Abstract
The autonomic nervous system is thought to modulate blood glucose homeostasis by regulating endocrine cell activity in the pancreatic islets of Langerhans. The role of islet innervation, however, has remained elusive because the direct effects of autonomic nervous input on islet cell physiology cannot be studied in the pancreas. Here, we used an in vivo model to study the role of islet nervous input in glucose homeostasis. We transplanted islets into the anterior chamber of the eye and found that islet grafts became densely innervated by the rich parasympathetic and sympathetic nervous supply of the iris. Parasympathetic innervation was imaged intravitally by using transgenic mice expressing GFP in cholinergic axons. To manipulate selectively the islet nervous input, we increased the ambient illumination to increase the parasympathetic input to the islet grafts via the pupillary light reflex. This reduced fasting glycemia and improved glucose tolerance. These effects could be blocked by topical application of the muscarinic antagonist atropine to the eye, indicating that local cholinergic innervation had a direct effect on islet function in vivo. By using this approach, we found that parasympathetic innervation influences islet function in C57BL/6 mice but not in 129X1 mice, which reflected differences in innervation densities and may explain major strain differences in glucose homeostasis. This study directly demonstrates that autonomic axons innervating the islet modulate glucose homeostasis.
Keywords: diabetes, beta cell, alpha cell, insulin, glucagon
The autonomic nervous system is generally thought to modulate pancreatic islet hormone secretion to adjust glucose homeostasis in response to food intake or stress (1, 2). The overall effect of parasympathetic stimulation is an increase in insulin secretion (3–8), whereas the net effect of sympathetic stimulation is a lowering of plasma insulin concentration (9–13). In most examined species, the pancreatic islet is richly innervated by autonomic axons (1, 14–17), but the role of direct autonomic input to the islet is unclear because the autonomic nervous system may use several other mechanisms to influence glucose homeostasis. Experimentally, it is not easy to distinguish the effects autonomic activation elicits locally in the islet from those it elicits in other organs, which can indirectly affect islet function (e.g., incretin secretion from the intestine or activation of the adrenal medulla). Achieving selective stimulation of the pancreatic innervation is difficult and invasive, as it requires electrical activation of the mixed autonomic nerves along a pancreatic artery while blocking the joint preganglionic cholinergic nerves (10, 11), but even this approach may not stimulate exclusively axons innervating the islet. Because it has not been possible to dissociate the neural effects on islet function from other confounding effects, the role of autonomic innervation of the islet has not been defined.
Three formal criteria are used to confirm that a substance serves as neurotransmitter between an axon and an effector cell: (i) it is present in the axon, (ii) it is released from the axon, and (ii) specific receptors for the substance must be present on the effector cell (18). For parasympathetic, cholinergic innervation of the mouse islet, these criteria have been partially met. β-Cell–specific genetic deletion of muscarinic receptors demonstrated that activation of muscarinic receptors exerts a strong influence on β-cell function that is critical for maintaining glucose homeostasis (19). Recently, we established that axons expressing cholinergic markers contact α- and β-cells in the mouse islet (20). However, it has not been demonstrated that stimulated parasympathetic axons release acetylcholine and affect cells in the islet. The experimental approach used in most studies in which the vagus nerve is stimulated and cholinergic antagonists are applied exogenously can influence multiple organs and may affect glucose homeostasis indirectly (16). Thus, criterion ii has not been met, and it is very likely that selective stimulation of parasympathetic axons in the islet and measurement of its effects on islet cell function cannot be accomplished in the pancreas in vivo.
Our strategy was to transplant islets into a site that allows local and noninvasive manipulation of autonomic input while islet cell function can be monitored. We hypothesized that our experimental platform in which islets are transplanted into the anterior chamber of the eye for functional imaging (21, 22) could also be used to study the role of islet innervation. After transplantation, noradrenergic and cholinergic nerve axons from the iris innervated intraocular islet grafts in patterns reflecting those of the islets in the pancreas. Autonomic input to the islet could be specifically activated via the pupillary reflex by changing ambient illumination (23, 24). Islet graft responses to nervous input could be manipulated with topical drug application via eye drops. We were able to determine that nervous input is important for islet function in C57BL/6 mice but less so in 129X1 mice. Our results demonstrate that the autonomic input to intraocular islet grafts can be modulated noninvasively and locally to study the effects of islet innervation on glucose homeostasis.
Results
Intraocular Islet Grafts Are Innervated.
Islets from C57BL/6 mice were transplanted into the anterior chamber of the eye of C57BL/6 mice as previously described (21, 22). Islets engrafted on the iris and retained their cell composition and shape (22). Immunohistochemical staining of intraocular islet isografts 90 d after transplantation showed innervation by sympathetic and parasympathetic axons immunoreactive for tyrosine hydroxylase (TH) and for vesicular acetylcholine transporter (vAChT), respectively (Fig. 1A). Sympathetic and parasympathetic innervation densities were similar to those observed in islets in the pancreas (Fig. 1B). Importantly, β-cells in intraocular islet grafts were innervated by parasympathetic axons (Fig. 1C), as they are in the native pancreas (20).
