Abstract
Background
Children with cancer in middle-income countries have inferior outcomes to those in high-income countries. The magnitude and drivers for this survival gap are not well understood. We sought to describe patterns of clinical presentation, magnitude of treatment abandonment, and survival in children with sarcoma in Central America.
Methods
Retrospective review of hospital-based registries from national pediatric oncology referral centers. Patients with newly diagnosed osteosarcoma, Ewing sarcoma (Ewing), rhabdomyosarcoma (RMS), and soft tissue sarcomas (STS) between 1/1/00-12/31/09 were included. Survival analysis was performed using standard definitions of overall and event-free survival (OS and EFS) and with abandonment included as an event (AOS and AEFS).
Results
A total of 785 new cases of pediatric sarcoma were reported (264 osteosarcoma, 175 Ewing, 240 RMS, and 106 STS). Metastatic disease at presentation was high (osteosarcoma 38%, Ewing 39%, RMS 29% and STS 21%). Treatment abandonment rate was high, particularly among patients with extremity bone sarcomas (osteosarcoma 30%, Ewing 15%, RMS 25% and STS 15%). Of 559 patients experiencing a first event, 59% had either relapse or progressive disease. The 4-year OS was 40% (SE±3%) and EFS was 30% (SE±2%), but further decreased to 31% (SE±2%) and 24% (SE±2%), when abandonment was taken into account.
Conclusion
High rate of metastases and treatment abandonment, and difficulty with upfront treatment effectiveness are important contributors to poor survival of children with pediatric sarcomas in Central America. Initiatives for early diagnosis, psychosocial support, quality improvement, and multidisciplinary care are warranted to improve outcomes.
Keywords: pediatric sarcoma, developing countries, treatment abandonment, outcomes research, international outreach
BACKGROUND
Eighty percent of children with cancer live in low-and middle-income countries (LMC)1. In developed countries, childhood cancer survival has steadily improved reaching 81% for all children with cancer and 70% for children with sarcoma2, 3. Improved survival for pediatric sarcoma in high income countries (HIC) reflects improved access to chemotherapy, surgery and radiation, as well as better diagnostic techniques, risk-adapted approaches and optimization of supportive care and palliation4. In developing countries, advances have been hampered by low socioeconomic conditions that result in delayed diagnosis and fractionated care. Information on clinical presentation, epidemiology, delivery of cancer care and outcomes for children with sarcoma in LMCs is scarce5–8; a detailed analysis of all these elements is needed. International organizations have called for action to address cancer in the developing world9–11. Great variability in cancer outcomes has been documented not only between developed and developing countries, but also between countries with similar government investment in health care12, 13; highlighting the need to improve our understanding of regional disparities and to develop new models of care for children with cancer in LMC. Successful models of care for treatment of childhood leukemia in resource-limited settings have been developed14. However, an expanded model that accounts for the complexity and multidisciplinary nature of solid tumor care is critically needed. Pediatric sarcomas collectively represent only between 6–16% of pediatric cancers2, 3, however, due to the complexity of diagnostic and treatment, they can serve as a benchmark to study healthcare systems and the influence of quality improvement or multidisciplinary care paradigms on cancer outcomes in LMC.
The Central American Association of Pediatric Hematologists and Oncologists (AHOPCA) is a regional collaboration that has successfully implemented protocol-based treatment, studied causes of treatment abandonment, and improved survival in childhood leukemia in the region15, 16. However, despite parallel development of pediatric leukemia and solid tumor programs, improvements in outcomes for children with solid tumors, have not always matched those of children with pediatric leukemia.
In Guatemala, the implementation of a soft tissue sarcoma protocol based on IRS-IV proved deliverable, yet despite access to chemotherapy, surgery and radiation, the outcomes reported in developed countries were not reproducible in this lower-income setting8, 17. Similar challenges in matching published outcomes have been documented for treatment of rhabdomyosarcoma in Turkey18, for treatment of osteosarcoma in Brazil19 and other solid tumors in Nigeria, Morocco and South Africa20–22.
The goal of this study was to describe patterns of clinical presentation, magnitude of treatment abandonment, and survival in children with sarcoma in Central America in order to document and guide development of focused initiatives aimed at addressing the expected survival gap.
METHODS
Study design
Retrospective review of previously collected de-identified data obtained from hospital-based registries from seven pediatric oncology centers in Central America. Based on nature of the data this study was reviewed and considered exempt from further review by the Office of Human Subject Research Studies at the Dana Farber Cancer Institute.
The centers
Six of seven pediatric oncology centers involved were pediatric cancer national referral centers and members of AHOPCA: Hospital Nacional de Niños, Costa Rica, Hospital Nacional de Niños Benjamin Bloom, El Salvador, Unidad Nacional de Oncologia Pediatrica, Guatemala, Hospital Materno Infantil, Honduras, Hospital Infantil “La Mascota”, Nicaragua, and Hospital del Niño de Panama (HNP), Panama. Centers serve the general population, including children from indigenous background, economically constrained households, and distant locations. Data from Hospital de Especialidades Pediatricas (HEP), the second largest center in Panama, was also included.
Data source
Hospital- based cancer registries stored in POND4KIDS23. Data collection and entry had been previously approved by local ethics committees. Data had been prospectively collected since 2005 and retrospectively collected for cases diagnosed before 2005. Data from HEP, Panama, was provided in Excel without linking identifiers.
Analytic cohort
Patients with newly diagnosed osteosarcoma, Ewing sarcoma (Ewing), rhabdomyosarcoma (RMS), and soft tissue sarcomas (STS) between Jan/1/00-Dec/31/09 were included. Patients with sarcoma as a secondary cancer were excluded. An age cut-off was not pre-established and all cases presenting to the pediatric oncology center and entered into the database were analyzed.
