Abstract
Aerosol deposition plays an important role in climate and biogeochemical cycles by supplying nutrients to the open ocean, in turn stimulating ocean productivity and carbon sequestration. Aerosol particles also contain elements such as copper (Cu) that are essential in trace amounts for phytoplankton physiology but that can be toxic at high concentrations. Although the toxicity of Cu associated with aerosols has been demonstrated in bioassay experiments, extrapolation of these laboratory results to natural conditions is not straightforward. This study provides observational evidence of the negative effect of aerosols containing high Cu concentrations on marine phytoplankton over a vast region of the western Mediterranean Sea. Direct aerosol measurements were combined with satellite observations, resulting in the detection of significant declines in phytoplankton biomass after atmospheric aerosol events characterized by high Cu concentrations. The declines were more evident during summer, when nanoflagellates predominate in the phytoplankton population and stratification and oligotrophic conditions prevail in the study region. Together with previous findings concerning atmospheric Cu deposition, these results demonstrate that the toxicity of Cu-rich aerosols can involve large areas of the world’s oceans. Moreover, they highlight the present vulnerability of oceanic ecosystems to Cu-rich aerosols of anthropogenic origins. Because anthropogenic emissions are increasing, large-scale negative effects on marine ecosystems can be anticipated.
Keywords: atmospheric dust, trace metal
Biological productivity in most of the world’s oceans is limited by the availability of light and nutrients. In the euphotic zone at the ocean surface, phytoplankton growth is thus controlled by the supply of nutrients. Processes governing upwelling and vertical mixing in the ocean can enhance vertical fluxes of nutrient-rich deep water into the euphotic zone. Nutrients may also be of continental origin, fertilizing the ocean through riverine, groundwater, or atmospheric pathways. For example, nitrogen (N) aerosol deposition fluxes in the North Atlantic subtropical were shown to enhance phytoplankton growth and biological production (1, 2). In ocean areas where biological production is mainly inhibited by iron (Fe) deficiency (3), the atmospheric deposition of Fe plays a central role in increasing plankton productivity (4, 5). In addition to N and Fe, a recent study has demonstrated that aerosol deposition also contributes other bioavailable metals such as cobalt, manganese, and nickel (6).
Aerosol particles consist of natural and anthropogenic components that can exert important effects when deposited in the sea. Although the partial dissolution of the deposited particles increases the input of certain elements required for phytoplankton growth, an excess of these elements can be harmful to the extent that they negatively impact ecosystem health (7, 8). For example, Cu is highly toxic even at relatively low concentrations (9), and its toxic effects can be exerted following its release from aerosol particles. In fact, bioassay experiments have demonstrated that the addition of aerosols containing high copper concentrations to ocean water samples can have detrimental effects on phytoplankton communities (10). Nonetheless, the bulk oceanic response to Cu aerosol deposition has only been assessed based on a coupled atmosphere–ocean model (10). This lack of direct evidence is in itself a cause for major concern because human activities have increased the amount of atmospheric aerosols and specifically those carrying Cu and other metals (11, 12). The major input of anthropogenic Cu in the atmosphere is the metal industry, but fugitive emissions from urban agglomerations (e.g., due to the abrasion products of brake pads in vehicles) contribute significantly as well (13).
Results and Discussion
The western Mediterranean Sea (Fig. 1) receives some of the highest fluxes of atmospheric aerosols from natural and anthropogenic sources (14, 15). The Sahara desert in Africa is the major source of natural aerosols to the Mediterranean and the main external source of nutrients for the sea’s surface waters during summer (14). Conversely, anthropogenic aerosol components are predominantly transported from Europe (16). Representative data regarding regional aerosols in the western Mediterranean Sea (17), obtained from Montseny station (northwestern Mediterranean; Fig. 1), show episodic pulse-like events of aerosol components (Fig. S5) as result of the contrasting atmospheric inputs from natural and anthropogenic origins.
