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Courtois S, Cappellano CM, Ball M, Francou FX, Normand P, Helynck G, Martinez A, Kolvek SJ, Hopke J, Osburne MS, August PR, Nalin R, Guerineau M, Jeannin P, Simonet P, Pernodet JL. Recombinant environmental libraries provide access to microbial diversity for drug discovery from natural products. Appl. Environ. Microbiol. 2003;69:49–55. doi: 10.1128/AEM.69.1.49-55.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby HA, Heiniger EK, Harwood CS, Escalante-Semerena JC. Reversible N(epsilon)-lysine acetylation regulates the activity of acyl-CoA synthetases involved in anaerobic benzoate catabolism in Rhodopseudomonas palustris. Mol. Microbiol. 2010;76:874–888. doi: 10.1111/j.1365-2958.2010.07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby HA, Pelletier DA, Hurst GB, Escalante-Semerena JC. System-wide studies of N-lysine acetylation in Rhodopseudomonas palustris reveal substrate specificity of protein acetyltransferases. J. Biol. Chem. 2012;287:15590–15601. doi: 10.1074/jbc.M112.352104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion EA, Marina P, Yu L. Constructing recombinant DNA molecules by PCR. In: Ausubel FM, Brent RER, Kingston DD, Moore JG, Seidman JA, Smith a., Struhl K, editors. Current protocols in molecular biology. Greene Publishing Associates & Wiley Interscience; New York, N.Y.: 2007. pp. Unit 3.17.11–13.17.12. [DOI] [PubMed] [Google Scholar]
- Erb TJ, Berg IA, Brecht V, Muller M, Fuchs G, Alber BE. Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10631–10636. doi: 10.1073/pnas.0702791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T, Ito M, Tomita K. Purification and characterization of acetoacetyl-CoA synthetase from Zoogloea ramigera I-16-M. Eur. J. Biochem. 1982;127:423–428. doi: 10.1111/j.1432-1033.1982.tb06889.x. [DOI] [PubMed] [Google Scholar]
- Gardner JG, Grundy FJ, Henkin TM, Escalante-Semerena JC. Control of acetyl-coenzyme A synthetase (AcsA) activity by acetylation/deacetylation without NAD(+) involvement in Bacillus subtilis. J. Bacteriol. 2006;188:5460–5468. doi: 10.1128/JB.00215-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity J, Gardner JG, Hawse W, Wolberger C, Escalante-Semerena JC. N-lysine propionylation controls the activity of propionyl-CoA synthetase. J. Biol. Chem. 2007;282:30239–30245. doi: 10.1074/jbc.M704409200. [DOI] [PubMed] [Google Scholar]
- Geelen MJ, Lopes-Cardozo M, Edmond J. Acetoacetate: a major substrate for the synthesis of cholesterol and fatty acids by isolated rat hepatocytes. FEBS Lett. 1983;163:269–273. doi: 10.1016/0014-5793(83)80833-3. [DOI] [PubMed] [Google Scholar]
- Gu T, Orita S, Han M. Caenorhabditis elegans SUR-5, a novel but conserved protein, negatively regulates LET-60 Ras activity during vulval induction. Mol. Cell. Biol. 1998;18:4556–4564. doi: 10.1128/mcb.18.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RW, Bond MA. Kinetics of decarboxylation of acetoacetic acid. Aust. J. Chem. 1967;20:1823–1828. [Google Scholar]
- Horswill AR, Escalante-Semerena JC. Characterization of the propionyl-CoA synthetase (PrpE) enzyme of Salmonella enterica: Residue Lys592 is required for propionyl-AMP synthesis. Biochemistry. 2002;41:2379–2387. doi: 10.1021/bi015647q. [DOI] [PubMed] [Google Scholar]
- Hulmes DJ. The collagen superfamily - diverse structures and assemblies. Essays Biochem. 1992;27:49–67. [PubMed] [Google Scholar]
- Ikeuchi Y, Kitahara K, Suzuki T. The RNA acetyltransferase driven by ATP hydrolysis synthesizes N4-acetylcytidine of tRNA anticodon. Embo J. 2008;27:2194–2203. doi: 10.1038/emboj.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Fukui T, Kamokari M, Saito T, Tomita K. Purification and characterization of acetoacetyl-CoA synthetase from rat liver. Biochim. Biophys. Acta. 1984;794:183–193. [PubMed] [Google Scholar]
- Jenkins LS, Nunn WD. Genetic and molecular characterization of the genes involved in short-chain fatty acid degradation in Escherichia coli: the ato system. J. Bacteriol. 1987;169:42–52. doi: 10.1128/jb.169.1.42-52.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S, Ochi K. Comparative ribosomal protein (L11 and L30) sequence analyses of several Streptomyces spp. commonly used in genetic studies. Int. J. Syst. Bacteriol. 1998;48:597–600. doi: 10.1099/00207713-48-2-597. [DOI] [PubMed] [Google Scholar]