Oncology treatment
Patients had received multimodal treatment on a variety of published or regionally adapted protocols. Standard chemotherapy agents, pediatric surgeons, radiation therapy and subsidies for pediatric cancer care were available in all countries. For example, RMS treatment was based on IRS-IV and osteosarcoma treatment on amputation and chemotherapy with cisplatin, doxorubicin, ifosfamide, and etoposide. Limb-sparing procedures and high-dose methotrexate were only reliably available in Costa Rica.
Generalizability
Costa Rica is the only AHOPCA-member country with a national cancer registry. Therefore, the pediatric sarcoma incidence reported in Costa Rica (9.7 cases/million)24 for the period of 1984–92 is the best regional estimate available. In the United States the incidence is reported to be higher (16 cases/million)25. Based on the population less than 15 years of age (14.8 million in 2009), between 143 and 237 new cases of pediatric sarcoma would be expected per year in the region during the analytic period. Because the incidence of sarcoma increases during adolescence, the number expected to present to a pediatric center could be higher and depend on the maximum age allowed at the center.
Outcomes and important definitions
AHOPCA-centers have shared agreement on cancer outcomes reported into POND4KIDS: treatment refusal, treatment abandonment, relapse, progressive disease, secondary malignancy, lost to follow-up and death26. Treatment refusal and treatment abandonment are not usually included in analysis of cancer outcomes in HICs, but are noteworthy outcomes in LMCs27. Treatment refusal involves upfront decline of treatment and does not allow initiation of therapy. Treatment abandonment occurs after initiation of treatment and has been defined in AHOPCA as missing 4 or more consecutive weeks of treatment. Refusal and abandonment were analyzed as one event (“abandonment”) as recommended by the International Society of Pediatric Oncology Working Group on Treatment Abandonment28. Date of abandonment was the last date that the patient was known to participate in cancer care. Palliative care was considered continued care until death and was not counted as abandonment. Patients lost to follow-up after completion of therapy not counted as abandonment.
Subdivision of data
To evaluate changes over time, patients were divided into two treatment eras (2000–04 and 2005–09), based on existence of more mature twinning, more established psychosocial strategies and socioeconomic supports, and improved access to multimodal therapy in the second era. To evaluate the role of disease extent on outcomes, patients were divided based on presence or absence of metastases at diagnosis into Localized or Metastatic. To evaluate the role of disease location on outcomes, patients were divided into: bone sarcoma (osteosarcoma and Ewing of bone located in extremity or other- skull, chest wall, spine or pelvis), soft-tissue sarcoma (extra-osseous Ewing, rhabdomyosarcoma and STS in the extremity or other- located elsewhere).
Statistical Methods
Descriptive statistics were used to describe patient characteristics. A chi-square test was used to compare proportions. Cumulative incidence of abandonment curves were generated and Pepe-More test performed to test for statistically significant differences between the curves (p<0.05). Four survival analyses were performed: 1) Event-free survival (EFS) with events defined as relapse, progressive disease, secondary malignancy, or death; 2) Abandonment-sensitive event-free survival (AEFS) with events defined as abandonment of therapy, relapse, progressive disease, secondary malignancy, or death; 3) Overall survival (OS) with event defined as death, and 4) Abandonment-sensitive overall survival (AOS) with events defined as abandonment and death. Time to event was calculated from the time of diagnosis until the first event or until last contact if no event occurred. Survival results are presented as 4-year point estimate ± standard error due stability of the curves at that time point. Kaplan-Meier curves were generated and a Log rank test performed to test for statistically significant difference between the curves. P values of p<0.05 were considered significant.
RESULTS
Cohort
Seven hundred and ninety one (791) children were diagnosed with sarcoma in the seven pediatric oncology centers, between 1/1/2000 and 12/31/2009. Six patients (0.7%) were excluded because sarcoma represented a second malignancy. Among those included in the analysis, 323 (43%) were diagnosed between 2000–2004 and 458 (61%) between 2005–2009 (4 patients had missing data). Patients were from Costa Rica (115), El Salvador (102), Guatemala (259), Honduras (94), Nicaragua (151), and Panama (from HNP and 11 from HEP). Of the 785 patients included in the analysis, 182 (23%) abandoned treatment. The summary of patient characteristics is presented in Table I by abandonment status. Median age of the cohort was 10.2 years (range 0.1–23.6 years). Mean age and age range were similar between the countries analyzed.
Table I.
Patient Characteristics by Treatment Abandonment Status
| Abandonment Status | Patients who did not abandon treatment | Patients who abandoned treatment | |
|---|---|---|---|
| n=785 | 603 | 182 | |
| Osteosarcoma | n | 184 | 80 |
| Age (mean ±SD) | 12.0 ±0.3 | 12.6 ±0.3 | |
| Male | 47% | 51% | |
| Metastasis at Diagnosis | 37% | 42% | |
| Location in extremity | 98% | 97% | |
| Ewing Sarcoma | n | 148 | 27 |
| Age (mean ±SD) | 9.6 ±0.3 | 8.6 ±1.0 | |
| Male | 50% | 56% | |
| Metastasis at Diagnosis | 41% | 28% | |
| Primary bone, extremity | 50% | 71% | |
| Extraosseous, extremity | 29% | 25% | |
| Rhabdomyosarcoma | n | 181 | 59 |
| Age (mean ±SD) | 6.4 ±0.3 | 5.9 ±0.7 | |
| Male | 55% | 63% | |
| Metastasis at Diagnosis | 28% | 33% | |
| Surgical Group | |||
| I | 7% | 5% | |
| II | 10% | 12% | |
| III | 52% | 53% | |
| IV | 24% | 27% | |
| Not available | 6% | 3% | |
| Tumor Histology | |||
| Embryonal | 60% | 63% | |
| Alveolar | 30% | 27% | |
| Other | 9% | 8% | |
| Not available | 1% | 2% | |
| Location in extremity | 17% | 22% | |
| Soft-Tissue Sarcoma | n | 90 | 16 |
| Age (mean ±SD) | 9.4 ±0.5 | 8.1±1.2 | |
| Male | 57% | 44% | |
| Metastasis at Diagnosis | 20% | 29% | |
| Location in extremity | 38% | 23% |
Burden of disease
A high proportion of patients presented with metastatic disease (osteosarcoma 38%, Ewing 39%, RMS 29% and STS 21% compared to in HICs (20%, 25%, 18%, and 15% respectively)17, 29–31. Decrease in metastatic disease was noted by era for all diagnoses, but trend was not statistically significant (osteosarcoma 39% to 38%, Ewing 42% to 37%, RMS 33% to 26%, and STS 25% to 19%). Only 17% of RMS were classified as surgical group I–II (compared to 33% in HIC)17. By histology, the proportion of alveolar subtype RMS (ARMS) was similar to that in HIC (30% vs 32% respectively)17. However, analysis by country revealed a higher rate of ARMS in Guatemala compared to the other countries (48% of RMS in Guatemala vs 17% in El Salvador, 22% in Nicaragua, 27% in Costa Rica, 28% in Honduras, and 29% in Panama).