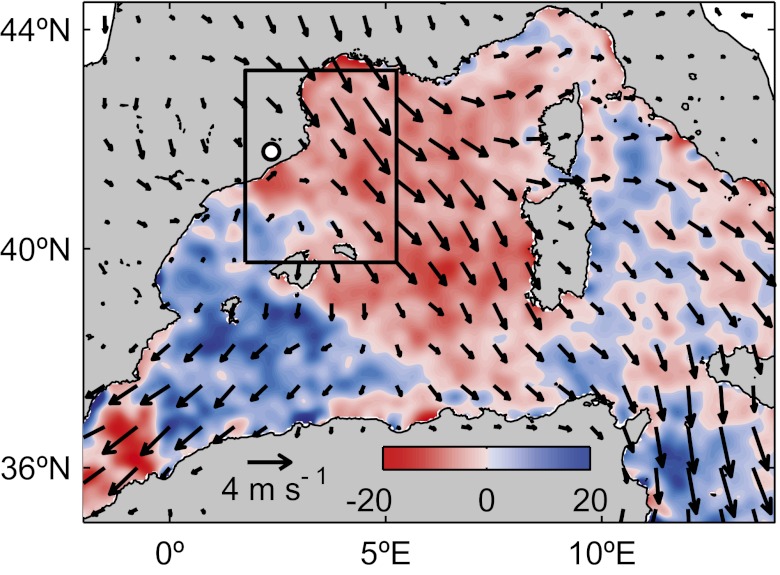
Fig. 1.
Median effect of Cu aerosol pulses on satellite daily chlorophyll variation (ΔChl) in the western Mediterranean Sea. Color scale represents the calculated median ΔChl (%) during Cu aerosol pulses. The arrows are the calculated median surface wind speed (m s−1) and direction during the pulses. The white dot indicates the location of Montseny station. The rectangle delimited by a back line is the 3.5 × 3.5° box area in which ΔChl was averaged.
To evaluate the toxic effect of Cu on marine phytoplankton, we focused on Cu aerosol pulses, herein defined as Cu events in which the Cu concentration is higher than the mean concentration plus the SD. Merged satellite chlorophyll (Chl) data were used as an indicator of total phytoplankton biomass (18). Daily Chl variations (ΔChl) were calculated during Cu pulses, as the toxic response of phytoplankton to Cu was previously shown to be evident already on the first day of a Cu event (9, 10, 19). As seen in Fig. 1, median phytoplankton declines were observed for 57% of the sea grid points in the western Mediterranean Sea. The main cluster of declining grid points was located near Montseny station, in the track established by the northerly winds (Tramontane). The predominance of these winds during Cu pulses favors the transport of aerosols from Europe, suggesting that anthropogenic Cu aerosols are of major relevance in the dynamics of open-ocean phytoplankton in the western Mediterranean (Fig. 1).
We also analyzed the bulk response of phytoplankton biomass in the western Mediterranean Sea to each Cu pulse. The ΔChl during each Cu pulse was averaged over a 3.5 × 3.5° box area adjacent to Montseny station (Fig. 1) to reduce the effect on phytoplankton of other processes with shorter spatial scales. Aerosol transport and deposition during major pulses in the Mediterranean usually have spatial scales larger than that of the selected box area (16). We note that large-scale processes such as storms can also influence phytoplankton dynamics. However, there is no significant relationship between Cu aerosol pulses and wind speed (SI Text). Fig. 2 presents the relationship between each Cu pulse and the corresponding ΔChl value in the box area. This observed relationship yields a linear correlation coefficient of r = –0.637 (P = 0.001), confirming the negative effect of aerosols with high Cu concentrations on marine phytoplankton.
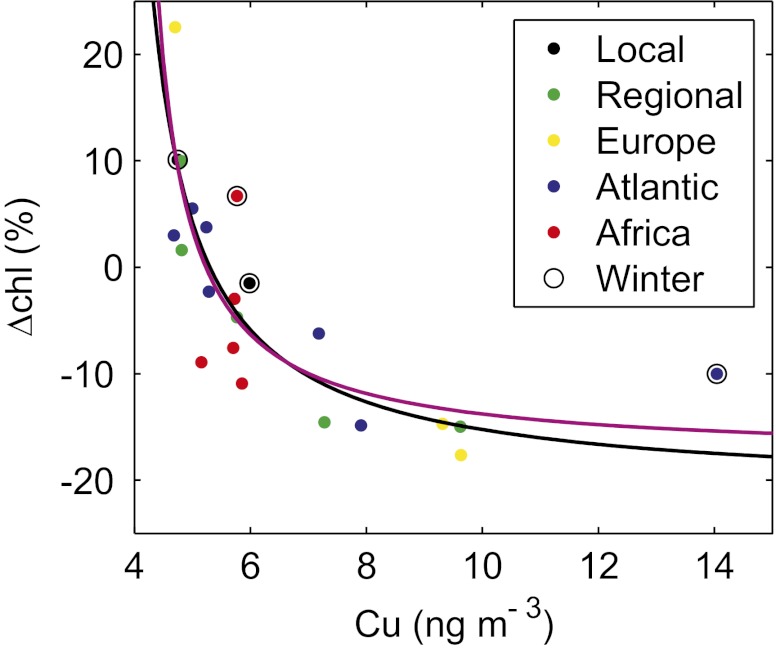
Fig. 2.
