Abstract
Titin-based passive stiffness is post-translationally regulated by several kinases that phosphorylate specific spring elements located within titin’s elastic I-band region. Whether titin is phosphorylated by calcium/calmodulin dependent protein kinase II (CaMKII), an important regulator of cardiac function and disease, has not been addressed. The aim of this work was to determine whether CaMKIIδ, the predominant CaMKII isoform in the heart, phosphorylates titin, and to use phosphorylation assays and mass spectrometry to study which of titin’s spring elements might be targeted by CaMKIIδ. It was found that CaMKIIδ phosphorylates titin in mouse LV skinned fibers, that the CaMKIIδ sites can be dephosphorylated by protein phosphatase 1 (PP1), and that under baseline conditions, in both intact isolated hearts and skinned myocardium, about half of the CaMKIIδ sites are phosphorylated. Mass spectrometry revealed that both the N2B and PEVK segments are targeted by CaMKIIδ at several conserved serine residues. Whether phosphorylation of titin by CaMKIIδ occurs in vivo, was tested in several conditions using back phosphorylation assays and phospho-specific antibodies to CaMKIIδ sites. Reperfusion following global ischemia increased the phosphorylation level of CaMKIIδ sites on titin and this effect was abolished by the CaMKII inhibitor KN-93. No changes in the phosphorylation level of the PEVK element were found suggesting that the increased phosphorylation level of titin in IR might be due to phosphorylation of the N2B element. The findings of these studies show for the first time that titin can be phosphoryalated by CaMKIIδ, both in vitro and in vivo, and that titin’s molecular spring region that determines diastolic stiffness is a target of CaMKIIδ.
Introduction
Abnormal cardiac filling caused by increased diastolic chamber stiffness is an important factor in the pathophysiology of a range of cardiac diseases, including the highly prevalent heart failure with preserved ejection fraction (HFpEF) syndrome[1]. However, the mechanisms that determine the level of diastolic stiffness and its various tuning mechanisms are not completely understood. An important determinant of diastolic stiffness is the giant protein titin that spans from Z-disk to M-band of the cardiac sarcomere and has an elastic I-band region that functions as a diastolic stiffness generating molecular spring[2]. Titin’s molecular spring region can be restructured through alternative splicing that gives rise to titin isoforms with spring elements that vary in length and generate different levels of passive stiffness [3]. However, changes in splicing are slow processes that require days to weeks to be accomplished and in recent years it has become apparent that phosphorylation-based mechanisms exists that rapidly tune stiffness. The two main spring elements of cardiac titin, the so-called N2B and PEVK elements, are both targeted by kinases. The PEVK spring element has been shown to be phosphorylated by protein kinase Cα (PKCα), a key player in contractile dysfunction and heart failure [4, 5]; single molecule, single cell and myocardial tissue experiments in wildtype and PEVK KO mice have shown that PKCα phosphorylation of the PEVK element increases passive stiffness [6–8]. The N2B element of titin is also a kinase substrate whose mechanical properties change following phosphorylation. Protein kinase A (PKA), which is stimulated by the β-adrenergic pathway, phosphorylates the large unique sequence of the N2B element which reduces passive stiffness [9, 10]. Similar to PKA, protein kinase G (PKG), a cGMP-dependent kinase that is part of signaling cascades initiated by nitric oxide (NO) and natriuretic peptides (NPs), phosphorylates the unique sequence of the N2B element and reduces passive stiffness; the PKG phosphorylation site in humans (S469) is also a residue targeted by PKA [11].
In this study we focused on the Ca2+ and calmodulin dependent serine/threonine kinase (CaMKII) that is activated by increases in cellular Ca2+. Four isoforms have been described (α, β, δ, γ) of which CaMKIIδ is the predominant isoform in the heart[12]. CaMKIIδ phosphorylates several Ca2+-handling proteins including phospholamban (PLB)[13, 14], ryanodine receptor (RyR2)[15–17], and L-type Ca2+ channel (LTCC)[18], as well as myofilament proteins including TnT[19] and MyBP-C[20]. Our aim was to determine whether CaMKIIδ also phosphorylates titin, and to use phosphorylation assays and mass spectrometry to study which of titin’s spring elements might be phosphorylated by CaMKIIδ. We found that CaMKIIδ phosphorylates titin in mouse LV skinned fibers, that the CaMKIIδ sites can be dephosphorylated by protein phosphatase 1 (PP1), and that about half of the CaMKIIδ sites are phosphorylated under baseline conditions in isolated hearts and skinned myocardium. We also found that the N2B and PEVK segments are targeted by CaMKIIδ at several conserved sites. Whether phosphorylation of titin by CaMKIIδ occurs in vivo was tested in several conditions, including ischemia reperfusion (IR), that is known to activate CaMKIIδ [21]. IR induced an increase in the phosphorylation level of CaMKIIδ sites on titin and this was abolished by the CaMKII inhibitor KN-93. Findings of this work have been presented previously in abstract format[22].
Material and Methods
In vitro phosphorylation assay of skinned myocardium
All experiments were performed on 3 month old male C57BL/6J mice and were approved by the University of Arizona IACUC and followed the U.S. National Institutes of Health “Using Animals in Intramural Research” guidelines for animal use. Skinned fibers isolated from the left ventricular (LV) wall[23] were incubated for 2h at 30°C with 0.05 U/µl human CaMKIIδ (expressed in insect cells, Invitrogen, CA USA), in kinase buffer (25 mM BES, 1 mM CaCl2, 5 µM calmodulin, 4 mM NaATP, 4 mM MgCl2, 1 mM DTT, 50 µM protein kinase A inhibitor (Sigma), 5 mM NaF, 1 mM Na3VO4 pH 7.0), and 10 µCi of [γ-32P]ATP (specific activity 3,000 Ci/mmol, Perkin-Elmer). Some fibers were dephosphorylated by incubation with 0.75 U/µl of protein phosphatase 1 (PP1; recombinant rabbit muscle α-isoform, Calbiochem), 2h at 30°C, followed by extensive washing and then incubation with CaMKIIδ. The reaction was stopped by adding solubilization buffer (6 M urea, 1.5 M thiourea, 2.25% SDS, 56.25 mM DTT, 0.0225% bromophenol blue, 35% glycerol, 0.0825 mg/ml leupeptin, 1 mM E-64, 0.015 mM PMSF, 37.5 mM Tris-HCl pH 6.8) and the proteins were separated on 2–7% SDS-PAGE gel gradient. The gels were stained with Coomassie blue, dried, scanned, and exposed to X-ray film and analyzed. The titin optical density (OD) of the autoradiograph was normalized to that of the Coomassie blue-stained gel, to normalize for protein loading. In a subset of experiments Western Blots were used to detect phosphorylation of S26 and S170 in the PEVK region of the N2B cardiac titin isoform. Skinned fibers were solubilized and proteins were separated on 0.8% agarose gel and transferred to PVDF membrane (Millipore). The membranes were stained with Ponceau S (Sigma) to determine the level of transferred proteins. The membranes were probed with phospho-specific rabbit polyclonal antibodies against titin’s p-S26 and p-S170[24]. Secondary antibodies conjugated with fluorescent dyes (Biotium, Hayward, Ca, USA) with infrared excitation spectra were used for detection and membranes scanned and analyzed using an Odyssey Infrared Imaging System (Li-Cor Biosciences).