Treatment abandonment
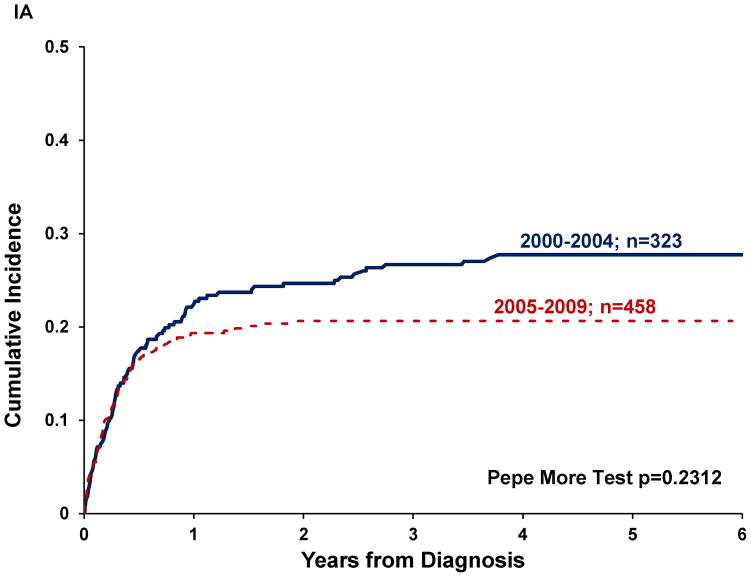
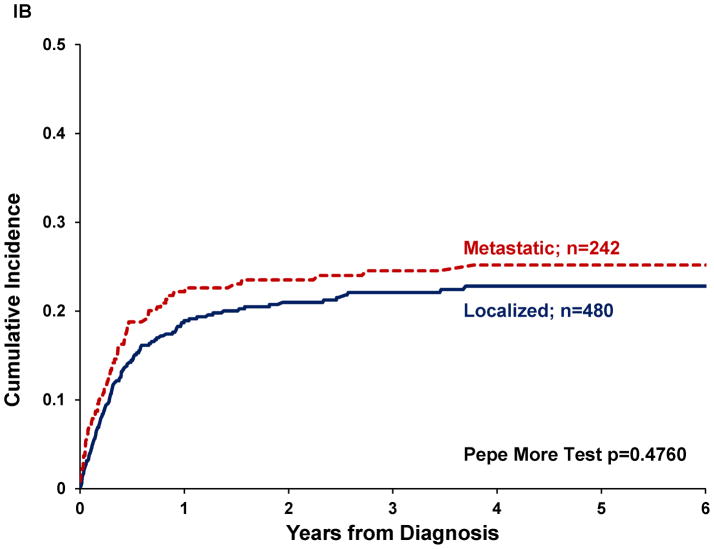
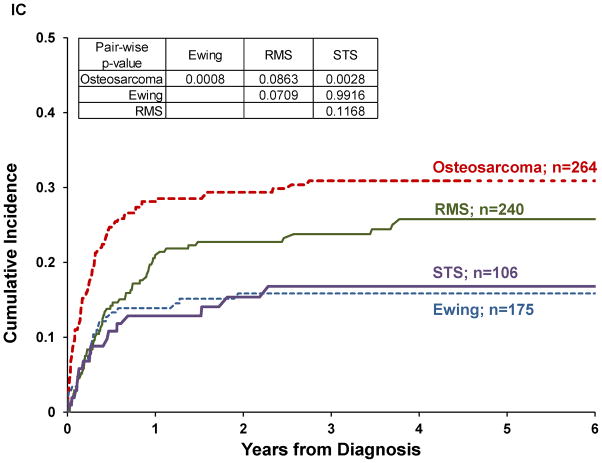
Twenty-three percent of patients abandoned treatment. Abandonment rate differed significantly by diagnosis (p=0.0094) and was higher for osteosarcoma and RMS (30% and 25% respectively) than for Ewing and STS (15% each). Abandonment rate decreased between the two eras (p=0.0193), but decrease was only significant for RMS and STS (p=0.011 and 0.0411, respectively, see Table II). Cumulative incidence (CI) of abandonment was not significantly different by era (p=0.2312; Figure 1a) or by disease extent (p=0.4760, Figure 1b). Pairwise comparisons between diagnoses revealed a higher CI of abandonment for osteosarcoma compared to Ewing (p=0.0008) or STS (p=0.0028), but not significantly different from RMS (Figure 1c). The CI of abandonment in the first six months was steeper for osteosarcoma compared to all other diagnoses, including RMS. Furthermore, 54% of patients with osteosarcoma and 56% of patients with Ewing who abandoned therapy did so in the first three months after diagnosis, compared to 30% for RMS and 41% for STS.
Table II.
