Abstract
Objective
Generation and differentiation of new oligodendrocytes in demyelinated white matter is the best described repair process in the adult human brain. However, remyelinating capacity falters with age in patients with multiple sclerosis. (MS). Since demyelination of cerebral cortex is extensive in brains from MS patients, we investigated the capacity of cortical lesions to remyelinate and directly compared the extent of remyelination in lesions that involve cerebral cortex and adjacent subcortical white matter.
Methods
Postmortem brain tissue from 22 patients with MS (age 27 to 77 years) and 6 subjects without brain disease were analyzed. Regions of cerebral cortex with reduced myelin were examined for remyelination, oligodendrocyte progenitor cells, reactive astrocytes, and molecules that inhibit remyelination.
Results
“New” oligodendrocytes that were actively forming myelin sheaths were identified in 30/42 remyelinated subpial cortical lesions, including lesions from three patients in their 70's. Oligodendrocyte progenitor cells were not decreased in demyelinated or remyelinated cortices when compared to adjacent normal-appearing cortex or controls. In demyelinated lesions involving cortex and adjacent white matter, the cortex showed greater remyelination, more actively remyelinating oligodendrocytes and fewer reactive astrocytes. Astrocytes in the white-matter, but not in cortical portions of these lesions, significantly up-regulate CD44, hyaluronan, and versican, molecules that form complexes that inhibit oligodendrocyte maturation and remyelination.
Interpretation
Endogenous remyelination of the cerebral cortex occurs in individuals with MS regardless of disease duration or chronological age of the patient. Cortical remyelination should be considered as a primary outcome measure in future clinical trials testing remyelination therapies.
Keywords: multiple sclerosis, remyelination
INTRODUCTION
Multiple sclerosis (MS) is an inflammatory-mediated demyelinating disease of the human central nervous system and the major cause of non-traumatic neurological disability in young adults1. Demyelinated lesions in white matter are the pathologic and radiologic hallmark of postmortem brains from patients with MS2 but only weakly correlate with neurological disability (reviewed in3). In addition to white matter demyelination, other pathologic changes must contribute to neurologic decline in MS patients. In this regard, it is established that gray matter is demyelinated in individuals with MS4-9. Cortical demyelination can exceed white matter demyelination in postmortem brains from individuals with MS6, and cortical atrophy is among the best predictors of neurologic disability in individuals with MS10,11.
Demyelination need not be permanent in the lesions of MS (for review see12). Early in the clinical disease course, generation of new myelin-forming cells and myelin can be prominent features of white-matter lesions13-15. Most chronically-demyelinated lesions in white matter, however, do not show evidence for active remyelination16-20. Remyelination in chronic white-matter lesions can be limited by reduced recruitment of oligodendrocyte progenitor cells, failure of oligodendrocyte progenitor cells to generate oligodendrocytes, and failure of oligodendrocyte to differentiate into myelin forming cells17,21,22. These limitations could be regulated by a variety of cellular and environmental factors, including epigenetic changes in oligodendrocytes23, an abnormal molecular composition of the chronically-demyelinated axon17,24,25, an imbalance of growth factors, or increased expression of the myelination inhibitors24,26,27. A pathologic hallmark of demyelinated white matter in brains from patients with MS is the sclerotic astroglial scar. Reactive astrocytes in these scars express and organize extracellular matrix proteins that inhibit recruitment of oligodendrocyte progenitor cells and differentiation of oligodendrocytes28. Central to this astrocyte-based inhibition is the induced expression of CD44, an astrocyte surface-membrane protein that binds and/or organizes the glycosaminoglycan, hyaluronan29. Hyaluronan also forms complexes with versican, a member of the lectican family of chondroitin sulfate proteoglycans28. CD44 and hyaluronan are significantly increased in white-matter lesions of MS, and hyaluronan inhibits oligodendrocyte differentiation in vitro and remyelination in rodents26,30.
Remyelination has two significant consequences for axons; it restores saltatory conduction of nerve impulses (for review see31), and it prolongs the survival of previously-demyelinated axons32. Elucidation of the mechanisms or environments that contribute to failed or successful remyelination should lead to novel therapeutics that delay the progression of permanent neurological disability in patients with MS. This report describes abundant and active remyelination of cortical lesions in postmortem brains from patients with MS. Successful cortical remyelination occurs in an environment of increased oligodendrocyte progenitor cells, fewer reactive astrocytes, and minimal expression of extracellular matrix molecules known to inhibit oligodendrocyte production and differentiation.
METHODS
Human tissues
This research was approved by the Cleveland Clinic Institutional Review Board. Tissue donation from patients with MS was obtained with consent from the patient or next of kin. Control tissues were obtained from patients who had autopsies at Cleveland Clinic. Clinical and tissue details are presented in Supplementary Tables S1 and S2. We studied tissue from the brains of 22 patients with MS (13 females and 10 males, age of death, 27-77 years). The most recent Expanded Disability Status Scale recorded prior to death ranged from 6.0-9.5 (mean, 8.3). Postmortem intervals ranged from 2.8-17.7 hours (mean, 6.4). The controls (four females and two males) ranged in age from 46-74 years and postmortem time of 11-36 hours (mean, 21.0). One cerebral hemisphere was cut into one cm coronal sections in the fresh state, and alternate slices were flash frozen for biochemical studies or fixed in 4% paraformaldehyde for 2-5 days. The opposite hemisphere was fixed intact for several months.