Observed effect of Cu aerosol pulses on satellite daily chlorophyll variation (ΔChl) averaged in the 3.5 × 3.5° box area (Fig. 1). The dots represent the spatially averaged ΔChl (%) for each Cu pulse, the various colors indicate the aerosol sources, and the open circles are the Cu pulses during winter (December to March). The lines are the regression of the observed values with (magenta line) and without (black line) African aerosols to Eq. 5 using a nonlinear least-squares method.
However, it was not clear that the toxic effect of Cu on phytoplankton is linear. To analyze this effect, we used a simple equation describing phytoplankton population dynamics, in which the growth of these microorganisms is balanced by losses due to mortality or grazing as follows:
 |
where P denotes the phytoplankton population density, t is time, and µ and m are the specific growth and loss rates of the phytoplankton, respectively. It was assumed that the phytoplankton population over a vast area of the ocean is in equilibrium at t = 0, before the addition of Cu through aerosol deposition, such that µ(t = 0) = µ0 ∼ m and P(t = 0) = P0.
The availability of Cu in the ocean depends on the fractional solubility of the aerosol Cu deposited in the surface waters, which in turn strongly varies according to the source-dependent composition of the aerosol particles (20). The fractional solubility of Cu for African aerosols ranges from 1% to 7%, whereas anthropogenic aerosols have higher values of 10–100% (20). Although Cu pulses in this region are of both African and European origin, most of them are anthropogenically (local, regional, European, or Atlantic) derived (Fig. 2). African aerosol events in the western Mediterranean Sea are often associated with clouds in the lower atmosphere, which negatively affect the quality of our satellite Chl data. The effects of the five Cu pulses from Africa in which good-quality Chl data were available did not differ from the effects of Cu aerosols originating from other sources (Fig. 2). However, taking into account the different solubility, the Cu concentration in the sea for African aerosols would be much lower than that for anthropogenic aerosols. Furthermore, the observed ΔChl for these African aerosols could be caused by an artifact due to residual dust in the atmosphere (SI Text). We thus used the aerosol Cu concentration as a proxy of the Cu concentration in the sea and the calculations were performed twice, including and excluding the African aerosols.
In addition, speciation of the Cu added to the water column is important because only certain forms of Cu (mostly free Cu ions) are biologically available (21). Therefore, the negative effect on phytoplankton does not depend on the total Cu concentration but rather on the concentrations of the biologically available forms (7, 22). The latter are controlled primarily by Cu-binding organic ligands, which include phytoplankton cell exudates and dissolved organic matter (22, 23). Consequently, the speciation of Cu is not straightforward. In the ocean, correlations between ligands and primary productivity have been reported (24), although the ligands become Cu-saturated when the toxic effects of Cu appear (25, 26). Accordingly, we assumed that biologically available forms of Cu are linearly related to the total Cu concentration when Cu becomes toxic, after surpassing a given Cu threshold.