In vitro kinase assay of titin recombinant proteins
The human titin recombinant fragments N2B, PEVK (from cardiac N2B isoform), Ig8-15, Ig84-91 and the murine recombinant N2B were expressed in E coli as describe [25]. The purified recombinant proteins were incubated with 0.05 U/µl human recombinant CaMKIIδ (Invitrogen, CA USA), kinase buffer (see above), and 10 µCi of [γ-32P]ATP (Perkin-Elmer), 2h at 30°C. The proteins were solubilized (0.5 M Tris-HCl pH 6.8, 10% glycerol, 2% SDS, 0.1 mM 2-mercaptoethanol, 0.01% bromophenol blue) and separated on 4–20% gradient or 12% SDS-PAGE. The gels were Coomassie blue stained, dried, scanned, exposed to X-ray film, and analyzed as described above. We also probed the phosphorylation levels of PEVK’s S26 and S170 in recombinant protein using Western blots. The human titin recombinant fragment PEVK expressed in E coli including WT, mutants S26A, S170A, and S26A/S170A[24] were incubated for 2h at 30°C with 0.05 U/µl human recombinant CaMKIIδ (Invitrogen, CA USA) in kinase buffer (see above), and 10 µCi of [γ-32P]ATP (Perkin-Elmer). The proteins were solubilized (0.5 M Tris-HCl pH 6.8, 10% glycerol, 2% SDS, 0.1 mM 2-mercaptoethanol, 0.01% bromophenol blue) and separated on 10% SDS-PAGE. The gels were Coomassie blue stained, dried, scanned, exposed to X-ray film, and analyzed as described above. Additionally some gels were also transferred to PVDF membrane (Millipore) and blotted with phospho-specific antibodies against p-S26 and p-S170 (for details, see above).
Tandem mass spectrometry coupled to liquid chromatography
Nonphosphorylated and CaMKIIδ-phosphorylated human and murine titin N2B recombinant proteins (see above) were electrophoresed and Coomassie blue stained. The N2B bands were excised from the gels and digested with trypsin or chymotrypsin. Tandem mass spectrometry coupled to liquid chromatography (LC-MS/MS) analyses were carried out (LTQ Orbitrap Velos Thermo Scientific Inc., MA USA) and tandem MS spectra of peptides were analyzed with TurboSEQUEST. Various possible modifications such as alkylation of cysteine residues and of methionine residues and phosphorylation were included in the search parameters.
Isoproterenol stimulation of intact cardiac myocytes
Cells were isolated as described previously [26, 27]. In brief, the heart was cannulated via the aorta and perfused for 4 min with perfusion buffer (in mM: 113 NaCl, 4.7 KCl,0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4, 12 NaHCO3, 10 KHCO3, 10 HEPES, 5.5 glucose, 5 BDM, 10 taurine, 20 Creatine, 5 Adenosine, 5 Inosine adjust pH to 7.4 at 37°C), followed by perfusion with digestion buffer (perfusion buffer plus 0.06 mg/ml of TM liberase [Liberase TM research grade medium thermolysin concentration; Roche Applied Science, IN USA], and 12.5 µM CaCl2) for 8–10 min. The left ventricle was cut into small pieces that were triturated several times with a transfer pipette and then filtered through a 300-µm nylon mesh filter. The cells were gravity pelleted and Ca2+ was reintroduced to a final concentration of 1.8 mM. Some of the cells were solubilized and electrophoresed [28, 29] to determine titin content. Activation of CaMKIIδ was determined indirectly by the measurement by Western Blot of the phosphorylation level of the specific CaMKIIδ target Thr17 on phospholamban, PLB [30, 31]. The cardiomyocytes were stimulated as described by Erickson et al [32] with some modifications. Freshly prepared calcium tolerant cardiomyocytes in minimum essential medium α (MEM α) (Invitrogen) supplemented with 1.8 mM calcium chloride were transferred to Petri dish coated with 0.01% poly-L-lysine (Sigma). The myocytes were allowed to adhere for 30 min at 37°C, 5% CO2 in a CO2 incubator (Thermo Scientific). The cell suspension was paced (1 Hz) and 1 µM Okadaic acid (Sigma) was added and the cells incubated for 10 min. The cells were electrically stimulated (paced) using a 6 well C-Dish electrode assembly in conjunction with a C-Pace EP cell culture stimulator (IonOptix). 1 µM isoproterenol (Sigma) was added to the cardiomyocytes and the cells paced at 1 Hz for 20 min. One set of cells were pre-incubated with 1 µM of the CaMKIIδ inhibitor KN93 (Calbiochem) for 10 min before the isoproterenol was added. Then the frequency of stimulation was raised to 4 Hz and the cells were paced for an additional 20 min. The medium was removed and the cells were solubilized by adding solubilization buffer (see above) and heated at 65°C for 5 min. The proteins were separated by SDS-PAGE (5%) and transferred to PDVF membrane. The membranes were blotted with rabbit anti-p-Thr17 (Badrilla, Leeds, UK) and mouse anti-PLB (Badrilla, Leeds, UK) as a loading control (in select experiments mouse anti-GAPDH (Abcam) was used as a second loading control. Secondary antibodies conjugated with fluorescent dyes (Biotium, Hayward, Ca, USA) with infrared excitation spectra were used for detection and membranes scanned and analyzed using an Odyssey Infrared Imaging System (Li-Cor Biosciences).
Ischemia-reperfusion (IR) experiments
Isolated hearts were perfused according to the Langendorff technique, at constant temperature (37°C), at 90mmHg constant pressure (~3 mL/min), and using a pacing rate of 450 beats/min [33]. The mechanical activity of the heart was assessed by passing into the left ventricle a latex balloon connected to a pressure transducer. After stabilization the hearts were perfused for 20 min (pre-ischemia), global ischemia was produced by interruption of the coronary flow for 20 min. after which perfusion was restored (reperfusion). The hearts were flash frozen after different periods of reperfusion. To evaluate the influence of CaMKII we used the cell permeable CaMKII inhibitor KN93 (IR-KN) (Calbiochem). For the IR-KN group, 5 µM KN93 was added in all solutions. After stabilization, the IR and IR-KN hearts were subject to global no-flow ischemia for 20 minutes; control hearts continued to be perfused. After ischemia, hearts were reperfused for 3 minutes and hearts were then rapidly removed and flash frozen in liquid nitrogen or rapidly dissected and permeabilized for back-phosphorylation assays (see above). For titin protein analysis, 0.8% agarose electrophoresis was performed. The muscle samples were solubilized (see above) and electrophoresed. The 0.8% agarose gels were run at 15 mA, 3 h 20 min at 4°C. The proteins were transferred to PVDF membrane. The membranes were stained with Ponceau S (Sigma) to visualize total transferred proteins. The membranes were probed with phospho-specific rabbit polyclonal antibodies against titin’s p-S26 or p-S170[24]. The activation level of CaMKIIδ was determined indirectly by the measurement of the phosphorylation level of the specific target Thr17 on phospholamban (PLB) by Western Blot, as stated above. Proteins were separated by SDS-PAGE (15%) and transferred to PVDF membrane (Millipore). The membranes were blotted with rabbit anti-p-Thr17 and mouse anti-PLB (for details, see above).
Statistical Analysis
Data are presented as mean ±SE. Statistical tests for significance were t-tests and ANOVA, as appropriate. Probability values <0.05 were taken as significant.