Tissue staining
Immunohistochemistry was performed as previously described17,21,33. All immunostaining for morphological analysis was performed on 30 μm free-floating sections of fixed tissue that was cryoprotected, frozen, and cut on a sliding microtome. Primary antibodies were rat anti-proteolipid protein (PLP, Wendy Macklin, University of Colorado, Denver), mouse anti-MHC Class II, rabbit anti-GFAP (Dako, Glostrup, Denmark), mouse anti-human NG2 (BD Biosciences, San Jose, CA), rabbit anti-Caspr (James Trimmer, University of California, Davis, CA), and CD44 (American Type Culture Collection, Manassas, VA). Appropriate biotinylated (Vector Laboratories, Burlingame, CA) and fluorescently-labeled (Invitrogen, Carlsbad, CA) secondary antibodies were used. Hyaluronan was localized using biotinylated hyaluronan binding protein (EMD Biosciences, San Diego, CA). Digital brightfield images were obtained using a Zeiss Axiophot microscope. Immunofluorescently-labeled tissues were analyzed using a Leica SP5 confocal microscope (Leica Microsystems, Exton, PA).
Quantitative immunohistochemistry
Subpial lesions
Forty-two subpial cortical lesions (ranging in size from 0.2 to 110 mm2) were identified in PLP stained cortical sections from 10 MS brains (Supplementary Table S1). These subpial lesions contained variable amounts of demyelination/remyelination. Total lesion area occupied by PLP staining was calculated using a slide scanner (PathScan Enabler IV, Meyer Instruments, Houston, TX) and ImageJ (NIH, http://rsweb.nih.gov/ij). Actively-myelinating oligodendrocytes were defined as cells with PLP-positive cell bodies and processes extending to short and thin myelin internodes, and their presence in a lesion was assessed by systematically sampling 40× fields (0.3 mm2).
Leukocortical lesions
Leukocortical lesions include both cerebral cortex and adjacent subcortical white matter. Sixty-four leukocortical lesions (with similar areas of white and gray matter demyelination) from 14 MS brains were analyzed in this study (Supplementary Table S1). MHC Class II staining of the white matter lesions was used to stage each leukocortical lesion as acute or chronic using established criteria34. The gray-matter and white-matter portions of each lesion were analyzed separately. Lesions with active remyelination were defined as those containing more than five actively-myelinating oligodendrocytes. Statistical analysis compared the log transformed oligodendrocyte density difference (within lesion difference between gray-matter portion and white matter portion) using a linear mixed model to include effects of data from multiple lesions from individual brains.
Quantification of Oligodendrocyte Progenitor Cells
The density of NG2-positive oligodendrocyte progenitor cells was compared in cortical sections from two control brains (Supplementary Table S2) and six MS brains (Supplementary Table S1). Since NG2 staining is sensitive to prolonged paraformaldehyde fixation 21,33, all tissues were obtained from short fixed (2-5 days) brain slices. MS sections contained cortices which were normally myelinated, demyelinated, or remyelinated. Normal-appearing cortex and cortical lesions were analyzed in the same slides from six MS brains. Five slides had cortical lesions with and without remyelination, and one slide had lesions without remyelination. Four cortical areas were analyzed from one control brain and three from the other control brain. Multiple fields (3-6 for each section) measuring 0.0576 mm2 were counted using a 40x objective and the average value used for statistical analysis. Differences were compared using the Mann-Whitney U test.
mRNA and Protein analysis
Cryostat sections (14 μm) of five fresh frozen tissue blocks with leukocortical lesions from five patients with MS and eight blocks containing cortex and white matter from three controls (Supplementary tables S1 and S2) were stained for PLP and MHC Class II to identify the gray- and white-matter portions of leukocortical lesions (WML, GML), adjacent normal-appearing white and gray matter (NAWM, NAGM), and control white and gray matter (CWM, CGM). These areas were dissected from each other by scoring the frozen block with a scalpel prior to cutting 60 μm sections. Total RNA was prepared as previously described35. Biotinylated cRNA probes were hybridized to U133AB arrays (Affymetrix Inc., USA), and the intensity values for GFAP, CD44, and versican were obtained from the resultant scanned microarray files. Lysates for Western blot analysis were obtained by the same procedure (n=3 for each tissue), and membranes were incubated with primary antibodies to CD44 and versican (Millipore Inc., USA). The amount of protein loaded on the white and gray matter blots as well as the exposure time was kept similar to cross compare between the two tissue types. Differences between groups were considered significant at P<0.05 using the Mann-Whitney U test.