Phytoplankton regulates the uptake rate of nutrient metals to maintain their intracellular concentrations at the levels needed for growth and metabolism. As phytoplankton grow, their metal uptake increases to maintain cellular metal concentrations. The metal uptake rate (V) is related to the external metal concentration ([M]) by the saturation kinetics equation (22) as follows:
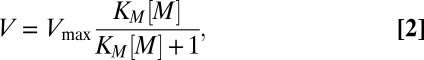 |
where Vmax is the maximum rate and KM is the half-saturation constant. However, phytoplankton cells do not limit their uptake of metals to those used as nutrients but also accumulate nonnutritive and even toxic metals, such as Cu. In this case, increasing cellular concentrations of toxic Cu progressively inhibit algal photosynthesis by altering electron transport and by inactivating a fraction of the PSII reaction centers (27–29). In other words, Cu does not generally cause mortality but instead reduces the phytoplankton growth rate. Accordingly, the effect of toxic Cu can be modeled by decreasing the specific growth rate as metal uptake increases as a function of the added Cu concentration ([Cu]) when the latter becomes higher than the Cu threshold ([Cu]lim) as follows:
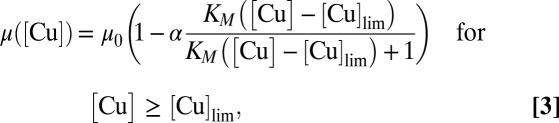 |
where α relates metal uptake to the decrease in growth rate. After the addition of Cu, the phytoplankton population is thus described by the following:
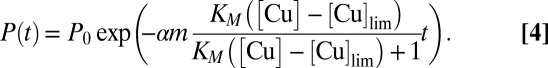 |
Assuming a linear relationship between phytoplankton and Chl, the ΔChl during Cu pulses is as follows:
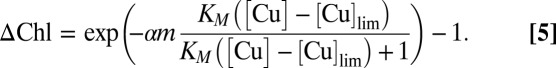 |
Regression analysis identified the close agreement between Eq. 5 and the data displayed in Fig. 2 (r2 = 0.701 for calculation with African aerosols and r2 = 0.810 without African aerosols, P < 0.001 in both cases), indicating the toxic effect of Cu aerosols on phytoplankton. The obtained values (SI Text) show that the negative effect of Cu on the phytoplankton growth rate is notable at metal concentrations above [Cu]lim = 5.23 ng m−3, corresponding to a dry deposition flux of 9.04 μg Cu m−2 d−1.
However, this value has to be considered as an approximate threshold because the sensitivity to Cu toxicity varies among phytoplankton species (28). For example, diatoms are usually more tolerant than dinoflagellates to elevated Cu concentrations (19, 30, 31). In the western Mediterranean Sea, cyanobacteria, picoeukaryotes, diatoms, and flagellates belonging to different algal groups often coexist, although their relative abundances are marked by strong seasonal variations in that diatoms predominate in winter and nanoflagellates in spring and summer (32). Our results suggest that Chl is less vulnerable to Cu pulses during winter, in agreement with the dominance of diatoms during this period and their Cu tolerance. Nevertheless, other factors can also explain this lack of sensitivity in winter: the mixing depth is deeper in winter and the deposited Cu mixes with a greater volume of water; or phytoplankton growth is limited by light availability and Cu thus plays a smaller role.
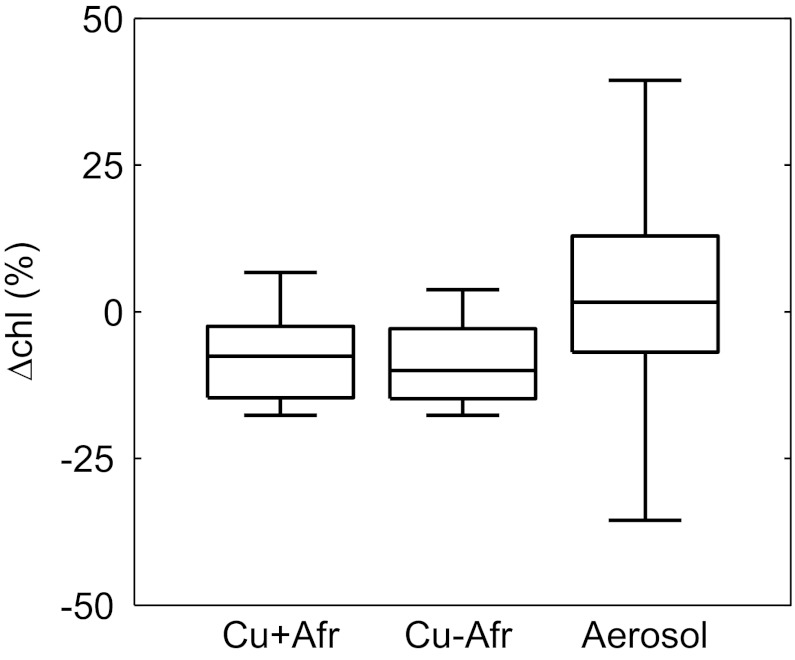
In fact, the response to Cu is not the main cause of Chl variability in the Mediterranean Sea. Chl mirrors the seasonal physical forcing, displaying a typical temperate cycle in which there is an increase in winter and very low values during summer. Other oceanographic processes that drive significant variability in the phytoplankton biomass are vertical mixing associated with strong atmospheric forcing, deep convection, and mesoscale frontal activity such as filaments and eddies (33–35). This variability when aerosol data were available is clearly higher than the variability caused by Cu pulses above [Cu]lim (Fig. 3). Nevertheless, the distribution of ΔChl shifts substantially toward negative values when only Cu pulses are considered. The difference in the median variability caused by these Cu pulses with respect to the other processes is significant (P = 0.002 for calculation with African aerosols and P = 0.003 without African aerosols). Additional tests on the consistency and robustness of our results are given in SI Text.