Results
CaMKIIδ phosphorylates cardiac titin
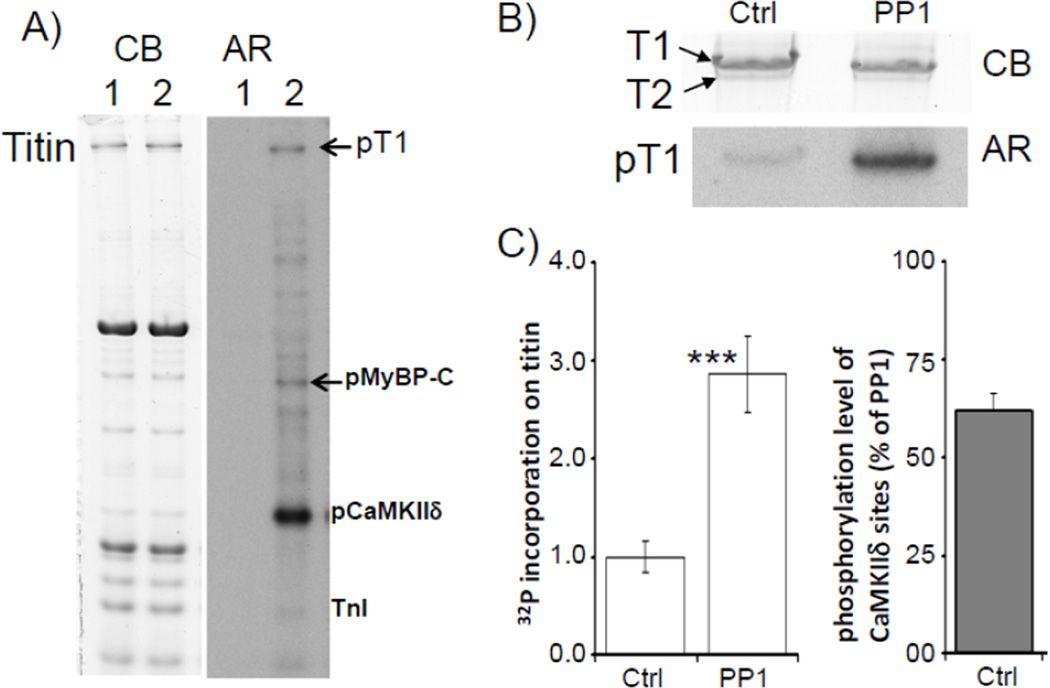
To determine whether titin is phosphorylated by CaMKIIδ a back-phosphorylation assay was performed on mouse LV skinned cardiac fibers. The fibers were incubated with CaMKIIδ and Ca2+/calmodulin in the presence of [γ-32P]ATP. Results showed phosphorylation of MyBP-C, a well-known CaMKIIδ substrate, a weak signal from TnI, and a strong CaMKIIδ auto-phosphorylation signal[34]) (Fig.1A). Importantly, titin was also phosphorylated by CaMKIIδ. Phosphorylation of titin was mainly on T1, the full length titin molecule, and not on T2 (Fig. 1B). (T2 is a degradation product that is likely to form during sample preparation and that corresponds largely to the A-band portion of the molecule[35].) We also pre-treated skinned muscle with protein phosphatase 1 (PP1) and found that this significantly enhanced CaMKIIδ phosphorylation by nearly 3- fold (Fig. 1B and Fig. 1C, left). From the results of the back-phosphorylation assay, the baseline phosphorylation level of the CaMKIIδ accessible sites was estimated ((PP1- Ctrl)/PP1) at 60.3±6.0% (Fig. 1C, right). In summary, the results showed that 1) CaMKIIδ phosphorylates titin, 2) CaMKIIδ sites on titin can be dephosphorylated by PP1, and 3) the baseline phosphorylation level of CaMKIIδ accessible sites in mouse skinned myocardium is relatively high.
Figure 1. Titin is phosphorylated by CaMKIIδ.
A) Mouse skinned LV muscle fibers incubated with CaMKIIδ. Left panel: Coomassie blue (CB) stained 2–7% gradient gel. Right panel: corresponding autoradiograph (AR). Lane 1: skinned fibers in kinase buffer (containing Ca2+/Calmodulin, and [γ-32P]ATP). Lane 2: skinned fibers in kinase buffer plus CaMKIIδ. B) Effect of pre-incubation of mouse skinned cardiac fibers with protein phosphatase 1 (PP1). Top: lane 1 (Ctrl) skinned fibers incubated with CaMKIIδ. Lane 2 (PP1) skinned fibers pre-treated with PP1 and then incubated with CaMKIIδ. Upper panel: Coomassie blue (CB) stained gel; Lower panel: corresponding autoradiograph (AR). C) Left: pre-incubation with PP1 resulted in a significant increase in CaMKIIδ induced 32P incorporation on titin. Right: Phosphorylation level of CaMKIIδ accessible sites under control conditions (expressed as percentage of 32P incorporation on titin in PP1 pretreated and then CaMKIIδ phosphorylated skinned muscle). (N=12, *** p<0.001 in t-test of PP1 treated vs. ctrl muscle. T1: intact titin; T2: titin degradation product.)
CaMKIIδ phosphorylates titin’s N2B and PEVK elements
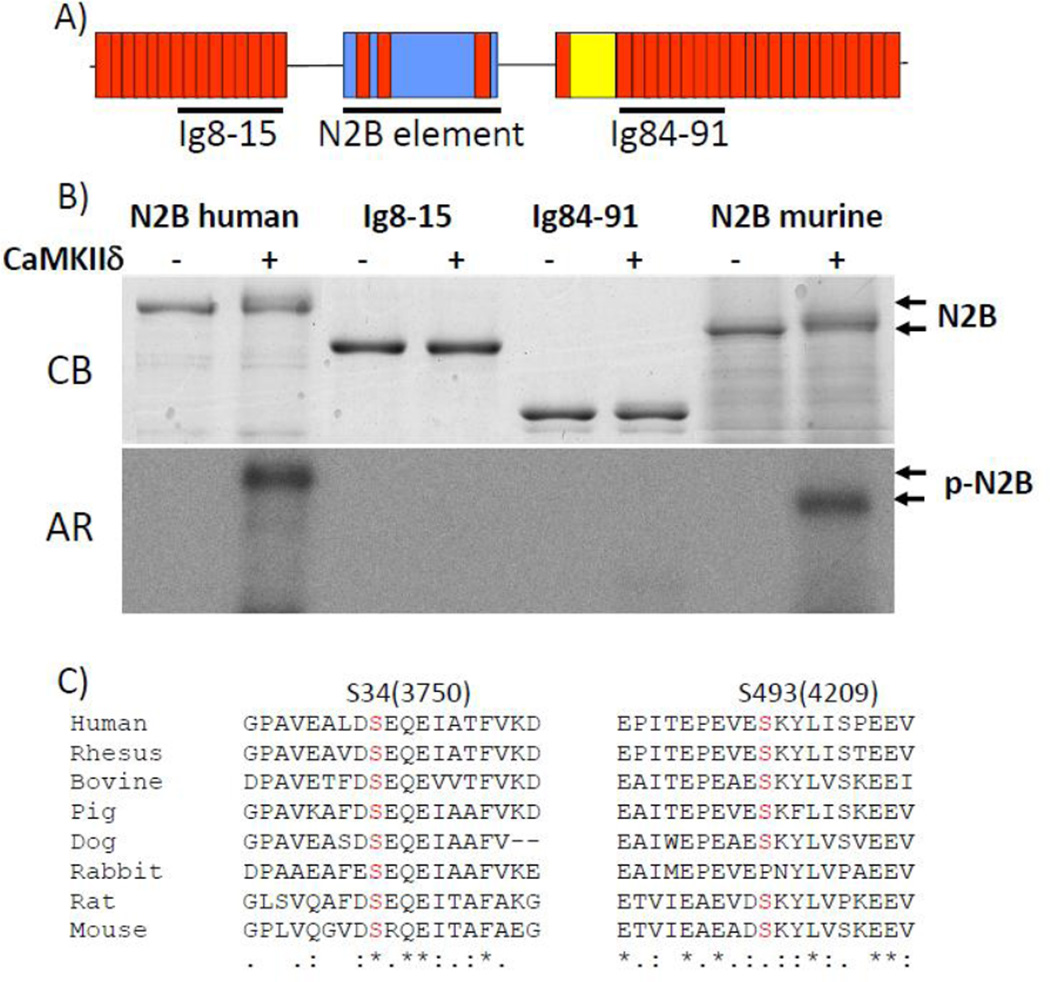
The finding that phosphorylation of titin takes place on T1 suggests that CaMKIIδ phosphorylates sites in the I-band region of the titin molecule. It was therefore tested whether titin’s spring elements are CaMKIIδ targets. In vitro phosphorylation assays were performed with recombinant proteins that comprise titin’s I-band segments: tandem immunoglobulin segments representing both the proximal and distal tandem Ig segment (Ig8-15 and Ig27-34), the N2B element (both the human and mouse versions), including its flanking Ig domains (Ig24, 25 and 26), and the human PEVK and its flanking Ig domains (see Fig. 2A and Fig. 3A). It was found that CaMKIIδ does not phosphorylate the tandem Ig segments (Fig. 2B), but that it does phosphorylate the N2B (Fig. 2B) and PEVK elements (Figs. 3 and 4).