Proportion of Patients Who Abandoned Treatment by Disease and Treatment Era
| N | Patients who abandoned treatment (%) | p-value | ||
|---|---|---|---|---|
| All Diagnoses | 2000–2004 | 323 | 27.0% | 0.0193 |
| 2005–2009 | 458 | 20.0% | ||
| Osteosarcoma | 2000–2004 | 107 | 32.0% | 0.6343 |
| 2005–2009 | 155 | 29.0% | ||
| Ewing Sarcoma | 2000–2004 | 68 | 13.0% | 0.5952 |
| 2005–2009 | 105 | 16.0% | ||
| Rhabdomyosarcoma | 2000–2004 | 112 | 32.0% | 0.011 |
| 2005–2009 | 128 | 18.0% | ||
| Soft-Tissue Sarcoma | 2000–2004 | 36 | 25.0% | 0.0411 |
| 2005–2009 | 70 | 10.0% | ||
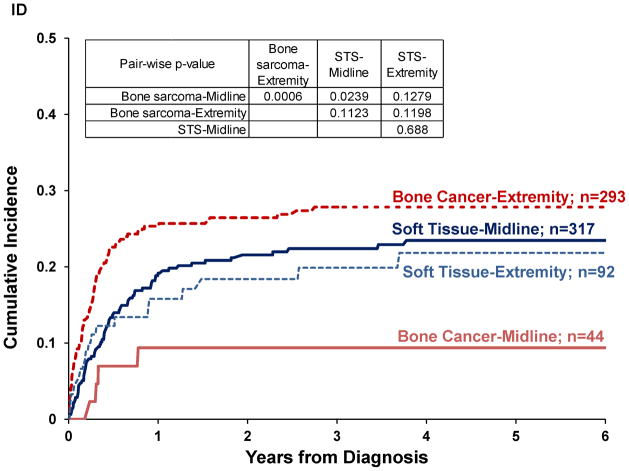
Figure I. Cumulative incidence of abandonment in pediatric sarcomas in Central America by.
(A) treatment era (n=781), (B) disease extent (n=722), (c) diagnosis (n=785), (D) disease location (n=746).
Pairwise comparisons by disease location showed higher CI of abandonment for patients with bone sarcoma located in the extremity compared to elsewhere (p=0.0006; Figure 1d). For sarcomas arising in soft tissues, CI of abandonment was not significantly different by disease location in the extremity vs. elsewhere (p=0.6880). CI of abandonment in the first six months was steeper for patients with bone sarcoma in the extremity than for tumors located elsewhere. Fifty-three percent of patients with bone sarcoma located in the extremity that abandoned therapy did so in the first three months after diagnosis compared to 45% for soft-tissue sarcoma located in the extremity, 34% for soft-tissue sarcoma located elsewhere, 22% for bone sarcoma located elsewhere.
Survival Analysis
Of 559 patients who experienced a first event: 329 (59%) experienced progressive disease or relapse, 161 (29%) abandoned therapy, 68 (12%) died and 1 developed a secondary malignancy. The median follow up for patients alive at last contact was 2.2 years (0 days -10.7 years). Of the 785 patients, 8 were excluded from the event-free survival (EFS) analysis and 7 from the overall survival (OS) analysis due to missing data.
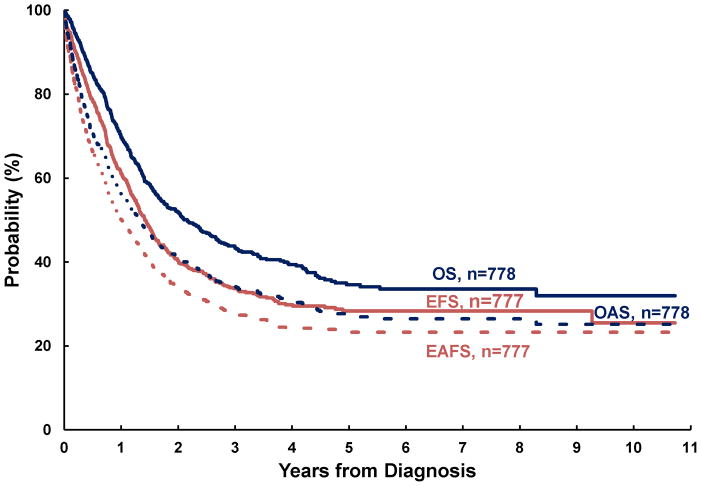
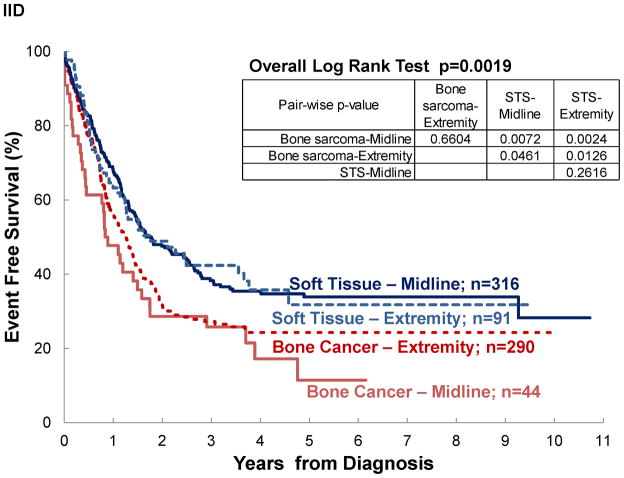
Figure II shows the overview of survival analyses for pediatric sarcoma in Central America. The estimated 4-year OS and EFS were 40% (±3%) and 30% (±3%), respectively. With abandonment included as an event, estimated survival decreased to 31% (±2%) and 24% (±2%) respectively (AOS and AEFS). Four-year OS estimates by disease were 31% (±4%) for osteosarcoma, 36% (±6%) for Ewing, 44% (±5%) for RMS, and 53% (±8%) for STS. By disease location, 4-year OS estimates were 32% (±4%) for bone sarcomas and 46% (±4%) for soft-tissue sarcoma. For contrast the 5-year survival in HIC is 68% for malignant bone tumors and 73% for soft-tissue sarcomas3.