Fig. 3.
Box plots of satellite daily chlorophyll variation (ΔChl) averaged in the 3.5 × 3.5° box area (Fig. 1) for the days with Cu pulses greater than [Cu]lim = 5.23 ng m−3 (labeled as “Cu + Afr” for calculation with African aerosols and “Cu − Afr” without African aerosols) and for the days when aerosol data were available (excluding Cu pulses, labeled as “Aerosol”). The central lines represent the medians, the edges of the boxes are the 25th and 75th percentiles, and the whiskers extend to the most extreme data points. The significance of the separation of the median values between Cu pulses and nonpulses is 0.002 for calculation with African aerosols and 0.003 without African aerosols.
Conclusion
Anthropogenic emissions of Cu into the atmosphere have sharply increased over the past century and are currently ∼10 times higher than natural emissions (11). This increase can partly explain the global decline in phytoplankton over the past 100 y (18). In fact, large oceanic areas may be similarly vulnerable to Cu, because Cu concentrations in the form of aerosol depositions that are roughly the same or even higher than those determined in the western Mediterranean Sea may occur throughout the subtropical Atlantic Ocean, the northern Indian Ocean, the west Pacific Ocean, and other marine waters in the proximity of highly industrialized regions such as North America and East Asia (10), suggesting the relevance of our findings at a global scale.
Our results provide evidence of the negative effect of Cu aerosols of anthropogenic origins on marine phytoplankton over a vast area of the western Mediterranean Sea, based on the available data (aerosol Cu concentration and satellite Chl). However, the processes and interactions between atmospheric Cu chemistry and phytoplankton dynamics are highly complex. At this stage, we point to anthropogenic aerosols as a new threat to marine phytoplankton, in contrast to multiple examples of ocean fertilization via aerosol inputs (1, 5). Nevertheless, additional studies on Cu deposition, solubility, and speciation, and the effects of Cu on phytoplankton at the species level are required to fully understand the magnitude of this threat. Moreover, it is unlikely that the negative impact of aerosols is limited to marine phytoplankton; rather, toxicity is likely to also encompass other processes in the marine ecosystem and in biogeochemical cycles.
Methods
Aerosol Composition Data.
Samples of suspended air aerosols were collected over several 24-h periods on quartz microfiber filters (Schleicher and Schuell; QF20) using high-volume samplers (30 m3 h−1) and DIGITEL PM2.5 cutoff inlets. The study site was Montseny station, located northwest of the Mediterranean Sea, in Catalonia (Spain) in forested park land (41°46'N, 02°21'E; Fig. 1). This site was selected because it is representative of the regional background aerosols in the western Mediterranean Sea (17). Total particulate mass concentrations of aerosols finer than 2.5 μm (PM2.5) were determined by standard gravimetric procedures (36). Concentrations of aluminum (Al), Fe, titanium (Ti), calcium (Ca), sodium (Na), vanadium (V), nickel (Ni), zinc (Zn), and Cu were measured using ICP-MS (X Series II; Thermo) and ICP-AES (IRIS Advantage TJA Solutions; Thermo) following standard procedures (37). The data analyzed herein were obtained from October 2003 to December 2010, with a median sampling interval of 4 d. Although the series contains a few data gaps, more than 89% of the 408 analyzed samples were collected with an interval of <10 d. The aerosol pulse for each analyzed component was defined as an event in which the concentration was higher than the mean concentration plus the SD for each component.