RESULTS
Remyelination in Cortical Lesions of MS
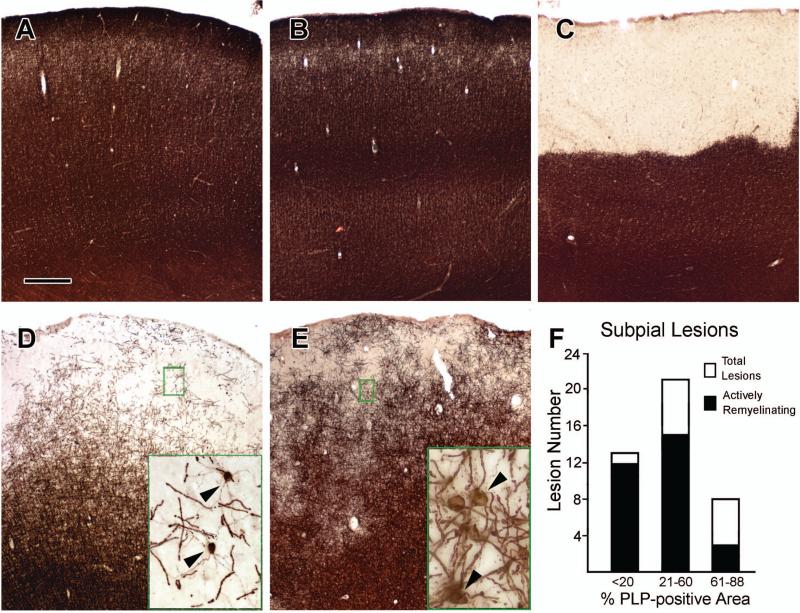
Immunohistochemical staining for proteolipid protein (PLP) demonstrated that all cortical areas examined from control brains (Fig 1A) and many cortical areas from brains of patients with MS (Fig 1B) contain abundant myelin. The most prevalent demyelinated lesion in the cerebral cortex extends from the pial surface into deeper cortical layers and is known as a subpial or Type III cortical lesion5. Figure 1C shows a subpial lesion that is completely demyelinated. Many subpial lesions were not completely demyelinated but had a reduced density of PLP-positive myelin internodes (Fig 1D and 1E, Fig 2A). We quantified the area occupied by PLP staining in 42 of these “partially-myelinated” subpial cortical lesions from 10 brains. Thirteen contained ≤20% percent of cortical area occupied by PLP staining, 21 contained 21-60%, and 8 contained 61-88% (Fig 1F). These subpial lesions often contained PLP-positive oligodendrocyte cell bodies, which extended thin PLP-positive processes to multiple short and thin myelin internodes (Fig 1D, inset, 2C, and 2E). This cellular PLP staining pattern is similar to that seen during active stages of myelin formation in the developing brain36. We interpret this pattern as evidence of active remyelination of axons by oligodendrocytes. Seventy-one percent (30/42) of the subpial cortical lesions contained oligodendrocytes that displayed features of active myelination (Fig 1F, Supplementary Table S3). The extent of active myelination varied from lesion to lesion within a single brain. Active remyelination of axons was present in patients of all ages examined, including two 77-year-olds (Supplementary Table S3). There was no obvious correlation between the extent of remyelinated lesions and age at death. These data support the generation and differentiation of oligodendrocytes in the human cerebral cortex through the eighth decade of life.
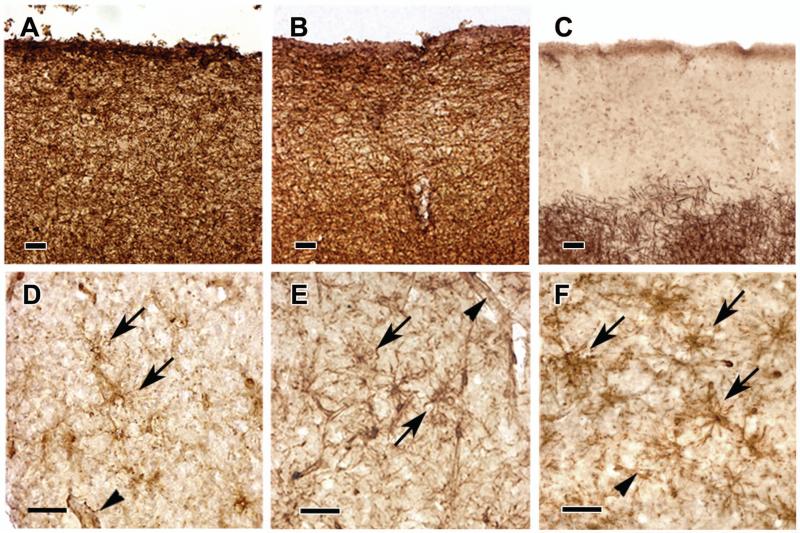
Figure 1. Remyelination in Subpial Cortical Lesions.
Brains from control patients without neurological disease have dense PLP immunoreactivity in all cerebral cortical layers (Panel A). Many regions of cortex in patients with MS show a similar pattern in PLP immunoreactivity (Panel B). A subpial demyelinated lesion with complete loss of PLP immunoreactivity is shown in Panel C. Many subpial lesions are not completely demyelinated, but contain PLP-positive myelin internodes (Panels D and E). Frequently, these areas contain actively-myelinating oligodendrocytes with PLP-positive cell bodies and processes extending to myelin internodes (Panels D and E, arrowheads in insets). Actively-myelinating oligodendrocytes are most frequent in lesions where PLP-positive myelin occupied 60% or less of the lesion area (Panel F). The scale bar represents 400 μm for Panels A-E.