Figure 2. The N2B element of cardiac titin is phosphorylated by CaMKIIδ.
A) Domain organization of cardiac titin’s I-band region (N2B isoform) and location of recombinant proteins used in kinase assay: Ig8-15, Ig84-91, and the N2B element (both the human and murine versions were used). B) Upper panel: Coomassie blue (CB) stained gel. Lower panel: corresponding autoradiograph (AR) of recombinant proteins incubated in kinase buffer (containing Ca2+/Calmodulin, and [γ-32P]ATP) without (−) and with CaMKIIδ (+). C) Conserved Serines that are phosphorylated by CaMKIIδ (numbering based on N2B unique sequence in human).
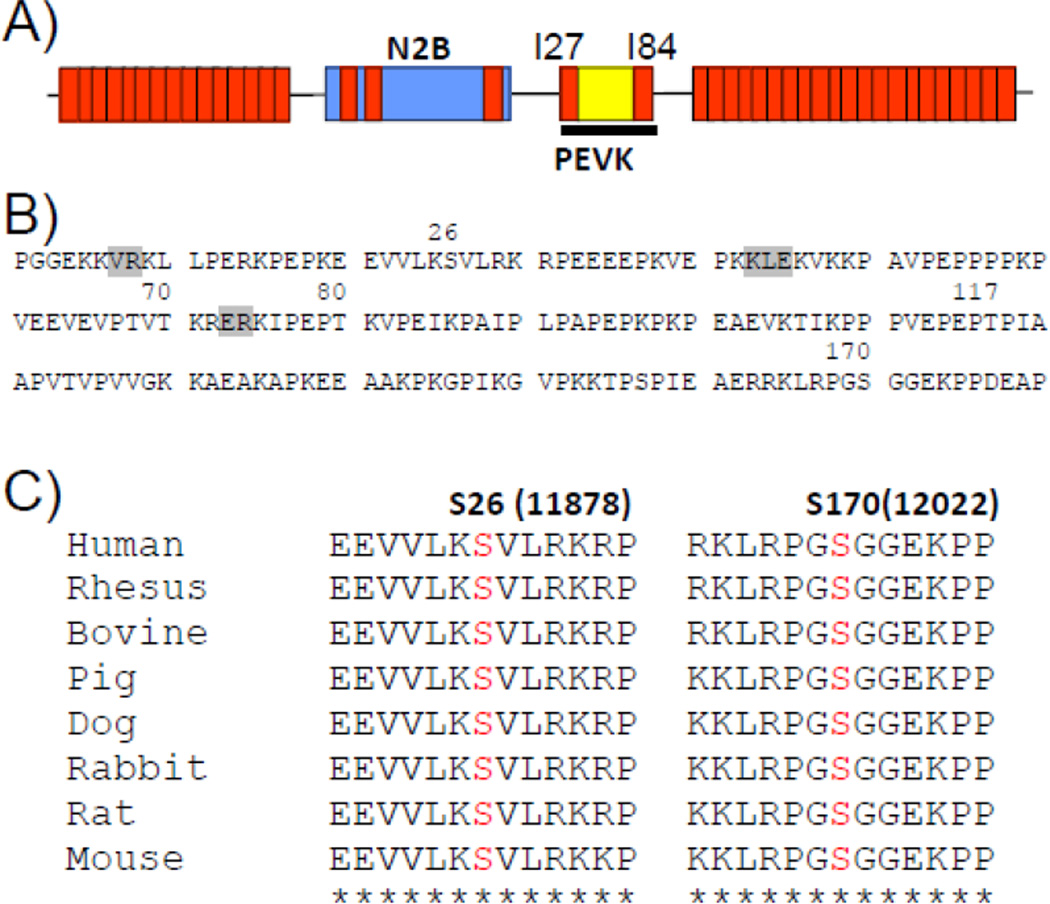
Figure 3. CaMKIIδ phosphorylates titin’s PEVK spring element.
A) Schematic diagram showing location of PEVK element in extensible region of cardiac N2B isoform. B) Amino acid sequence of PEVK element (human) with indicated CaMKIIδ phosphorylation sites identified by mass spectrometry. Five phosphorylation sites were identified (S26, T70, T80, T117, S170) of which S26 and S170 are highly conserved (C). (Residues shown in grey were not covered in mass spectrometry analysis.)
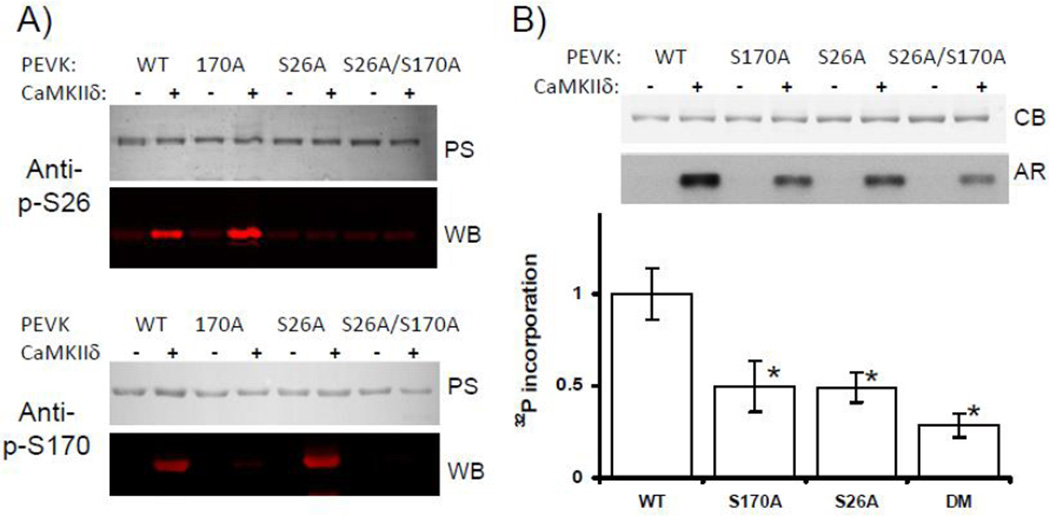
Figure 4. Evaluation of PEVK S26 and PEVK S170 as CaMKIIδ phosphorylation sites.