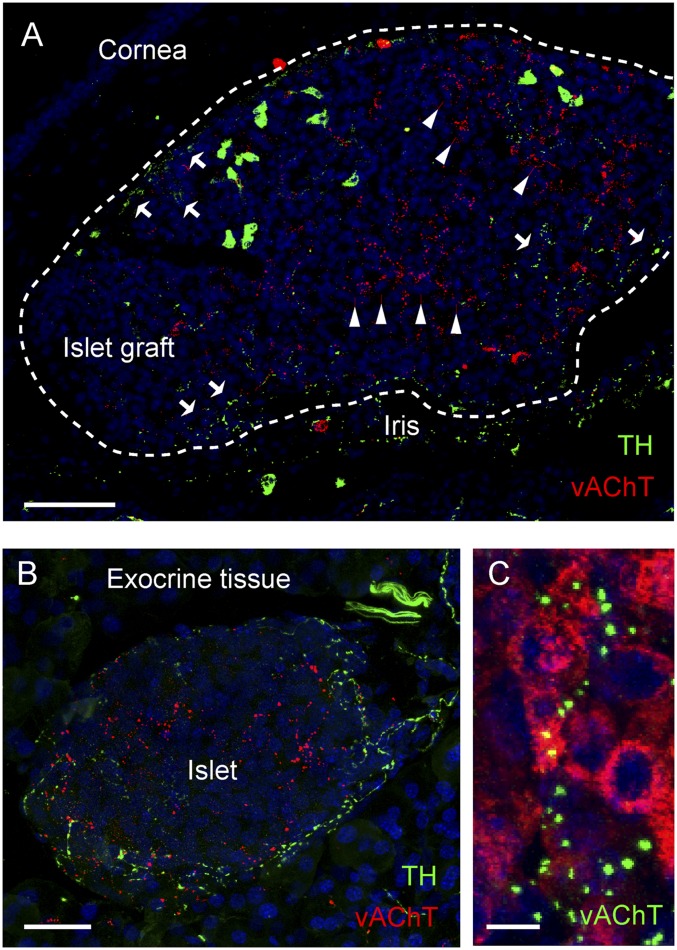
Fig. 1.
Mouse pancreatic islets of Langerhans transplanted into the anterior chamber of the mouse eye are reinnervated. (A) Maximal projection of a Z-stack of confocal images of intraocular islet grafts 90 d after transplantation shows sympathetic and parasympathetic axons immunoreactive for TH (green) and vAChT (red), respectively. Arrows and arrowheads, respectively, point at sympathetic and parasympathetic axonal terminal fields. Islet graft is outlined. (B) Maximal projection of a Z-stack of confocal images of a pancreatic section shows the distribution of TH and vAChT immunoreactive axons in the mouse islet. (C) High-magnification Z-stack of confocal images of vAChT axonal varicosities (green) closely apposed to β-cells stained for insulin (red). Shown are maximal projections of Z-stacks of confocal images (Z-depth, 38 µm; Z-step, 0.7 µm). (Scale bars: A, 50 μm; B, 20 μm; C, 5 μm.)
As early as 3 d after transplantation, a few projections of TH and vAChT immunoreactive axons of the iris could be seen close to the islet grafts (Fig. S1). The time course of sympathetic reinnervation paralleled that of revascularization (Fig. S1). At day 15, axons could be seen inside the islet grafts, mostly in close association with blood vessels (Fig. S1). Between days 15 and 30, the number of axons along blood vessels increased (Fig. S1). After 30 d, axons could be seen in the islet parenchyma, gradually increasing their density and complexity to reach a plateau by ∼90 d. Parasympathetic axons innervated the islet graft following a similar pattern, first present along vessels and later also in the parenchyma, but with slower kinetics, as very few axons could be seen in the islet graft before day 30 (Fig. S1).
In Vivo Assessment of Parasympathetic Fibers.
Histological studies can provide snapshots of innervation, but to understand the dynamic processes of reinnervation and structural adaptation during pathophysiological conditions, in vivo experiments are indispensable. To assess the feasibility of imaging islet innervation in vivo, we transplanted islets into the eyes of transgenic mice with the endogenous choline acetyltransferase (ChAT) transcriptional regulatory elements (cholinergic gene locus) driving EGFP expression (25). After engraftment, in vivo imaging showed GFP labeled neuroinsular complexes associated with islets in the anterior chamber of ChAT-GFP mice (Fig. 2A). These cholinergic neurons extended processes along the surface of the islet graft. A similar innervation pattern was observed for pancreatic islets in situ (Fig. 2B) (20).
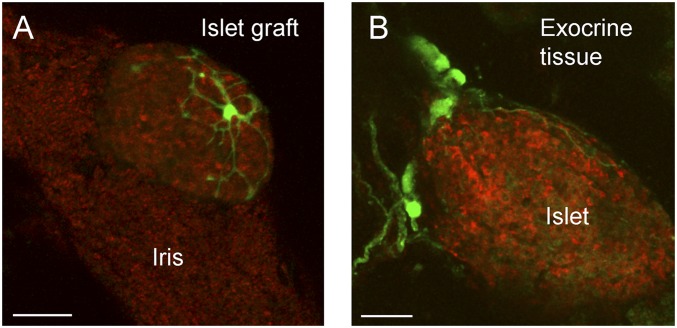
Fig. 2.