Figure II. Survival Analysis.
Event-free survival (EFS), abandonment-sensitive event-free survival (AEFS), overall survival (OS) and abandonment-sensitive overall survival (AOS) for pediatric sarcoma in Central America.
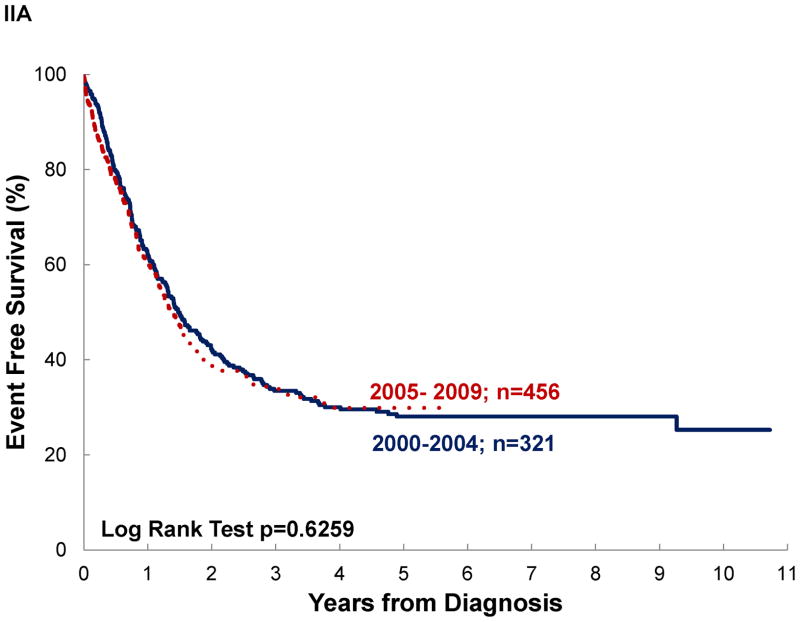
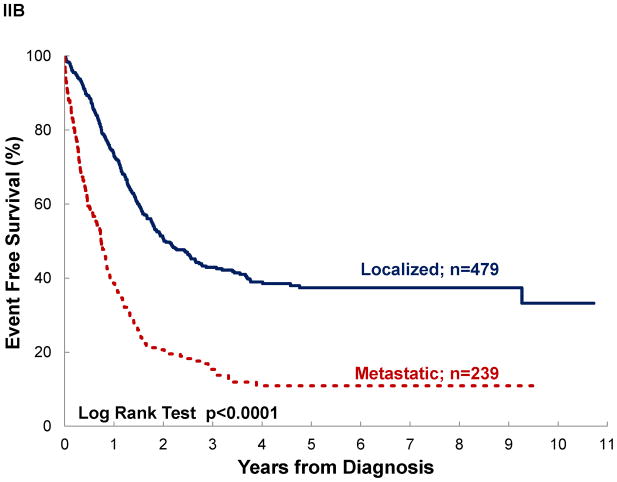
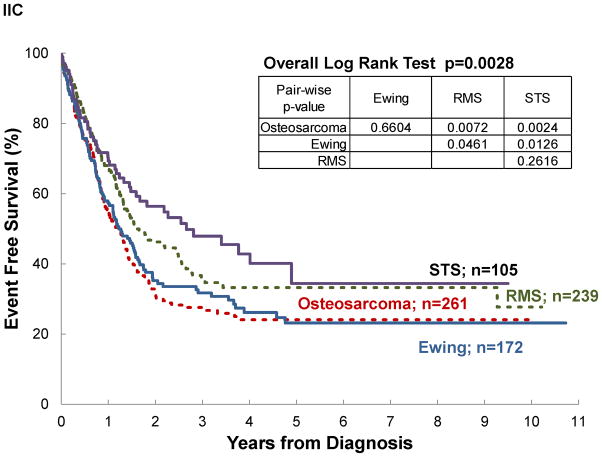
Significant differences in survival were noted by disease extent, diagnosis and disease location, but not by treatment era, even for patients with localized disease (See Table III and Figure III). Assuming negative outcome after abandonment, failing to include it as an event would have overestimated survival by 3–14% (see OS vs AOS and EFS vs AEFS). A difference ≥10% between OS and AOS was noted for localized disease, rhabdomyosarcoma, and STS. No differences ≥10% was observed between EFS and AEFS. Survival differed significantly by diagnosis. Patients with osteosarcoma and Ewing sarcoma experienced the poorest outcomes. In osteosarcoma, this was particularly strong when abandonment was included as an event (AOS for osteosarcoma vs RMS p=0.0007; vs STS p=0.0011; vs Ewing p=0.0669). Disease location in bone vs elsewhere was associated with decreased survival, particularly when abandonment was taken into account (AOS for Extremity vs Other was 28% vs 35%; p=0.0239 and EAFS for Extremity vs Other was 22% vs 27%; p=0.0376).
Table III.