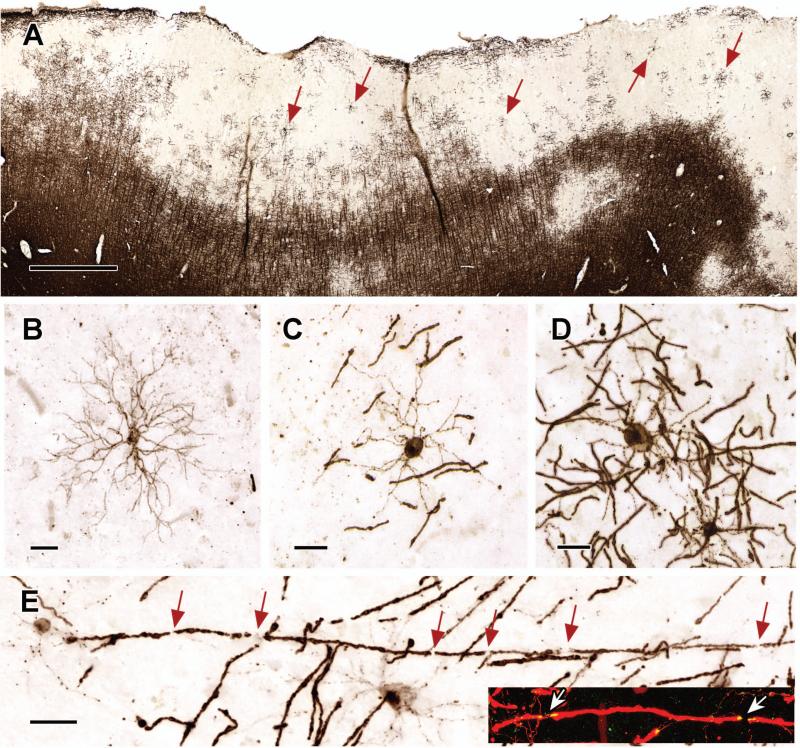
Figure 2. Active Remyelination in Subpial Cortical Lesions.
Subpial cortical lesions contain oligodendrocytes in various stages of differentiation (Panel A, arrows). Premyelinating oligodendrocytes with PLP-positive cell bodies and multiple processes are present (Panel B), but actively-myelinating oligodendrocytes with PLP-positive cell bodies and processes extending to multiple myelin internodes are more prevalent (Panels C and D). Myelin-internodal lengths are relatively short (Panels C and D), which supports the interpretation that these oligodendrocytes are actively remyelinating axons. Individual axons are ensheathed by multiple-short myelin internodes (Panel E, inset, arrows indicate nodes of Ranvier). Remyelinated fibers also show molecular maturation (Panel E, inset) as demonstrated by the appropriate distribution of the paranodal protein, Caspr (yellow in Panel E, inset; red in inset is PLP staining). The scale bar in Panel A represents 1 mm; the scale bars in Panels B - E represent 20 μm.
Subpial cortical lesions contained oligodendrocytes in various stages of differentiation. Premyelinating oligodendrocytes, which have multiple radially-oriented processes and no obvious association with axons17,36, are rare in adult mammalian brain. However, they were present in many remyelinating subpial cortical lesions (Fig 2B). Premyelinating oligodendrocytes have one of two fates: they either myelinate axons, or they undergo programmed cell death36. Dying premyelinating oligodendrocytes were rare in subpial cortical lesions; however, we identified numerous oligodendrocytes that extended PLP-positive processes to short and thin myelin internodes (Fig 2C), a histologic hallmark of remyelination. Other oligodendrocytes extended processes to longer and thicker myelin internodes (Fig 2D). Functional maturation of short remyelinated internodes is supported by the assembly of paranodal/nodal complexes (Fig 2E, arrows), characterized by the expression of the paranodal protein, Caspr (for review see37.
Cortical Remyelination Exceeds White Matter Remyelination in Lesions of Similar Age
White matter lesions can be classified as acute or chronic based upon the density and distribution of MHC Class II-positive macrophages and microglia, but to date, there are no established criteria to judge the age of lesions located entirely in the cortex. A subset of lesions involve both the cortex and subcortical white matter5, and it is reasonable to infer that the demyelination in both parts of these lesions are of the same age. We identified 64 leukocortical lesions (Fig 3, Supplementary Fig S1, Supplementary Table S4). Seven lesions had abundant phagocytic macrophages in the white matter portion (Supplementary Fig S1B and S1D) and were classified as acute5,34. The cortical portion contained process-bearing activated microglia that were increased at the cortical lesion border (Supplementary Fig S1B and S1C). Five of these seven acute leukocortical lesions (71%) showed no evidence of remyelination. Two had small foci of remyelination in the cortex but not the white matter.