- Kawamoto Y, Shinozuka K, Kunitomo M, Haginaka J. Determination of ATP and its metabolites released from rat caudal artery by isocratic ion-pair reversed-phase high-performance liquid chromatography. Anal. Biochem. 1998;262:33–38. doi: 10.1006/abio.1998.2729. [DOI] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Khan SR, Gaines J, Roop RM, 2nd, Farrand SK. Broad-host-range expression vectors with tightly regulated promoters and their use to examine the influence of TraR and TraM expression on Ti plasmid quorum sensing. Appl. Environ. Microbiol. 2008;74:5053–5062. doi: 10.1128/AEM.01098-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T, Bibb MJ, Buttner MJ, Chater K, Hopwood DA. Practical Streptomyces Genetics. John Innes Foundation; Norwich, England: 2000a. Growth and preservation of Streptomyces; pp. 43–62. [Google Scholar]
- Kieser T, Bibb MJ, Buttner MJ, Chater K, Hopwood DA. Practical Streptomyces Genetics. John Innes Foundation; Norwich, England: 2000b. Introduction of DNA into Streptomyces; pp. 229–252. [Google Scholar]
- Kieser T, Bibb MJ, Buttner MJ, Chater K, Hopwood DA. Practical Streptomyces Genetics. John Innes Foundation; Norwich, England: 2000c. Media, buffers, and suppliers; pp. 405–420. [Google Scholar]
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lang PT, Fortune SM, Sassetti CM, Alber T. Cyclic AMP regulation of protein lysine acetylation in Mycobacterium tuberculosis. Nat. Struct. Mol. Biol. 2012 doi: 10.1038/nsmb.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RA, Laing E, Allenby N, Bucca G, Brenner V, Harrison M, Kierzek AM, Smith CP. Metabolic and evolutionary insights into the closely-related species Streptomyces coelicolor and Streptomyces lividans deduced from high-resolution comparative genomic hybridization. BMC Genomics. 2010;11:682. doi: 10.1186/1471-2164-11-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Kolvek SJ, Yip CL, Hopke J, Brown KA, MacNeil IA, Osburne MS. Genetically modified bacterial strains and novel bacterial artificial chromosome shuttle vectors for constructing environmental libraries and detecting heterologous natural products in multiple expression hosts. Appl. Environ. Microbiol. 2004;70:2452–2463. doi: 10.1128/AEM.70.4.2452-2463.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon MD, Guan C, Handelsman J, Thomas MG. Metagenomic analysis of Streptomyces lividans reveals host-dependent functional expression. Appl. Environ. Microbiol. 2012;78:3622–3629. doi: 10.1128/AEM.00044-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulik K, Felsberg J, Kudrnacova E, Bezouskova S, Setinova D, Stodulkova E, Zidkova J, Zidek V. CobB1 deacetylase activity in Streptomyces coelicolor. Biochem. Cell Biol. 2012;90:179–187. doi: 10.1139/o11-086. [DOI] [PubMed] [Google Scholar]
- Nambi S, Basu N, Visweswariah SS. Cyclic AMP-regulated protein lysine acetylases in mycobacteria. J. Biol. Chem. 2010;285:24313–24323. doi: 10.1074/jbc.M110.118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Pauli G, Overath P. ato Operon: a highly inducible system for acetoacetate and butyrate degradation in Escherichia coli. Eur. J. Biochem. 1972;29:553–562. doi: 10.1111/j.1432-1033.1972.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Reizer J, Saier MH., Jr. Modular multidomain phosphoryl transfer proteins of bacteria. Curr. Opin. Struct. Biol. 1997;7:407–415. doi: 10.1016/s0959-440x(97)80059-0. [DOI] [PubMed] [Google Scholar]
- Rocco CJ, Dennison KL, Klenchin VA, Rayment I, Escalante-Semerena JC. Construction and use of new cloning vectors for the rapid isolation of recombinant proteins from Escherichia coli. Plasmid. 2008;59:231–237. doi: 10.1016/j.plasmid.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez LB, Galperin MY, Muller M. Acetyl-CoA synthetase from the amitochondriate eukaryote Giardia lamblia belongs to the newly recognized superfamily of acyl-CoA synthetases (Nucleoside diphosphate-forming) J. Biol. Chem. 2000;275:5794–5803. doi: 10.1074/jbc.275.8.5794. [DOI] [PubMed] [Google Scholar]
- Seow KT, Meurer G, Gerlitz M, Wendt-Pienkowski E, Hutchinson CR, Davies J. A study of iterative type II polyketide synthases, using bacterial genes cloned from soil DNA: a means to access and use genes from uncultured microorganisms. J. Bacteriol. 1997;179:7360–7368. doi: 10.1128/jb.179.23.7360-7368.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KJ, Rather PN, Hare RS, Miller GH. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirling EB, Gottlieb D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966;16:313–340. [Google Scholar]