Survival Analysis
| 4-year OS | 4-year AOS* | 4-year EFS | 4-year AEFS* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | OS±SE (%) | p-value | AOS± SE (%) | p-value | N | EFS± SE (%) | p-value | AEFS ±SE (%) | p-value | ||
| Overall | 778 | 40±3 | 31±2 | 777 | 30±3 | 24±2 | |||||
| Disease Extent | Localized Disease | 479 | 52±3 | <0.0001 | 42±3 | <0.0001 | 479 | 39±3 | <0.0001 | 33±3 | <0.0001 |
| Metastatic Disease | 240 | 17±4 | 12±3 | 239 | 11±3 | 8±2 | |||||
| Treatment Era | 2000–2004 | 321 | 41±3 | 0.41 | 30±3 | 0.92 | 321 | 30±3 | 0.63 | 23±3 | 0.93 |
| 2005–2009 | 457 | 39±5 | 32±4 | 456 | 30±4 | 26±4 | |||||
| Localized Disease, by treatment era | 2000–2004 | 195 | 56±4 | 0.13 | 42±4 | 0.77 | 195 | 41±4 | 0.27 | 33±4 | 0.67 |
| 2005–2009 | 284 | 49±6 | 42±5 | 284 | 38±5 | 33±5 | |||||
| Diagnosis | Osteosarcoma | 262 | 31±4 | 0.004** | 23±3 | 0.0007** | 261 | 24±4 | 0.0028** | 19±3 | 0.0005** |
| Ewing Sarcoma | 173 | 36±6 | 31±5 | 172 | 26±5 | 23±5 | |||||
| Rhabdomyosarcoma | 238 | 44±5 | 34±4 | 239 | 33±4 | 27±4 | |||||
| Soft-Tissue Sarcoma | 105 | 53±8 | 42±8 | 105 | 43±8 | 35±7 | |||||
| Disease Location# | Bone Sarcoma | 335 | 32±4 | 0.0005 | 25±3 | 0.0006 | 334 | 23±4 | 0.0003 | 20±3 | 0.0004 |
| Soft-Tissue Sarcoma | 407 | 46±4 | 36±3 | 407 | 36±4 | 29±3 | |||||
| Extremity | 383 | 35±4 | 0.044 | 28±3 | 0.0239 | 381 | 27±4 | 0.0727 | 22±3 | 0.0376 | |
| Other Location | 359 | 44±4 | 35±4 | 360 | 33±4 | 27±3 | |||||
AOS is abandonment-sensitive overall survival and AEFS is abandonment-sensitive event-free survival (see “Statistical Definitions” in methods for details)
Global test for differences in survival curves across diagnoses. Pairwise analysis done (not shown) to detect individual differences.
Disease Location (see “Subdivision of Data” in methods for details)
Figure III. Event-free survival of pediatric sarcoma in Central America.
by: (A) treatment era, (B) disease extent, (C) diagnosis, and (D) disease location; (n=777).
Analysis by country
Treatment abandonment and survival differed by country. Details of this analysis and correlation with cancer-infrastructure and health and economic indicators will be reported separately. In brief, treatment abandonment rate ranged from 1% in Costa Rica to 38% in Honduras. Abandonment-sensitive OS (AOS) ranged from 23.5% in Panama and 47.1% in Costa Rica.
DISCUSSION
This retrospective review of pediatric sarcoma outcomes in Central America documents a significant survival gap despite improved access to multimodal therapy and indicates that high burden of metastatic disease at diagnosis, high rate of treatment abandonment, and difficulty with upfront treatment effectiveness are likely drivers for the poor outcomes observed. To our knowledge, this study reviews the largest cohort of pediatric sarcoma cases from the developing world and provides relevant information for comprehensive cancer survival analysis in developing countries.
Due to lack of regional cancer registries, generalizability of our cohort cannot be fully ascertained. However, based on reported cancer incidence from Costa Rica, the cohort could represent up to two thirds of the expected cases, particularly during the second analytic period. Therefore, our results are likely the closest approximation to the true overall clinical presentation and outcomes of children with pediatric sarcoma in Central America.
One interesting variation in disease distribution was noted; while RMS histology for the cohort was similar compared to HIC, the proportion of alveolar RMS was notably higher (48%) in Guatemala. This supports prior observations of increased incidence of retinoblastoma, decreased incidence of neuroblastoma and high proportion of alveolar rhabdomyosarcoma in Guatemala8, 32. Comprehensive etiological studies merging cancer-epidemiology and tumor biology in LMCs are lacking and potential genetic and environmental modifiers of tumor behavior have not been fully explored. Studies confirming pathologic diagnostic accuracy, followed by studies integrating genetics, environment, and cancer biology would be of great value to the region.
A high proportion of patients presented with metastatic disease. In sarcomas, advanced disease translates into higher upfront risk-group assignment, more chemotherapy, difficulty achieving complete surgical resection and increased reliance on radiation therapy to achieve adequate local control. Therefore, advanced disease impacts outcomes and imposes further pressure on already overburdened healthcare systems. Advanced disease at presentation is most often attributed to delayed diagnosis and this is the leading argument for national media and educational campaigns. Short term impact of such programs has been documented in Honduras, where a retinoblastoma education program linked to a national vaccination campaign increased referrals and decreased presentation with extraocular spread33. Bundled strategies that educate child advocates, the general public and health care providers on referral process, treatment availability and improved curability of low stage disease, has the potential to impact this driver of poor outcomes and merits further support.
Central America has pioneered research on delivery of pediatric cancer care in resource-limited settings and has shown great improvement in survival and treatment abandonment for children with leukemia15, 16. However, treatment abandonment in pediatric sarcoma remains a concern with rate consistently higher in pediatric sarcoma (average 20%) than for leukemia (average 6%) in recent years (AHOPCA unpublished data provided by Dr. Baez). This study shows that although the proportion of patients with pediatric sarcoma that abandon therapy has decreased over time, these patients remain at high-risk group for treatment abandonment. Also, that lack of inclusion of treatment abandonment as an event leads to overestimation of overall and event-free survival. Relevant socioeconomic and demographic drivers of abandonment have been described15, 27, 34. Our study focuses on clinical characteristics that influence abandonment. Occurrence and timing to abandonment appear to be mostly influenced by the primary diagnosis and disease location; not by age, gender, or disease extent. Patients with bone tumors of the extremities (particularly osteosarcoma) abandon treatment most frequently and do so earlier than patients with other types of sarcoma; presumably reflecting a rejection of amputation. Unfortunately, due to high cost of endo-prosthesis, lack of bone allografts and limited skilled personnel, amputation is the primary modality available for local control in all countries except Costa Rica. Strategies for strengthening of rehabilitation and reintegration programs for children that have sustained amputation and possibly increasing access to limb-sparing procedures could be of great impact.