A) PEVK WT, and PEVK mutants S170A, S26A, and S170A/S26A were incubated with CaMKIIδ and the phosphorylation status of S26 and S170 evaluated in WBs with phospho-specific anti-p-S26 (top) and anti-p-S170 (bottom) antibodies[6]. Results show that both sites are phosphorylated by CaMKIIδ. B) Proteins were incubated in kinase buffer (containing Ca2+/Calmodulin, and [γ-32P]ATP) without (−) and with CaMKIIδ (+). Proteins were then electrophoresed, Coomassie blue stained (top), and exposed to autoradiographic film (bottom). Top: phosphorylation results in incorporation of 32P in all proteins, but the level varies and is highest in the WT protein. Bottom: Quantitative analysis of 32P incorporation (normalized to protein loading) shows that the single mutants incorporate only about half the WT amount. The phosphorylation level in the double mutant is ~75% less than in WT PEVK. See text for details. (N=5, * p<0.05 in ANOVA vs WT; PS: Ponceau S; WB: western blot; CB: Coomassie blue; AR: Autoradiograph.)
In order to identify the amino acid residues of the N2B element that are phosphorylated by CaMKIIδ, nano-LC tandem MS/MS was used. All residues in the N2Bunique sequence (except one, see Supplemental Figure 1, residue in grey) were covered in the digests and mass spectrometry detected 9 serine and threonine sites that had been phosphorylated by CaMKIIδ (Supplemental Figure 1). A sequence alignment using the N2B sequence of a wide range of species revealed that only two serine residues (S34 and S493 in human N2B unique sequence) are highly conserved (Fig. 2C).
An in vitro kinase assay was also performed on the PEVK element, using the 180 residue PEVK sequence of the human cardiac N2B isoform and its flanking Ig domains I27 and I84 (Fig. 3A). (Note that preliminary phosphorylation studies revealed that reproducible results devoid of spurious phosphorylation events are only obtained when the flanking Ig domains are present.) The recombinant protein was phosphorylated by CaMKIIδ and was then analyzed by nano-LC tandem MS/MS. Five sites were found to be phosphorylated (S26, T70, T80, T117, and S170, see Fig. 3B) of which only S26 and S170 are highly conserved (Fig. 3C). Of these sites S170 (LRPGSG) is a consensus sequence for CaMKIIδ (X-R-X-X-S/T-X)[12]. Intriguingly, both S26 and S170 have previously been found to be phosphorylated by PKCα[6], suggesting that both CaMKIIδ and PKCα can phosphorylate overlapping titin residues.
Using phospho-specific antibodies to the PEVK’s S26 and S170 sites we confirmed that these two sites are phosphorylated by CaMKIIδ in wildtype PEVK (Fig. 4A top and bottom, left two lanes) whereas mutation of serine to alanine abolished this effect (Fig. 4A, top and bottom). We also studied the phosphorylation level of the two sites in skinned muscle fibers and found that CaMKIIδ increased the level of S26 by 28±11.3% (n=4) and S170 by 31.9±12.3% (n=4), respectively.
Mass spectrometry on the PEVK had revealed that in addition to S26 and S170, CaMKIIδ also phosphorylates T70, T80 and T117. We addressed the importance of these additional sites in phosphorylation assays with [γ-32P]ATP and using PEVK WT and S26A, S170A and S26A/S170A PEVK mutants, reasoning that their importance would be revealed by 32P incorporation in the single and double mutants. Mutating S26 and S170 individually to alanine abolished ~50% of the 32P incorporation. Because only S170 is part of a CaMKIIδ consensus sequence, we anticipated that it might be a better substrate and would incorporate a larger amount of 32P than S26. However, the obtained results do not support this notion. Interestingly, mutating the S26 and S170 sites simultaneously abolished ~75% of the 32P incorporation (Fig. 4B). This suggests that some or all of T70, T80, and T117 residues are phosphorylated by CaMKIIδ and that mutating the two serines alters the likelihood that they become phosphorylated (see also Discussion). However, S26 and S170 are the dominant CaMKIIδ targets in the PEVK element.
In vivo CaMKIIδ phosphorylation
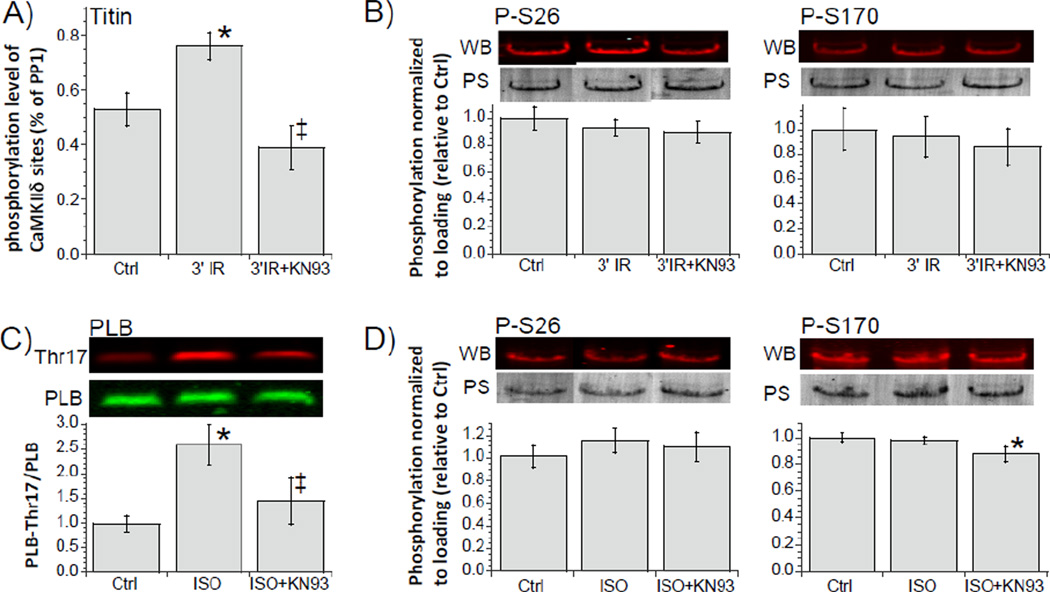
Whether CaMKIIδ phosphorylates titin in vivo was addressed in mouse isolated hearts that underwent an ischemia-reperfusion (IR) protocol, a condition that is wellknown to activate CaMKIIδ [12, 31, 36]. It has been shown in the mouse that in IR CaMKIIδ is highly activated within minutes of the start of reperfusion and that its activity peaks when reperfusing for ~3 min [31]. We used back-phosphorylation assays with CaMKIIδ on mouse isolated hearts and found 1) an increase in titin phosphorylation following 3 min of reperfusion and 2) that this increase was absent when the experiments were carried out in the presence of the CaMKII inhibitor KN-93 (Fig. 5A). A WB analysis with the PEVK phospho-S26 and phospho-S170 antibodies showed no change in the phosphorylation level of S26 and S170 (Fig. 5B, left and right). We also studied intact cardiac myocytes that were twitch activated and stimulated with isoproterenol (ISO), a condition that has been shown in rabbit cardiac myocytes to cause CaMKIIδ activation [32, 37]. Whether this also occurs in mouse myocytes was tested by using the phosphorylation status of phospholamban (PLB) Thr17, a well-known target of CaMKIIδ [30]. Indeed we found that ISO increased the phosphorylation level of Thr17 and that this could be inhibited by KN-93 (Fig. 5C). We then probed the phosphorylation level of titin’s PEVK S26 and S170 and found that these sites were unaffected by ISO (Fig. 5D, left and right). Thus the experiments show that CaMKIIδ sites on titin are accessible in vivo but they do not support that S26 and S170 of the PEVK element are involved.