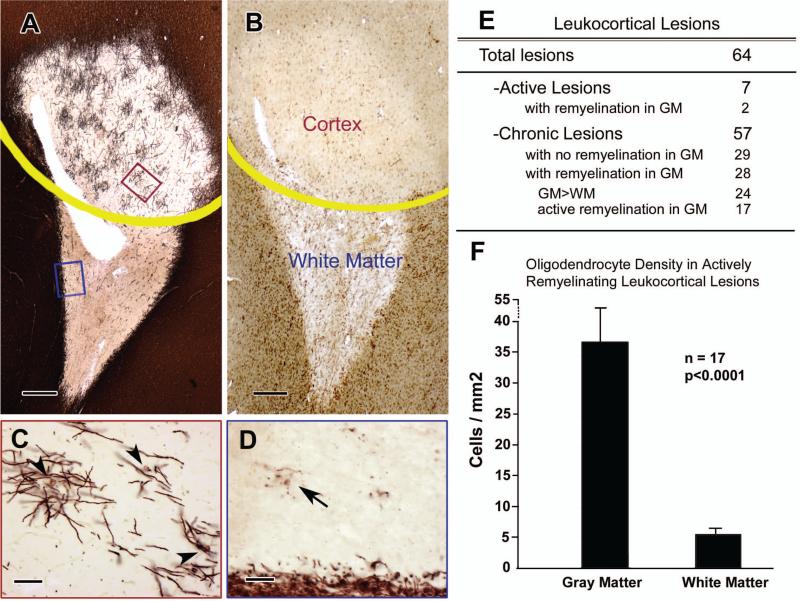
Figure 3. Remyelination in Chronic Leukocortical Lesions.
PLP immunohistochemistry of a leukocortical lesion shows significant remyelination in the demyelinated cortex compared to the demyelinated white matter (Panel A, yellow line shows the boundary between cortex and white matter). MHC Class II staining (Panel B) identifies this lesion as chronic. The PLP-positive cells in demyelinated cortex have features of actively-remyelinating oligodendrocytes (Panel C is high magnification of the red box in Panel A), whereas those in demyelinated white matter often extend dystrophic processes with no apparent connection to myelin internodes (Panel D is high magnification of the blue box in Panel A). Twenty-eight chronic leukocortical lesions (49%) show evidence of remyelination (Panel E). Of these, 24 showed a greater extent of remyelination in the gray matter (GM) compared to that in the white matter (WM), and 17 of these 24 (70%) show evidence of active remyelination. The density of oligodendrocytes in the gray-matter portion of the actively-remyelinating lesions greatly exceeds the oligodendrocyte density in the white-matter portion (Panel F, P< 0.0001). The scale bars in Panels A and B represent 400 μm; the scale bars in Panels C and D represent 50 μm; the error bars in Panel F represent standard error of mean.
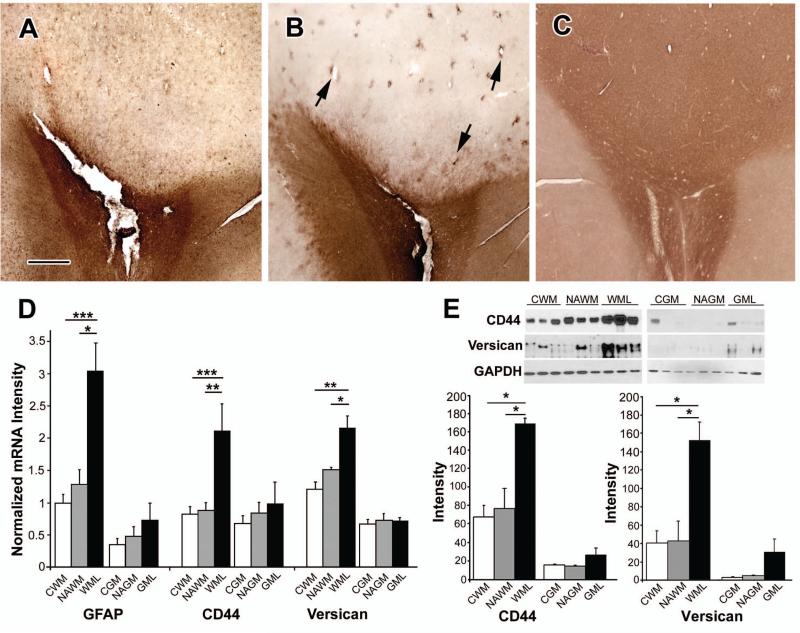
Fifty-seven of the 64 leukocortical lesions did not contain phagocytic macrophages in the white matter and were classified as chronic (Fig 3B). Twenty-eight of 57 (49%) chronic leukocortical lesions had evidence of remyelination in the cortical portion (Fig 3A and 3C) and variable amounts of remyelination in the white-matter portion. Overall, the extent of remyelination in gray matter exceeded that observed in the white matter in 24 of the 28 leukocortical lesions with remyelination in gray matter(Fig 3E). The morphology of the PLP-positive oligodendrocytes also differed in gray matter compared to white matter. The PLP-positive oligodendrocytes in the gray-matter portions were similar to remyelinating oligodendrocytes present in the subpial lesions with PLP-positive cell bodies and processes connected to thin and short myelin internodes. By contrast, the PLP-positive oligodendrocytes in the white-matter portions often had a dystrophic appearance, characterized by fragmented processes and no myelin internodes (Fig 3D). The density of oligodendrocytes in the gray-matter portion of leukocortical lesions was increased 6.8 fold compared to the white-matter portion (37.3/mm2 vs. 5.5/mm2, Fig 3F, P<0.0001). Since individual oligodendrocytes myelinate multiple axons, the increase in number of myelin internodes in the cortex was much greater than in the white matter. There was no obvious correlation between the extent of remyelination in either the gray- or white-matter portions of leukocortical lesions and age at death. These results indicate that the environment of chronically-demyelinated cerebral cortex is more conducive to oligodendrogenesis and remyelination than the environment of chronically-demyelinated white matter. The data described above raise the possibility that white-matter lesions contain inhibitors of oligodendrocyte production and differentiation that are expressed at lower levels in cortical lesions. Since reactive astrocytes are a source of molecules that can inhibit brain repair and remyelination28, we compared the presence, distribution, and/or amounts of glial fibrillary acidic protein (GFAP), CD44, hyaluronan, and versican. Compared to the cortical portion, the white-matter portion had increased GFAP immunoreactivity (Fig 4A). CD44 and hyaluronan also showed intense and global increases in the white-matter portion, but not in the gray-matter portion of the lesions when compared to adjacent non-lesion areas (Fig 4B and 4C). Sparsely scattered CD44-positive astrocytes, most often associated with blood vessels, were present in some cortical lesions (Fig 4B, arrows). mRNAs encoding GFAP, CD44 and versican, were also significantly increased in the white-matter portion of leukocortical lesions compared to normal-appearing white matter and control white matter (Fig 4D). Absolute amounts of these mRNAs were less in gray-matter portions of leukocortical lesions, and there was no significant increase in gray matter lesions (Fig 4D). Western blot analysis showed similar changes in protein levels of CD44 and versican (Fig 4E).