Patients with rhabdomyosarcoma share similar long term incidence of abandonment as patients with osteosarcoma, but abandonment accumulates over longer period of time; suggesting that the drivers of early and late abandonment for bone and soft-tissue tumors are different. All in all, these findings suggest that abandonment is driven by factors beyond socio-demographics, are possibly disease/treatment specific, and that development of retention models designed for these high-risk patient populations is needed. However, metastatic disease and abandonment don’t fully explain the poor long-term outcomes observed. Survival did not significantly improve between eras, even for patients with localized disease and despite improved access to conventional therapy. Furthermore, 42% of all patients experienced progressive disease or relapse as first event, suggesting fundamental difficulty in effective delivery of upfront therapy. Focus on capacity building, quality improvement, communication, and coordination of multidisciplinary care is warranted to maximize upfront cancer care effectiveness and outcomes. This is particularly relevant in the management of solid malignancies, where sophisticated local control measures are at the core of therapy. Successful restructuring of care in order to effectively deliver complex frontline therapy has been reported for treatment of non-metastatic Ewing sarcoma35 and osteosarcoma36 in Chile.
In conclusion, we have identified high burden of metastatic disease, persistently high rate of treatment abandonment, and difficulty with upfront treatment effectiveness as major barriers to optimal care to children with sarcoma in Central America. While advances in care delivery for children with hematologic malignancies have resulted in significant improvements in outcomes, children with sarcomas have not benefited from those gains, likely due to deficiencies in the delivery of multidisciplinary care that is key in the management of solid malignancies. Several initiatives are ongoing to address these issues. The Pan-American Health Organization is working regionally on general and health care provider education using the Integrated Management of Childhood Illness Method. Collaboration with major academic centers in the US, Italy and Brazil is allowing for further education and capacity building opportunities in limb-salvage surgery as well as early referral of selected cases for limb-sparing procedures. Finally, web-based case discussions, detailed infrastructure assessment, quality control initiatives, assessment of local control effectiveness, and further analysis of inherent drivers of abandonment in pediatric sarcoma are the focus of the current AHOPCA Sarcoma Initiative and the core of our future studies.
Acknowledgments
The authors gratefully acknowledge support from the Dana-Farber/Children’s Hospital Cancer Center Global Health Initiative and St. Jude St. Jude Children’s Research Hospital International Outreach Program and thank AHOPCA’s data managers and clinical staff for their inspiring work.
Funding Sources: First author’s effort was supported by Pathophysiology of Human Blood Cells (T32 HL 7574) and Program in Cancer Outcomes Research Training (R25 CA092203); both National Institutes of Health training grants.
Footnotes
Financial disclosures: No disclosures.
References
- 1.Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer. 2008;112(3):461–72. doi: 10.1002/cncr.23205. [DOI] [PubMed] [Google Scholar]
- 2.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–34. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36(4):277–85. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Carroll WL, Finlay JL. Cancer in children and adolescents. Sudbury, Mass: Jones and Bartlett Publishers; 2010. [Google Scholar]
- 5.Tanko NM, Echejoh GO, Manasseh NA, Mandong MB, Uba AF. Paediatric solid tumours in Nigerian children: a changing pattern? Afr J Paediatr Surg. 2009;6(1):7–10. doi: 10.4103/0189-6725.48567. [DOI] [PubMed] [Google Scholar]
- 6.Bhurgri Y, Bhurgri H, Pervez S, et al. Epidemiology of soft tissue sarcomas in Karachi South, Pakistan (1995–7) Asian Pac J Cancer Prev. 2008;9(4):709–14. [PubMed] [Google Scholar]
- 7.Margaron FC, Poenaru D, Northcutt A. Pediatric cancer spectrum in Kenya: a histopathologic review. Pediatr Surg Int. 2010;26(8):789–94. doi: 10.1007/s00383-010-2639-9. [DOI] [PubMed] [Google Scholar]
- 8.Antillon F, Castellanos M, Valverde P, et al. Treating Pediatric soft tissue sarcomas in a country with limited resources: the experience of the Unidad Nacional de Oncologia Pediatrica in Guatemala. Pediatr Blood Cancer. 2008;51(6):760–4. doi: 10.1002/pbc.21699. [DOI] [PubMed] [Google Scholar]
- 9.Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle-income: a call to action. Lancet. 2010;376(9747):1186–93. doi: 10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro RC, Pui CH. Saving the children--improving childhood cancer treatment in developing countries. N Engl J Med. 2005;352(21):2158–60. doi: 10.1056/NEJMp048313. [DOI] [PubMed] [Google Scholar]
- 11.Masera G, Eden T, Schrappe M, et al. Statement by members of the Ponte di Legno Group on the right of children to have full access to essential treatment for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;43(2):103–4. doi: 10.1002/pbc.20135. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro RC, Steliarova-Foucher E, Magrath I, et al. Baseline status of paediatric oncology care in ten low-income or mid-income countries receiving My Child Matters support: a descriptive study. Lancet Oncol. 2008;9(8):721–9. doi: 10.1016/S1470-2045(08)70194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatenoud L, Bertuccio P, Bosetti C, Levi F, Negri E, La Vecchia C. Childhood cancer mortality in America, Asia, and Oceania, 1970 through 2007. Cancer. 2010;116(21):5063–74. doi: 10.1002/cncr.25406. [DOI] [PubMed] [Google Scholar]
- 14.Howard SC, Pedrosa M, Lins M, et al. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource-poor area. JAMA. 2004;291(20):2471–5. doi: 10.1001/jama.291.20.2471. [DOI] [PubMed] [Google Scholar]
- 15.Metzger ML, Howard SC, Fu LC, et al. Outcome of childhood acute lymphoblastic leukaemia in resource-poor countries. Lancet. 2003;362(9385):706–8. doi: 10.1016/S0140-6736(03)14228-6. [DOI] [PubMed] [Google Scholar]
- 16.Bonilla M, Moreno N, Marina N, et al. Acute lymphoblastic leukemia in a developing country: preliminary results of a nonrandomized clinical trial in El Salvador. J Pediatr Hematol Oncol. 2000;22(6):495–501. doi: 10.1097/00043426-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. [[accessed March 10, 2011].];PDQR Childhood Rhabdomyosarcoma Treatment. Available from URL: http://cancer.gov/cancertopics/pdq/treatment/childrhabdomyosarcoma/HealthProfessional.