Figure 5. Phosphorylation of titin in intact hearts (A and B) and intact cardiac myocytes (C and D).
A) Isolated hearts underwent a 20 min period of global ischemia followed by reperfusion for 3 min in absence and presence of the CaMKII inhibitor KN93. Left: back phosphorylation assays with CaMKIIδ show an increase in titin phosphorylation of the IR hearts and that this increase was inhibited by KN93. B) left and right: IR hearts analyzed with WB and p-S26 (left) and p-S170 (right) antibodies. C) Mouse intact cardiac myocytes were electrically paced at 4Hz in absence or presence of isoproterenol. p-Thr17 of PLB was increased in ISO treated cells and this effect was inhibited by KN93. D) left and right: pS26 (left) and pS170 (right) levels were not altered by ISO. (N varied between 5 and 8; *p<0.05 (ANOVA) vs. ctrl; ‡ p<0.05 (ANOVA) vs. ISO; PS: Ponceau S; WB: western blot; PLB antibody against unphosphorylated phospholamban that was used for loading control (See Methods).)
Discussion
By phosphorylating a wide range of calcium handling and myofilament proteins CaMKIIδ regulates systolic and diastolic function and plays important roles in the physiology and pathology of the heart[12, 38, 39]. Using phosphorylation assays and mass spectrometry the present study investigated whether CaMKIIδ phosphorylates also titin and we focused on titin’s PEVK and the N2B elements, two important spring elements within titin’s extensible I-band region. In both skinned fibers and in intact hearts CaMKIIδ phosphorylates titin and about half of the CaMKIIδ accessible sites are in their phosphorylated state under normal conditions. In vitro kinase assays showed that N2B and PEVK elements are both phosphorylated by CaMKIIδ, including at several wellconserved serine residues. Below we discuss these results in detail.
N2B element phosphorylation
Back-phosphorylation assays on skinned fibers showed that cMyBP-C and titin are strong CaMKIIδ substrates among the myofilament proteins, with titin 32P incorporation on T1 (full length titin molecule) but not T2 (A-band region of titin). This preferential phosphorylation behavior of titin suggests that the phosphorylation sites are in the Iband region of the sarcomere, which is consistent with previous work that has shown that titin’s spring elements are ‘phosphorylation hotspots’[6, 9, 11, 40]. The computer program GPS 2.1.2 of kinase specific phosphorylation sites [41] predicts multiple CaMKII phospho-sites in the N2B elements (results not shown) with S34 as a high confidence target. We therefore performed in vitro CaMKIIδ phosphorylation experiments on the N2B element. This revealed 9 phospho-sites in the N2B element including S34. None of these phospho-sites overlap with the N2B sites that are known to be targeted by other kinases (PKA/PKG: S469[11] and ERK2: S294, S244 and S202). Of the CaMKIIδ sites, only S34 and S493 are highly conserved (Fig. 2C) suggesting that these two sites might perform important functions. Currently we can only speculate what these functions might be. The N2B element is known to interact with the small heat shock protein αBcrystallin[42, 43] and with four-and-a-half LIM protein, FHL[44] and CaMKIIδ phosphorylation of the N2B element might regulate protein-protein interactions. It is also worth considering that phosphorylation of the N2B element by CaMKIIδ alters passive tension, analogous to the effect of PKA/PKG phosphorylation of the N2B element. The N2B element is a cardiac specific spring element that dominates the elasticity of titin at intermediate to long sarcomere lengths[45]. Extension of the N2B element follows wormlike-chain entropic behavior and is characterized by a persistence length (a measure of the bending rigidity) of ~0.65 nm[25, 46]. PKA/PKG phosphorylation induces structural transitions in the N2B element that increase the persistence length and thereby lower passive tension[47]. Regulation of passive tension through PKA/PKG phosphorylation is clinically important as hypo-phosphorylation of the PKA/PKG site contributes to elevated passive tension in HFpEF patients[1]. In summary, multiple residues in the N2B element are phosphorylated including two highly conserved sites (S34 might be most important). We speculate that CaMKIIδ phosphorylation triggers local structural transitions that, analogous to the changes that follow PKA/PKG phosphorylation, lower passive tension.
PEVK element phosphorylation
In vitro kinase assays, mass spectrometry, and skinned fiber studies also revealed that CaMKIIδ targets the PEVK region of titin and CaMKIIδ is the first known kinase that phosphorylates more than one spring element. Mass spectrometry revealed 5 PEVK sites, distributed more or less evenly along the PEVK sequence (Fig. 3B). Of these identified sites only S26 and S170 are predicted by ‘GPS 2.1.2 phosphosite [41]’ to be high confidence CaMKII sites (S170 is a consensus sequence for CaMKIIδ (X-R-X-X-S/TX)[12]) and both sites are highly conserved in a wide range of species (Fig. 3C). Site directed mutagenesis showed that individually mutating these serines to alanines abolished ~50% of WT 32P incorporation (Fig. 4B). Surprisingly, mutating both serines simultaneously did not fully abolish 32P incorporation, but resulted in ~25% 32P incorporation that remained. A possible explanation is provided by the previous work that showed that mutating S26 and S170 to alanines causes structural changes in the PEVK[48] and such changes might permit phosphorylation of one or more of the three threonines that mass spectrometry analysis had uncovered (T70, T80 and T177). Regardless, the phosphorylation assays on mutant PEVK showed that S26 and S170 are the most important CaMKIIδ sites. Interestingly, S26 and S170 were also previously shown to be phosphorylated by PKCα [6]. [Note that others have also found that the same amino acid can be phosphorylated by several kinases, for example S368 of the neuronal nicotinic receptor alpha4 subunits is phosphorylated by both PKA and PKC[49].] This interesting finding suggests that there might be crosstalk between CaMKIIδ and PKCα signaling on titin and warrants follow-up work, especially in heart failure conditions where both kinases display deranged signaling[4, 5, 38].
The PEVK structure is not well-defined, but the existence of structural motifs has been proposed[50]. Although the high proline concentration is likely to preclude the formation of sizeable α-helices and β-sheet structures, NMR and circular dichroism (CD) experiments suggest that polyproline helix-coil motifs are structural features of the PEVK[50]. The fact that the PEVK sequence, especially around S26 and S170, is highly conserved in a wide variety of species, also suggests that the PEVK is locally structured. Single molecule force spectroscopy has shown that prior to phosphorylation, the WT PEVK has a persistence length of ~1 nm and that phosphorylation reduces this value to ~ 0.5 nm[6, 48]. Serine-to-alanine mutations also significantly reduce persistence length in both single mutants and the double mutant[48], suggesting that both S26 and S170 are important in maintaining structural integrity. It has been proposed that the S26A and S170A mutations disrupt the stabilizing interactions involving the native serine residues, which increases the conformational entropy of the PEVK and results in a lower effective persistence length[48]. A reduction in persistence length is predicted to increase passive tension, and cellular and tissue studies on wildtype and PEVK deficient mice have indeed provided evidence that PKCα phosphorylation of S26 and S170 increases passive tension[6, 8]. The same effect is expected when the sites are phosphorylated by CaMKIIδ. In summary, the PEVK element is phosphorylated by CaMKIIδ, S26 and S170 are the main targets, and the effect of phosphorylation is likely to be an increase in passive tension.