Figure 4. Comparison of the Astrocytosis and Inhibitors of Myelination in the Gray- and White-Matter Portions of Leukocortical Lesions.
Gray-matter portions of leukocortical lesions have fewer reactive astrocytes compared to the white-matter portions. Panels A-C show the same lesion depicted in Figure 3. GFAP immunoreactivity is much greater in the white-matter portion of the lesion compared to the gray-matter portion (Panel A). The same pattern is seen in sections stained for CD44 (Panel B) and hyaluronan (Panel C). Occasional CD44-positive astrocytes were present in cortical lesions and frequently associate with blood vessels (Panel B, arrows). mRNAs encoding GFAP, CD44, and versican are significantly increased in white-matter portions of leukocortical lesions (WML) compared to normal-appearing white matter (NAWM) from the same patients with MS and white matter from controls without neurological diseases (CWM)(Panel D). Levels of these mRNAs are lower in the gray matter portions of leukocortical lesions (GML), normal appearing gray matter (NAGM), and control gray matter (CGM) (Panel D). Western blot analysis confirms increased protein levels of CD44 and versican in white-matter portions of leukocortical lesions (Panel E). The scale bar represents 500 μm for Panels A, B and C; the error bars in Panels D and E represent SEM; *= P<0.05, **= P<0.01 ***=P<0.005.
Oligodendrocyte Progenitor Cell Density is Not Decreased in Cortical Lesions
Since remyelination requires the generation of new oligodendrocytes, we quantified the density of oligodendrocyte progenitor cells in cerebral cortex of controls and patients with MS. We previously showed that oligodendrocyte density in chronic white-matter lesions was reduced compared to adjacent normal-appearing white matter21. Visual inspection of stained sections showed no obvious reduction or loss of oligodendrocyte progenitor cells in demyelinated cerebral cortex (Fig 5). This impression was confirmed by quantification of NG2 cell densities: 98.1± 19.6 in normal appearing gray matter, 109.9 ± 19.6 cells/mm2 in cortical lesions without remyelination, and 121.3 ± 27.2 cells/mm2 in lesions with remyelination. Although the mean NG2 cell density was increased in the lesions, this was not significantly different. NG2 cell density was higher in all MS tissues compared to density in two control brains (63.3 ± 0.3 cells/mm2). These data demonstrate that there is no decrease in the number of oligodendrocyte progenitor cells in cortical lesions compared to non-lesion cortex, and that their ability to produce myelinating oligodendrocytes may contribute to the greater repair capacity of cortical lesions when compared to chronic white matter lesions.
Figure 5. NG2-Postive Oligodendrocyte Progenitor Cells in Subpial Cortical Lesions.
Oligodendrocyte progenitor cell density is not decreased in subpial-cortical lesions of MS. PLP immunoreactivity in cerebral cortex is shown from a control subject (Panel A), normal-appearing cortex from a patient with MS (Panel B), and a subpial lesion with no evidence of remyelination (Panel C). Panels D, E and F represent NG2 immunohistochemistry in sections cut adjacent to those in Panels A, B and C, respectively. NG2-positive oligodendrocyte progenitor cells are observed in all areas. Arrows indicate NG2-positive cell bodies; arrowheads indicate NG2-positive cells of blood vessels. The scale bars represents 50 μm.
DISCUSSION
Generation of oligodendrocytes and new myelin in white matter lesions of MS is the best described and most abundant repair process in the adult human brain12. Although extensive remyelination can be identified in brains of MS patients of all ages18,19,38, it often fails, and data from animal models where both age of the subject and age of lesion onset can be controlled, demonstrate that this endogenous repair process becomes impaired with age27. Possible explanations for this reduction in brain repair include: (i) failure to recruit oligodendrocyte progenitor cells into the lesion, (ii) failure of oligodendrocyte progenitor cells to generate oligodendrocytes and (iii) failure of oligodendrocytes to remyelinate axons17,20,22. This report addressed these possibilities by comparing regions of the MS brain with successful and failed remyelination. We examined a total of 106 lesions involving cortex in the brains of 19 patients with MS who ranged in age from 27-77 yrs and found evidence of oligodendrocyte differentiation and ongoing myelin repair in 60 cortical lesions. In contrast to previous studies on remyelination in MS brain 18,19,38,39, we were able to directly compare the extent of remyelination in white matter versus gray matter in lesions of the same age by examination of contiguous leukocortical lesions. Oligodendrocyte generation and remyelination were significantly greater in the cortex. In addition, white-matter, but not cortical, portions of these lesions contained a significant increase in reactive astrocytes and associated extracellular matrix molecules that inhibit oligodendrocyte production and myelination26,30. These data establish that the adult human brain retains the ability to generate new oligodendrocytes and myelin into the 8th decade of life. In addition, they support the concept that myelin repair is limited by the astroglial sclerotic scar that is a pathologic hallmark of chronic white-matter lesions of MS.