Aerosol Dry Deposition Fluxes.
Ambient air concentrations were converted to dry deposition fluxes by multiplying the ambient air concentration of PM2.5 or of each metal by the deposition velocity. Deposition velocities vary from 10 cm s−1 for particles of 10 μm to 0.2 cm s−1 for particles of 0.5 μm (38, 39). We used a constant deposition velocity of 2 cm s−1. For example, during the Cu pulses the mean PM2.5 mass concentration was 16.67 μg m−3 and the mean Cu concentration 6.83 ng m−3, yielding dry deposition fluxes of 28.81 mg PM2.5 m−2 d−1 and 11.80 μg Cu m−2 d−1, respectively.
Aerosol Source Regions.
The different source regions of aerosol Cu pulses were determined according to a methodology described previously (17). Basically, data from meteorological maps, back-trajectories modeling, satellite and modeled dust concentration maps and simulations, and measured metal concentrations (normalized to Al as the crustal reference) were combined. Five source regions were identified: local, regional, regional with contributions from northern Europe, advection from the Atlantic Ocean, and African.
Chl Data.
Chl concentrations were obtained from the European Space Agency’s GlobColor project; specifically, remotely sensed level 3 data merged from multiple satellites (SeaWiFS, MERIS, and MODIS-Aqua) and derived using standard case 1 water algorithms (40). Satellite-retrieved Chl values obtained through these standard algorithms are, in the case of the Mediterranean Sea, subject to a calibration problem due to peculiarities in the environmental biooptical characteristics, such that a bias occurs compared with in situ observations (41, 42). Nevertheless, we focused our study on daily Chl variations rather than on absolute values. Detailed information regarding the processing of Chl data can be found on the GlobColor project Web site (www.globcolour.info). Daily Chl variation (ΔChl) at every pixel was defined as follows:
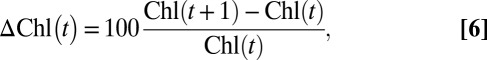 |
where t is the day of the Cu pulse. The ΔChl was averaged over a 3.5 × 3.5° boxed area adjacent to Montseny (Fig. 1). Only ΔChl data covering >50% of the ocean pixels in the 3.5 × 3.5° box were considered as good data.
Wind Data.
Daily-averaged surface wind data during the Cu pulses in the western Mediterranean Sea were obtained from the ERA-Interim reanalysis produced by the European Centre for Medium-Range Weather Forecasts (ECMWF) (43).
Supplementary Material
Acknowledgments
This work was partly supported by the Spanish Ministry of Agriculture, Food and Environment Grant PN384/2011 and the Spanish Ministry of the Science and Innovation Grants CTM2011-14036-E, CTM2009-08270, CGL2010-19464-CLI), CSD2007-00067, and CGL2008-06294/CLI. The work of A.J. was supported by a Ramón y Cajal grant from the Spanish Ministry of Economy and Competitiveness.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.N. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207567110/-/DCSupplemental.