In vivo titin phosphorylation
Effects seen in vitro with purified proteins or with skinned fibers to which exogenous kinases are added not necessarily translate to in vivo conditions where, for example, protein-protein interactions can mask phosphorylation sites or where proximity between kinase and substrate can be a limiting factor. Hence we studied intact mouse hearts that underwent an ischemia reperfusion (IR) insult and intact cells that were stimulated with isoproterenol (ISO). Both conditions are known to activate CaMKIIδ [31, 32, 36, 37], and this was confirmed in our study by the increased phosphorylation level of the CaMKIIδ site on PLB, Thr17. Titin phosphorylation was also increased, with the increase likely due to CaMKIIδ as the effect was abolished by the CaMKIIδ inhibitor KN-93 (Fig. 5A). Considering that no change was found in the phosphorylation levels of the PEVK S26 and S170 the in vivo increase in titin phosphorylation might be due to phosphorylation of the N2B element. Possible explanations for preferential phosphorylation are differences in substrate affinity and in distance between the phosphorylation sites on titin and CaMKIIδ. CaMKII is highly enriched along the Z-bands[12] and from previous immunoelectron microscopy studies[51] it can be estimated that within the physiological sarcomere length range of the heart (in the mouse ~1.8–2.2 um[52]) the C-terminus of the PEVK (where S170 is found) is ~150 nm away from the edge of the Z-disk[51]. This does not mean that the PEVK will never become phosphorylated as there is a low amount of cytosolic CaMKIIδ [53]and when activated chronically, this might phosphorylate the PEVK. On the other hand, the N-terminus of the N2B element (where S34 is found) is on average 100 nm closer to the Z-band than the PEVK[51]. It seems therefore reasonable to propose that due to its close proximity to the N-terminal end of titin’s spring region, CaMKIIδ preferentially phosphorylates the N2B element. It is interesting to note that one of the functions attributed to CaMKIIδ is the frequency dependent acceleration of relaxation (FDAR)[12, 54], by phosphorylating PLB and promoting Ca2+ reuptake from the cytosol by SERCA, maintaining thereby efficient ventricular filling at high heart rates. Preferential phosphorylation of the N2B element by CaMKIIδ and an ensuing decrease in titin-based passive tension would further augment ventricular filling. Thus it is possible that phosphorylation of PLB and titin, serves overlapping functions in maintaining diastolic function at high heart rates.
In summary, our work revealed the novel finding that CaMKIIδ phosphorylates titin, both in vitro and in vivo. Phosphorylation takes place on titin’s spring elements, suggesting a role for CaMKIIδ in passive tension modulation. Whether deranged expression and activity of CaMKIIδ in patients with structural heart disease and arrhythmias[38] contributes to titin-based diastolic dysfunction is an area that should be addressed next.
Supplementary Material
N2B unique sequence (human) with indicated CaMKIIδ phosphorylation sites identified by mass spectrometry. (Residues shown in grey were not covered in analysis.)
Acknowledgments
We thank past and present members of the Granzier laboratory for their help, the support by NIH (HL068821) and the generous support by the Allan and Alfie Norville Endowed Chair. George Tsaprailis acknowledges support by the Arizona Proteomics Consortium HEI grant (S10 RR028868-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 2.LeWinter MM, Wu Y, Labeit S, Granzier H. Cardiac titin: structure, functions and role in disease. Clin Chim Acta. 2007;375:1–9. doi: 10.1016/j.cca.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Freiburg A, Trombitas K, Hell W, Cazorla O, Fougerousse F, Centner T, et al. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res. 2000;86:1114–1121. doi: 10.1161/01.res.86.11.1114. [DOI] [PubMed] [Google Scholar]
- 4.Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 5.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, et al. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 6.Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, et al. PKC phosphorylation of titin's PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105:631–638. doi: 10.1161/CIRCRESAHA.109.198465. 17 p following 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson BR, Bogomolovas J, Labeit S, Granzier H. The Effects of PKCalpha Phosphorylation on the Extensibility of Titin's PEVK Element. J Struct Biol. 2010 doi: 10.1016/j.jsb.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson BD, Hidalgo CG, Gotthardt M, Granzier HL. Excision of titin's cardiac PEVK spring element abolishes PKCalpha-induced increases in myocardial stiffness. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90:1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol. 2005;125:257–271. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, et al. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 12.Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology. 2008;23:151–159. doi: 10.1152/physiol.00043.2007. [DOI] [PubMed] [Google Scholar]
- 13.Hagemann D, Kuschel M, Kuramochi T, Zhu W, Cheng H, Xiao RP. Frequencyencoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J Biol Chem. 2000;275:22532–22536. doi: 10.1074/jbc.C000253200. [DOI] [PubMed] [Google Scholar]
- 14.Mundina-Weilenmann C, Vittone L, Ortale M, de Cingolani GC, Mattiazzi A. Immunodetection of phosphorylation sites gives new insights into the mechanisms underlying phospholamban phosphorylation in the intact heart. J Biol Chem. 1996;271:33561–33567. doi: 10.1074/jbc.271.52.33561. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Colbran RJ, Anderson ME. Calmodulin kinase is a molecular switch for cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2001;98:2877–2881. doi: 10.1073/pnas.051449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 17.Ferrero P, Said M, Sanchez G, Vittone L, Valverde C, Donoso P, et al. Ca2+/calmodulin kinase II increases ryanodine binding and Ca2+-induced sarcoplasmic reticulum Ca2+ release kinetics during beta-adrenergic stimulation. J Mol Cell Cardiol. 2007;43:281–291. doi: 10.1016/j.yjmcc.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 19.Jaquet K, Fukunaga K, Miyamoto E, Meyer HE. A site phosphorylated in bovine cardiac troponin T by cardiac CaM kinase II. Biochim Biophys Acta. 1995;1248:193–195. doi: 10.1016/0167-4838(95)00028-s. [DOI] [PubMed] [Google Scholar]
- 20.Hartzell HC, Glass DB. Phosphorylation of purified cardiac muscle C-protein by purified cAMP-dependent and endogenous Ca2+-calmodulin-dependent protein kinases. J Biol Chem. 1984;259:15587–15596. [PubMed] [Google Scholar]
- 21.Vittone L, Mundina-Weilenmann C, Mattiazzi A. Phospholamban phosphorylation by CaMKII under pathophysiological conditions. Front Biosci. 2008;13:5988–6005. doi: 10.2741/3131. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo CG, Methawasin M, Chung C, Bogomolavas J, Gasch A, Labeit S, Granzier H. The Multifunctional Calcium/Calmodulin-Dependent Protein Kinase II Delta (CaMKIId) phosphorylates Titin N2B and PEVK Spring Elements. Biophysical Journal. 2012;102:559a. [Google Scholar]
- 23.Wu Y, Labeit S, Lewinter MM, Granzier H. Titin: an endosarcomeric protein that modulates myocardial stiffness in DCM. J Card Fail. 2002;8:S276–S286. doi: 10.1054/jcaf.2002.129278. [DOI] [PubMed] [Google Scholar]