In our evaluation of the efficiency of myelin repair, we asked two questions: is there any evidence for remyelination in a lesion and is there evidence for active oligodendrocyte differentiation and remyelination of axons at the time of death. The criteria we used for remyelination are established13,14 and include brain regions with reduced myelin staining, thin myelin sheaths, and short internodes. Our criteria for newly-generated oligodendrocytes and active myelin repair were based upon the immunohistochemical morphology of differentiating oligodendrocytes during normal development, which occurs in two stages36. Oligodendrocyte progenitor cells differentiate into premyelinating oligodendrocytes that extend multiple processes that do not immediately myelinate axons. These cells initiate abundant synthesis of myelin proteins that are transported into their processes. Once a premyelinating oligodendrocyte contacts a receptive axon, these proteins are inserted into developing myelin internodes. Therefore, active stages of myelin formation are characterized by the presence of both premyelinating oligodendrocytes and oligodendrocytes with myelin-protein-positive cell bodies and processes extending to short internodes. We identified both of these cell types in remyelinated lesions in postmortem brains and conclude that remyelination was ongoing at the time of death.
A previous paper reported that cortical lesions had more extensive remyelination than white matter lesions39. Our analysis of leukocortical lesions, in which the cortical and white-matter demyelination most likely began around the same time, extends these data and provides possible explanations for the greater capacity for remyelination of lesions in gray matter. In contrast to chronic white-matter lesions17, oligodendrocyte progenitor cell density was not reduced in cortical lesions (Fig 5). Therefore, oligodendrocyte progenitor cell recruitment does not appear to limit oligodendrocyte production in cortical lesions. In addition, minimal astrogliosis and low expression of extracellular matrix molecules that inhibit oligodendrocyte progenitor cell differentiation and remyelination (Fig 4) provide a more conducive environment for oligodendrocyte production and myelin repair in the cerebral cortex.
Our data have significant implications for clinical trials designed to enhance myelin repair in patients with MS. Enhancement of cortical remyelination in individuals with MS is a more attainable therapeutic target than enhancement of white matter remyelination because endogenous cortical remyelination occurs in most patients with MS regardless of chronological age or disease duration. Cortical remyelination as a primary outcome in myelin repair clinical trials could dramatically increase the chance of identifying a successful myelin repair therapy. A major challenge to this goal is the development of imaging modalities that can detect gray matter demyelination and remyelination in living MS patients, which is a focus of active investigation 40. The inhibitory effect of reactive extracellular matrix proteins on axonal regeneration is well-documented in spinal cord injury models41. Our data indicate that similar extracellular matrix proteins are likely to inhibit remyelination in white matter of individuals with MS. Therapeutic enhancement of white matter remyelination, therefore, would be most efficacious prior to glial scar formation. A possible therapeutic strategy might include antibodies that neutralize the inhibitory effects of extracellular matrix molecules.
Supplementary Material
Supplementary Figure 1. Failed Remyelination in Acute Leukocortical Lesions.
Most acute leukocortical lesions showed no evidence of remyelination by PLP immunostaining (Panel A). The abundant and even distribution of MHC Class II-positive cells in the demyelinated white matter (Panel B) classifies this lesion as acute. MHC Class II-positive cells in the cortex are predominantly process-bearing microglia (Panel C), whereas those in the white matter portion are phagocytic macrophages (Panel D). The scale bars in Panels A and B represent 400 μm; the scale bars in Panels C and D represent 50 μm.
ACKNOWLEDGEMENTS
We thank Cleveland Clinic Mellen Center clinical personnel and Lifebanc, Cleveland, OH, for assistance in identifying multiple sclerosis tissue donors, Cynthia Schwanger, RN, for coordinating the MS tissue donation program, Jar-Chi Lee, M.S. for consultation on statistical analysis, Jacqueline Chen, Ph.D., Richard M. Ransohoff, M.D., and Richard A. Rudick, M.D., for helpful comments, and Christopher Nelson, Ph.D., for editorial assistance. This work was supported by NIH/NINDS NS38867 (Project 2 and Tissue Acquisition Core A) and National Multiple Sclerosis Society RG 4348-A-7 grants awarded to BDT.