- 18.Karakas Z, Agaoglu L, Biner B, et al. Results of rhabdomyosarcoma treatment in a developing country. Acta Med Okayama. 2000;54(4):173–7. doi: 10.18926/AMO/32277. [DOI] [PubMed] [Google Scholar]
- 19.Petrilli AS, de Camargo B, Filho VO, et al. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006;24(7):1161–8. doi: 10.1200/JCO.2005.03.5352. [DOI] [PubMed] [Google Scholar]
- 20.Ekenze SO, Agugua-Obianyo NE, Odetunde OA. The challenge of nephroblastoma in a developing country. Ann Oncol. 2006;17(10):1598–600. doi: 10.1093/annonc/mdl167. [DOI] [PubMed] [Google Scholar]
- 21.Madani A, Zafad S, Harif M, et al. Treatment of Wilms tumor according to SIOP 9 protocol in Casablanca, Morocco. Pediatr Blood Cancer. 2006;46(4):472–5. doi: 10.1002/pbc.20436. [DOI] [PubMed] [Google Scholar]
- 22.Moore SW, Millar AJ, Hadley GP, et al. Hepatocellular carcinoma and liver tumors in South African children: a case for increased prevalence. Cancer. 2004;101(3):642–9. doi: 10.1002/cncr.20398. [DOI] [PubMed] [Google Scholar]
- 23.Quintana Y, Patel AN, Naidu PE, Howard SC, Antillon FA, Ribeiro RC. POND4Kids: A Web-based Pediatric Cancer Database for Hospital-based Cancer Registration and Clinical Collaboration. Stud Health Technol Inform. 2011;164:227–31. [PubMed] [Google Scholar]
- 24.Parkin DM. International incidence of childhood cancer. II. Lyon New York: International Agency for Research on Cancer; Distributed in the USA by Oxford University Press; 1998. International Agency for Research on Cancer. [Google Scholar]
- 25.Ries LA. SEER Cancer Statistics Review, 1973–1996. Bethesda, Md: National Cancer Institute; [Date Accessed April, 12, 2011, 1999.]. [Google Scholar]
- 26.Howard SC, Marinoni M, Castillo L, et al. Improving outcomes for children with cancer in low-income countries in Latin America: a report on the recent meetings of the Monza International School of Pediatric Hematology/Oncology (MISPHO)-Part I. Pediatr Blood Cancer. 2007;48(3):364–9. doi: 10.1002/pbc.21003. [DOI] [PubMed] [Google Scholar]
- 27.Arora RS, Eden T, Pizer B. The problem of treatment abandonment in children from developing countries with cancer. Pediatr Blood Cancer. 2007;49(7):941–6. doi: 10.1002/pbc.21127. [DOI] [PubMed] [Google Scholar]
- 28.Mostert S, Arora RS, Arreola M, et al. Abandonment of treatment for childhood cancer: position statement of a SIOP PODC Working Group. Lancet Oncol. 2011;12(8):719–20. doi: 10.1016/S1470-2045(11)70128-0. [DOI] [PubMed] [Google Scholar]
- 29.National Cancer Institute. [[accessed March 10, 2011].];PDQR Osteosarcoma/Malignant Fibrous Histiocytoma of Bone Treatment. Available from URL: http://cancer.gov/cancertopics/pdq/treatment/osteosarcoma/HealthProfessional.
- 30.National Cancer Institute. [[accessed March 10, 2011].];PDQR Ewing Sarcoma Family of Tumors Treatment. Available from URL: http://cancer.gov/cancertopics/pdq/treatment/ewings/HealthProfessional.
- 31.National Cancer Institute. [[accessed March 10, 2011].];PDQR Childhood Soft Tissue Sarcoma Treatment. Available from URL: http://cancer.gov/cancertopics/pdq/treatment/child-soft-tissue-sarcoma/HealthProfessional.
- 32.Deepa Bhojwani FA-K, Howard Scott. Childhood Cancer in the Developing World. In: Carroll WL, Finlay JL, editors. Cancer in children and adolescents. Sudbury, Mass: Jones and Bartlett Publishers; 2010. pp. 15–22. [Google Scholar]
- 33.Leander C, Fu LC, Pena A, et al. Impact of an education program on late diagnosis of retinoblastoma in Honduras. Pediatr Blood Cancer. 2007;49(6):817–9. doi: 10.1002/pbc.21052. [DOI] [PubMed] [Google Scholar]
- 34.Bonilla M, Rossell N, Salaverria C, et al. Prevalence and predictors of abandonment of therapy among children with cancer in El Salvador. Int J Cancer. 2009;125(9):2144–6. doi: 10.1002/ijc.24534. [DOI] [PubMed] [Google Scholar]
- 35.Villarroel M, Tordecilla J, Salgado C, et al. Multimodal therapy for children and adolescents with Ewing sarcoma: results of the First National Chilean Trial (1986–1991) Med Pediatr Oncol. 1997;29(3):190–6. doi: 10.1002/(sici)1096-911x(199709)29:3<190::aid-mpo5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Rivera GK, Quintana J, Villarroel M, et al. Transfer of complex frontline anticancer therapy to a developing country: the St. Jude osteosarcoma experience in Chile Pediatr Blood. Cancer. 2008;50(6):1143–6. doi: 10.1002/pbc.21444. [DOI] [PubMed] [Google Scholar]