- 24.Hudson B, Hidalgo C, Saripalli C, Granzier H. Hyperphosphorylation of mouse cardiac titin contributes to transverse aortic constriction-induced diastolic dysfunction. Circ Res. 2011;109:858–866. doi: 10.1161/CIRCRESAHA.111.246819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe K, Nair P, Labeit D, Kellermayer MS, Greaser M, Labeit S, et al. Molecular mechanics of cardiac titin's PEVK and N2B spring elements. J Biol Chem. 2002;277:11549–11558. doi: 10.1074/jbc.M200356200. [DOI] [PubMed] [Google Scholar]
- 26.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods in molecular biology. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 27.Granzier HL, Radke MH, Peng J, Westermann D, Nelson OL, Rost K, et al. Truncation of titin's elastic PEVK region leads to cardiomyopathy with diastolic dysfunction. Circ Res. 2009;105:557–564. doi: 10.1161/CIRCRESAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren CM, Jordan MC, Roos KP, Krzesinski PR, Greaser ML. Titin isoform expression in normal and hypertensive myocardium. Cardiovasc Res. 2003;59:86–94. doi: 10.1016/s0008-6363(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 29.Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004;94:505–513. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- 30.Koss KL, Kranias EG. Phospholamban: a prominent regulator of myocardial contractility. Circ Res. 1996;79:1059–1063. doi: 10.1161/01.res.79.6.1059. [DOI] [PubMed] [Google Scholar]
- 31.Valverde CA, Mundina-Weilenmann C, Reyes M, Kranias EG, Escobar AL, Mattiazzi A. Phospholamban phosphorylation sites enhance the recovery of intracellular Ca2+ after perfusion arrest in isolated, perfused mouse heart. Cardiovasc Res. 2006;70:335–345. doi: 10.1016/j.cardiores.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Erickson JR, Patel R, Ferguson A, Bossuyt J, Bers DM. Fluorescence resonance energy transfer-based sensor Camui provides new insight into mechanisms of calcium/calmodulin-dependent protein kinase II activation in intact cardiomyocytes. Circ Res. 2011;109:729–738. doi: 10.1161/CIRCRESAHA.111.247148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung CS, Bogomolovas J, Gasch A, Hidalgo CG, Labeit S, Granzier HL. Titin-Actin Interaction: PEVK-Actin-Based Viscosity in a Large Animal. J Biomed Biotechnol. 2011;2011:310791. doi: 10.1155/2011/310791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schworer CM, Colbran RJ, Soderling TR. Reversible generation of a Ca2+- independent form of Ca2+(calmodulin)-dependent protein kinase II by an autophosphorylation mechanism. J Biol Chem. 1986;261:8581–8584. [PubMed] [Google Scholar]
- 35.Granzier H, Helmes M, Trombitas K. Nonuniform elasticity of titin in cardiac myocytes: a study using immunoelectron microscopy and cellular mechanics. Biophys J. 1996;70:430–442. doi: 10.1016/S0006-3495(96)79586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vittone L, Mundina-Weilenmann C, Said M, Ferrero P, Mattiazzi A. Time course and mechanisms of phosphorylation of phospholamban residues in ischemia-reperfused rat hearts. Dissociation of phospholamban phosphorylation pathways. J Mol Cell Cardiol. 2002;34:39–50. doi: 10.1006/jmcc.2001.1488. [DOI] [PubMed] [Google Scholar]
- 37.Valverde CA, Mundina-Weilenmann C, Said M, Ferrero P, Vittone L, Salas M, et al. Frequency-dependent acceleration of relaxation in mammalian heart: a property not relying on phospholamban and SERCA2a phosphorylation. J Physiol. 2005;562:801–813. doi: 10.1113/jphysiol.2004.075432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattiazzi A, Kranias EG. CaMKII regulation of phospholamban and SR Ca2+ load. Heart Rhythm. 2011;8:784–787. doi: 10.1016/j.hrthm.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raskin A, Lange S, Banares K, Lyon RC, Zieseniss A, Lee LK, et al. A novel mechanism involving four and a half lim domain protein-1 and extracellular-signal-regulated kinase-2 regulates titin phosphorylation and mechanics. J Biol Chem. 2012 doi: 10.1074/jbc.M112.372839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue Y, Ren J, Gao X, Jin C, Wen L, Yao X. GPS 2.0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Mol Cell Proteomics. 2008;7:1598–1608. doi: 10.1074/mcp.M700574-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bullard B, Ferguson C, Minajeva A, Leake MC, Gautel M, Labeit D, et al. Association of the chaperone alphaB-crystallin with titin in heart muscle. J Biol Chem. 2004;279:7917–7924. doi: 10.1074/jbc.M307473200. [DOI] [PubMed] [Google Scholar]
- 43.Golenhofen N, Arbeiter A, Koob R, Drenckhahn D. Ischemia-induced association of the stress protein alpha B-crystallin with I-band portion of cardiac titin. J Mol Cell Cardiol. 2002;34:309–319. doi: 10.1006/jmcc.2001.1513. [DOI] [PubMed] [Google Scholar]
- 44.Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA, Zheng M, et al. An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest. 2008;118:3870–3880. doi: 10.1172/JCI34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trombitas K, Freiburg A, Centner T, Labeit S, Granzier H. Molecular dissection of N2B cardiac titin's extensibility. Biophys J. 1999;77:3189–3196. doi: 10.1016/S0006-3495(99)77149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Linke WA, Oberhauser AF, Carrion-Vazquez M, Kerkvliet JG, Lu H, et al. Reverse engineering of the giant muscle protein titin. Nature. 2002;418:998–1002. doi: 10.1038/nature00938. [DOI] [PubMed] [Google Scholar]
- 47.Kruger M, Linke WA. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil. 2006;27:435–444. doi: 10.1007/s10974-006-9090-5. [DOI] [PubMed] [Google Scholar]
- 48.Anderson BR, Bogomolovas J, Labeit S, Granzier H. The effects of PKCalpha phosphorylation on the extensibility of titin's PEVK element. J Struct Biol. 2010;170:270–277. doi: 10.1016/j.jsb.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wecker L, Guo X, Rycerz AM, Edwards SC. Cyclic AMP-dependent protein kinase (PKA) and protein kinase C phosphorylate sites in the amino acid sequence corresponding to the M3/M4 cytoplasmic domain of alpha4 neuronal nicotinic receptor subunits. J Neurochem. 2001;76:711–720. doi: 10.1046/j.1471-4159.2001.00041.x. [DOI] [PubMed] [Google Scholar]
- 50.Ma K, Kan L, Wang K. Polyproline II helix is a key structural motif of the elastic PEVK segment of titin. Biochemistry. 2001;40:3427–3438. doi: 10.1021/bi0022792. [DOI] [PubMed] [Google Scholar]
- 51.Trombitas K, Redkar A, Centner T, Wu Y, Labeit S, Granzier H. Extensibility of isoforms of cardiac titin: variation in contour length of molecular subsegments provides a basis for cellular passive stiffness diversity. Biophys J. 2000;79:3226–3234. doi: 10.1016/S0006-3495(00)76555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung C, Granzier H. Dissection of determinants of passive pressure and measurement of sarcomere length in the mouse left ventricle. Journal of Molecular and Cellular Cardiology. 2011;50:731–739. doi: 10.1016/j.yjmcc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac L-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am J Physiol. 1999;276:H2168–H2178. doi: 10.1152/ajpheart.1999.276.6.H2168. [DOI] [PubMed] [Google Scholar]
- 54.Wu Y, Luczak ED, Lee EJ, Hidalgo C, Yang J, Gao Z, et al. CaMKII effects on inotropic but not lusitropic force frequency responses require phospholamban. J Mol Cell Cardiol. 2012;53:429–436. doi: 10.1016/j.yjmcc.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
N2B unique sequence (human) with indicated CaMKIIδ phosphorylation sites identified by mass spectrometry. (Residues shown in grey were not covered in analysis.)