Grant Support: NIH/NINDS NS38867 and National Multiple Sclerosis Society RG 4348-A-7
REFERENCES
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Love S, Louis D, Ellison D. Greenfield's Neuropathology. 8th ed. Oxford University Press; London: 2008. [Google Scholar]
- 3.Goodin DS. Magnetic resonance imaging as a surrogate outcome measure of disability in multiple sclerosis: have we been overly harsh in our assessment? Ann Neurol. 2006;59:597–605. doi: 10.1002/ana.20832. [DOI] [PubMed] [Google Scholar]
- 4.Kidd D, Barkhof F, McConnell R, et al. Cortical lesions in multiple sclerosis. Brain. 1999;122:17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- 5.Peterson JW, Bo L, Mork S, et al. Transected neurites, apoptotic neurons and reduced inflammation in cortical MS lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 6.Bo L, Vedeler CA, Nyland HI, et al. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62:723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- 7.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 8.Vercellino M, Plano F, Votta B, et al. Grey matter pathology in multiple sclerosis. J Neuropathol Exp Neurol. 2005;64:1101–1107. doi: 10.1097/01.jnen.0000190067.20935.42. [DOI] [PubMed] [Google Scholar]
- 9.Gilmore CP, Donaldson I, Bo L, et al. Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: a comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J Neurol Neurosurg Psychiatry. 2009;80:182–187. doi: 10.1136/jnnp.2008.148767. [DOI] [PubMed] [Google Scholar]
- 10.Kutzelnigg A, Lassmann H. Cortical lesions and brain atrophy in MS. J Neurol Sci. 2005;233:55–59. doi: 10.1016/j.jns.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Rudick RA, Lee JC, Nakamura K, et al. Gray matter atrophy correlates with MS disability progression measured with MSFC but not EDSS. J Neurol Sci. 2009;282:106–111. doi: 10.1016/j.jns.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 13.Prineas JW, Barnard RO, Kwon EE, et al. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993;33:137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- 14.Raine CS, Wu E. Multiple sclerosis: remyelination in acute lesions. J Neuropathol Exp Neurol. 1993;52:199–204. [PubMed] [Google Scholar]
- 15.Lucchinetti C, Bruck W, Parisi J, et al. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain. 1999;122:2279–2295. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- 16.Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- 17.Chang A, Tourtellotte WW, Rudick R, et al. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 18.Patrikios P, Stadelmann C, Kutzelnigg A, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- 19.Patani R, Balaratnam M, Vora A, et al. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33:277–287. doi: 10.1111/j.1365-2990.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 20.Goldschmidt T, Antel J, Konig FB, et al. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72:1914–1921. doi: 10.1212/WNL.0b013e3181a8260a. [DOI] [PubMed] [Google Scholar]
- 21.Chang A, Nishiyama A, Peterson J, et al. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhlmann T, Miron V, Cuo Q, et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- 23.Shen S, Sandoval J, Swiss VA, et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mi S, Miller RH, Lee X, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- 25.Charles P, Reynolds R, Seilhean D, et al. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain. 2002;125:1972–1979. doi: 10.1093/brain/awf216. [DOI] [PubMed] [Google Scholar]
- 26.Back SA, Tuohy TM, Chen H, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 27.Rist JM, Franklin RJ. Taking ageing into account in remyelination-based therapies for multiple sclerosis. J Neurol Sci. 2008;274:64–67. doi: 10.1016/j.jns.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Sherman LS, Back SA. A ‘GAG’ reflex prevents repair of the damaged CNS. Trends Neurosci. 2008;31:44–52. doi: 10.1016/j.tins.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 30.Sloane JA, Batt C, Ma Y, et al. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A. 2010;107:11555–11560. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trapp BD, Stys PK. Virtual Hypoxia and Chronic Necrosis of Demyelinated Axons in Multiple Sclerosis. Lancet Neurol. 2009;8:280–291. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- 32.Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- 33.Staugaitis SM, Trapp BD. NG2-positive glia in the human central nervous system. Neuron Glia Biol. 2009;5:35–44. doi: 10.1017/S1740925X09990342. [DOI] [PubMed] [Google Scholar]
- 34.Bo L, Mork S, Kong PA, et al. Detection of MHC class II-antigens on macrophages and microglia, but not on astrocytes and endothelia in active multiple sclerosis lesions. J Neuroimmunol. 1994;51:135–146. doi: 10.1016/0165-5728(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 35.Dutta R, Chang A, Doud MK, et al. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol. 2011;69:445–454. doi: 10.1002/ana.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trapp BD, Nishiyama A, Cheng D, et al. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137:459–468. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- 38.Bramow S, Frischer JM, Lassmann H, et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain. 2010;133:2983–2998. doi: 10.1093/brain/awq250. [DOI] [PubMed] [Google Scholar]
- 39.Albert M, Antel J, Bruck W, et al. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17:129–138. doi: 10.1111/j.1750-3639.2006.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hulst HE, Geurts JJ. Gray matter imaging in multiple sclerosis: what have we learned? BMC Neurol. 2011;11:153. doi: 10.1186/1471-2377-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Failed Remyelination in Acute Leukocortical Lesions.
Most acute leukocortical lesions showed no evidence of remyelination by PLP immunostaining (Panel A). The abundant and even distribution of MHC Class II-positive cells in the demyelinated white matter (Panel B) classifies this lesion as acute. MHC Class II-positive cells in the cortex are predominantly process-bearing microglia (Panel C), whereas those in the white matter portion are phagocytic macrophages (Panel D). The scale bars in Panels A and B represent 400 μm; the scale bars in Panels C and D represent 50 μm.