- Soppa J. Protein acetylation in archaea, bacteria, and eukaryotes. Archaea. 2010;2010 doi: 10.1155/2010/820681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spange S, Wagner T, Heinzel T, Kramer OH. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int. J. Biochem. Cell. Biol. 2009;41:185–198. doi: 10.1016/j.biocel.2008.08.027. [DOI] [PubMed] [Google Scholar]
- Staack H, Binstock JF, Schulz H. Purification and properties of a pig heart thiolase with broad chain length specificity and comparison of thiolases from pig heart and Escherichia coli. J. Biol. Chem. 1978;253:1827–1831. [PubMed] [Google Scholar]
- Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- Starai VJ, Escalante-Semerena JC. Identification of the protein acetyltransferase (Pat) enzyme that acetylates acetyl-CoA synthetase in Salmonella enterica. J. Mol. Biol. 2004;340:1005–1012. doi: 10.1016/j.jmb.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Takenoya M, Nikolakakis K, Sagermann M. Crystallographic insights into the pore structures and mechanisms of the EutL and EutM shell proteins of the Eut-BMC. J. Bacteriol. 2010;192:6056–6063. doi: 10.1128/JB.00652-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thao S, Chen CS, Zhu H, Escalante-Semerena JC. N(epsilon)-Lysine acetylation of a bacterial transcription factor inhibits its DNA-binding activity. PLoS ONE. 2010;5:e15123. doi: 10.1371/journal.pone.0015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thao S, Escalante-Semerena JC. Biochemical and thermodynamic analyses of Salmonella enterica Pat, a multidomain, multimeric N(epsilon)-lysine acetyltransferase involved in carbon and energy metabolism. mBio. 2011a;2:e00216–00211. doi: 10.1128/mBio.00216-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thao S, Escalante-Semerena JC. Control of protein function by reversible N(epsilon)-lysine acetylation in bacteria. Curr. Opin. Microbiol. 2011b;14:200–204. doi: 10.1016/j.mib.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang AW, Escalante-Semerena JC. CobB, a new member of the SIR2 family of eucaryotic regulatory proteins, is required to compensate for the lack of nicotinate mononucleotide:5,6-dimethylbenzimidazole phosphoribosyltransferase activity in cobT mutants during cobalamin biosynthesis in Salmonella typhimurium LT2. J. Biol. Chem. 1998;273:31788–31794. doi: 10.1074/jbc.273.48.31788. [DOI] [PubMed] [Google Scholar]
- Tucker AC, Escalante-Semerena JC. Biologically active isoforms of CobB sirtuin deacetylase in Salmonella enterica and Erwinia amylovora. J. Bacteriol. 2010;192:6200–6208. doi: 10.1128/JB.00874-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetting MW, Carvalho L. P. S. d., Yu M, Hegde SS, Magnet S, Roderick SL, Blanchard JS. Structure and functions of the GNAT superfamily of acetyltransferases. Arch. Biochem. Biophys. 2005;433:212–226. doi: 10.1016/j.abb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ward JM, Janssen GR, Kieser T, Bibb MJ, Buttner MJ. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol. Gen. Genet. 1986;203:468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- Weinert BT, Wagner SA, Horn H, Henriksen P, Liu WR, Olsen JV, Jensen LJ, Choudhary C. Proteome-wide mapping of the Drosophila acetylome demonstrates a high degree of conservation of lysine acetylation. Science signaling. 2011;4:ra48. doi: 10.1126/scisignal.2001902. [DOI] [PubMed] [Google Scholar]
- Xu H, Hegde SS, Blanchard JS. Reversible acetylation and inactivation of Mycobacterium tuberculosis acetyl-CoA synthetase is dependent on cAMP. Biochemistry. 2011;50:5883–5892. doi: 10.1021/bi200156t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Keene DR, Bujnicki JM, Hook M, Lukomski S. Streptococcal Scl1 and Scl2 proteins form collagen-like triple helices. J. Biol. Chem. 2002;277:27312–27318. doi: 10.1074/jbc.M201163200. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Hasegawa S, Kitani T, Hidai K, Fukui T. Differential effects of obesity on acetoacetyl-CoA synthetase gene in rat adipose tissues. Eur. J. Lipid Sci. Technol. 2007;109:617–622. [Google Scholar]
- Yu BJ, Kim JA, Moon JH, Ryu SE, Pan JG. The diversity of lysine-acetylated proteins in Escherichia coli. J. Microbiol. Biotechnol. 2008;18:1529–1536. [PubMed] [Google Scholar]
- Zhang J, Sprung R, Pei J, Tan X, Kim S, Zhu H, Liu CF, Grishin NV, Zhao Y. Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol. Cell. Proteomics. 2009;8:215–225. doi: 10.1074/mcp.M800187-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.