References
- 1.Paerl HW. Enhancement of marine primary production by nitrogen-enriched acid-rain. Nature. 1985;315(6022):747–749. [Google Scholar]
- 2.Duarte CM, et al. Aerosol inputs enhance new production in the subtropical northeast Atlantic. J Geophys Res Biogeosci. 2006;111:G04006. [Google Scholar]
- 3.Martin JH, Fitzwater SE. Iron-deficiency limits phytoplankton growth in the northeast pacific subartic. Nature. 1988;331(6154):341–343. [Google Scholar]
- 4.Watson AJ, Bakker DCE, Ridgwell AJ, Boyd PW, Law CS. Effect of iron supply on Southern Ocean CO2 uptake and implications for glacial atmospheric CO2. Nature. 2000;407(6805):730–733. doi: 10.1038/35037561. [DOI] [PubMed] [Google Scholar]
- 5.Jickells TD, et al. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science. 2005;308(5718):67–71. doi: 10.1126/science.1105959. [DOI] [PubMed] [Google Scholar]
- 6.Mackey KRM, et al. Phytoplankton responses to atmospheric metal deposition in the coastal and open-ocean Sargasso Sea. Front Microbiol. 2012;3:00359. doi: 10.3389/fmicb.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morel FMM, Price NM. The biogeochemical cycles of trace metals in the oceans. Science. 2003;300(5621):944–947. doi: 10.1126/science.1083545. [DOI] [PubMed] [Google Scholar]
- 8.Twining BS, Baines SB. The trace metal composition of marine phytoplankton. Annu Rev Mar Sci. 2012;5:13.1–13.25. doi: 10.1146/annurev-marine-121211-172322. [DOI] [PubMed] [Google Scholar]
- 9.Moffett JW, Brand LE. Production of strong, extracellular Cu chelators by marine cyanobacteria in response to Cu stress. Limnol Oceanogr. 1996;41(3):388–395. [Google Scholar]
- 10.Paytan A, et al. Toxicity of atmospheric aerosols on marine phytoplankton. Proc Natl Acad Sci USA. 2009;106(12):4601–4605. doi: 10.1073/pnas.0811486106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong SM, Candelone JP, Patterson CC, Boutron CF. History of ancient copper smelting pollution during Roman and medieval times recorded in Greenland ice. Science. 1996;272(5259):246–249. [Google Scholar]
- 12.Rauch JN, Pacyna JM. Earth's global Ag, Al, Cr, Cu, Fe, Ni, Pb, and Zn cycles. Global Biogeochem Cycles. 2009;23:GB2001. [Google Scholar]
- 13.Schauer JJ, et al. Characterization of Metals Emitted from Motor Vehicles. Boston: Health Effects Institute; 2006. [PubMed] [Google Scholar]
- 14.Guerzoni S, et al. The role of atmospheric deposition in the biogeochemistry of the Mediterranean Sea. Prog Oceanogr. 1999;44(1–3):147–190. [Google Scholar]
- 15.Avila A, et al. Variation of soluble and insoluble calcium in red rains related to dust sources and transport patterns from North Africa to northeastern Spain. J Geophys Res Atmospheres. 2007;112:D05210. [Google Scholar]
- 16.Pey J, et al. Intense winter atmospheric pollution episodes affecting the Western Mediterranean. Sci Total Environ. 2010;408(8):1951–1959. doi: 10.1016/j.scitotenv.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 17.Pérez N, et al. Interpretation of the variability of levels of regional background aerosols in the Western Mediterranean. Sci Total Environ. 2008;407(1):527–540. doi: 10.1016/j.scitotenv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Boyce DG, Lewis MR, Worm B. Global phytoplankton decline over the past century. Nature. 2010;466(7306):591–596. doi: 10.1038/nature09268. [DOI] [PubMed] [Google Scholar]
- 19.Mann EL, Ahlgren N, Moffett JW, Chisholm SW. Copper toxicity and cyanobacteria ecology in the Sargasso Sea. Limnol Oceanogr. 2002;47(4):976–988. [Google Scholar]
- 20.Sholkovitz ER, Sedwick PN, Church TM. On the fractional solubility of copper in marine aerosols: Toxicity of aeolian copper revisited. Geophys Res Lett. 2010;37:L20601. [Google Scholar]
- 21.Sunda WG. In: Trace metal/phytoplankton interactions in the sea. Chemistry of Aquatic Systems: Local and Global Perspectives. Bidoglio G, Stumm W, editors. 1994. (Kluwer, Dordrecht, The Netherlands), pp 213–237. [Google Scholar]
- 22.Sunda WG, Huntsman SA. Processes regulating cellular metal accumulation and physiological effects: Phytoplankton as model systems. Sci Total Environ. 1998;219(2–3):165–181. [Google Scholar]
- 23.Shank GC, Skrabal SA, Whitehead RF, Kieber RJ. Strong copper complexation in an organic-rich estuary: The importance of allochthonous dissolved organic matter. Mar Chem. 2004;88(1–2):21–39. [Google Scholar]
- 24.Moffett JW, Zika RG, Brand LE. Distribution and potential sources and sinks of copper chelators in the Sargasso Sea. Deep-Sea Res. 1990;37(1):27–36. [Google Scholar]
- 25.Moffett JW, Brand LE, Croot PL, Barbeau KA. Cu speciation and cyanobacterial distribution in harbors subject to anthropogenic Cu inputs. Limnol Oceanogr. 1997;42(5):789–799. [Google Scholar]
- 26.Rijstenbil JW, Gerringa LJA. Interactions of algal ligands, metal complexation and availability, and cell responses of the diatom Ditylum brightwellii with a gradual increase in copper. Aquat Toxicol. 2002;56(2):115–131. doi: 10.1016/s0166-445x(01)00188-6. [DOI] [PubMed] [Google Scholar]
- 27.Bohner H, Böhme H, Böger P. Reciprocal formation of plastocyanin and cytochrome c-553 and the influence of cupric ions on photosynthetic electron transport. Biochim Biophys Acta. 1980;592(1):103–112. doi: 10.1016/0005-2728(80)90117-6. [DOI] [PubMed] [Google Scholar]
- 28.Brand LE, Sunda WG, Guillard RRL. Reduction of marine phytoplankton reproduction rates by copper and cadmium. J Exp Mar Biol Ecol. 1986;96(3):225–250. [Google Scholar]
- 29.Samson G, Morissette JC, Popovic R. Copper quenching of the variable fluorescence in Dunaliella tertiolecta—new evidence for a copper inhibition effect on PSII photochemistry. Photochem Photobiol. 1988;48(3):329–332. [Google Scholar]
- 30.Quigg A, Reinfelder JR, Fisher NS. Copper uptake kinetics in diverse marine phytoplankton. Limnol Oceanogr. 2006;51(2):893–899. [Google Scholar]
- 31.Levy JL, Stauber JL, Jolley DF. Sensitivity of marine microalgae to copper: The effect of biotic factors on copper adsorption and toxicity. Sci Total Environ. 2007;387(1–3):141–154. doi: 10.1016/j.scitotenv.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Siokou-Frangou I, et al. Plankton in the open Mediterranean Sea: A review. Biogeosciences. 2010;7(5):1543–1586. [Google Scholar]
- 33.MEDOC Observation of formation of deep water in Mediterranean-Sea, 1969. Nature. 1970;227(5262):1037–1040. [Google Scholar]
- 34.Levy M, Memery L, Madec G. The onset of a bloom after deep winter convection in the northwestern Mediterranean sea: Mesoscale process study with a primitive equation model. J Mar Syst. 1998;16(1–2):7–21. [Google Scholar]
- 35.Jordi A, Basterretxea G, Anglès S. Influence of ocean circulation on phytoplankton biomass distribution in the Balearic Sea: Study based on sea-viewing wide field-of-view sensor and altimetry satellite data. J Geophys Res Oceans. 2009;114:C11005. [Google Scholar]
- 36.Querol X, et al. Monitoring of PM10 and PM2.5 around primary particulate anthropogenic emission sources. Atmos Environ. 2001;35(5):845–858. [Google Scholar]
- 37.Pey J, et al. Geochemistry of regional background aerosols in the Western Mediterranean. Atmos Res. 2009;94(3):422–435. [Google Scholar]
- 38.Sehmel GA. Particle and gas dry deposition - a review. Atmos Environ. 1980;14(9):983–1011. [Google Scholar]
- 39.Vong RJ, Vong IJ, Vickers D, Covert DS. Size-dependent aerosol deposition velocities during BEARPEX' 07. Atmos Chem Phys. 2010;10(12):5749–5758. [Google Scholar]
- 40.O'Reilly JE, et al. Ocean color chlorophyll algorithms for SeaWiFS. J Geophys Res Oceans. 1998;103(C11):24937–24953. [Google Scholar]
- 41.Bosc E, Bricaud A, Antoine D. Seasonal and interannual variability in algal biomass and primary production in the Mediterranean Sea, as derived from 4 years of SeaWiFS observations. Global Biogeochem Cycles. 2004;18:GB1005. [Google Scholar]
- 42.Volpe G, et al. The colour of the Mediterranean Sea: Global versus regional bio-optical algorithms evaluation and implication for satellite chlorophyll estimates. Remote Sens Environ. 2007;107(4):625–638. [Google Scholar]
- 43.Dee DP, et al. The ERA-Interim reanalysis: Configuration and performance of the data assimilation system. Q J R Meteorol Soc. 2011;137(656):553–597. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.