In vivo imaging of islet innervation. (A) In vivo imaging of an intraocular islet graft 7 mo after transplantation into a mouse expressing GFP in cholinergic neurons (Materials and Methods). A cholinergic neuron (green) can be seen extending processes around the islet graft (backscatter image shown in red). (B) GFP labeled neurons (green) are seen associated with an islet (red) in a pancreatic slice. Shown are maximal projections of Z-stacks of confocal images (Z-depths, 104 µm in A; 102 µm in B; Z-step, 2 µm). (Scale bars: 50 μm.)
Noninvasive Manipulation of Parasympathetic Input to Islet.
We have previously shown that transplantation of pancreatic islets into the anterior chamber of the eye reestablishes normoglycemia in diabetic mice, which then respond appropriately to glucose challenges (22). Because islet grafts take over control of glucose homeostasis in these mice, glycemia can be used as readout for islet function. A striking feature of innervated tissue grafts in the anterior chamber of the eye is that their autonomic input can be regulated by light via the pupillary light reflex (23, 24). Stimulation with light increases cholinergic input to the iris to constrict the pupil, whereas noradrenergic axons are activated in the dark to dilate the pupil (26). We found that mice with intraocular islet grafts had consistently lower fed (nonfasting) glycemia than matched mice with the same amount of islets transplanted under the kidney capsule (Fig. 3A). Insulin and glucagon plasma concentrations were higher in mice with intraocular grafts (Fig. 3B and Fig. S2). We hypothesized that the lower fed glycemia and increased hormone secretion in mice with intraocular islet grafts were caused by stronger parasympathetic input during ambient light conditions. To test this hypothesis, we exposed mice with islet grafts to different light conditions and found that, when fed mice bearing intraocular islet grafts were changed from ambient light (∼500 lx) into darkness (∼1 lx), insulin secretion decreased in 11 of 14 mice (Fig. 3C) and glycemia increased correspondingly (Fig. 3D). Glycemia reached levels similar to those in mice transplanted with islets under the kidney capsule (Fig. 3 A and D), indicating that exposure to light chronically decreased glycemia in mice with intraocular islet grafts. That mice transplanted under the kidney capsule did not exhibit light-induced changes in glycemia confirms that the effects were specific for intraocular islets. Placing mice with intraocular grafts back in ambient illumination lowered glycemia again (Fig. 3D).
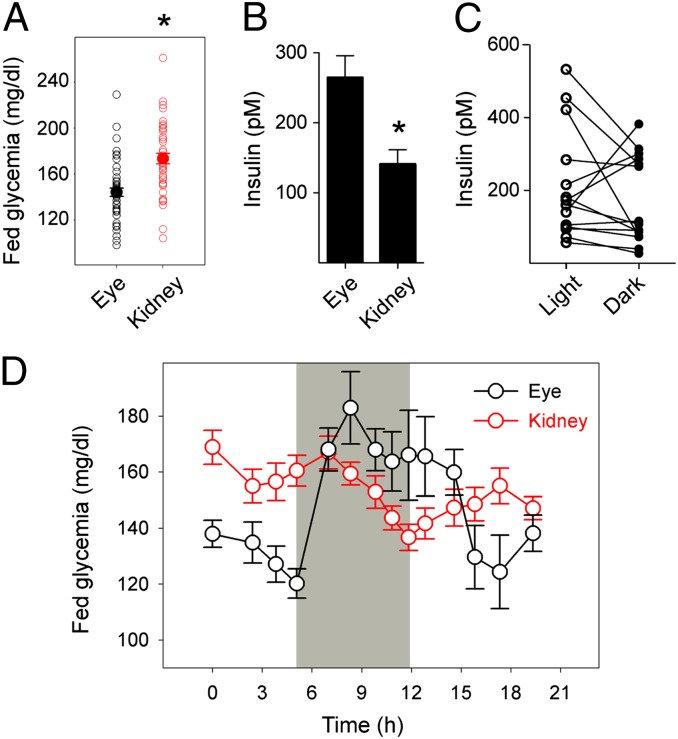
Fig. 3.
Modulating nervous input to the iris via the pupillary light reflex affects glycemia in mice with intraocular islet grafts. (A) Fed glycemia values of mice rendered diabetic with streptozotocin and transplanted with islets into the anterior chamber of the eye or under the kidney capsule. Values were obtained >2 mo after transplantation (n > 10 mice per group; *P < 0.05, Student t test). (B) Insulin plasma concentrations of mice shown in A (*P < 0.05, Student t test). (C) Insulin plasma concentrations in mice with intraocular islet grafts measured in ambient light and dark conditions. (D) Glycemia readings for mice with intraocular islet grafts (eye; black symbols) or under the kidney capsule (kidney;, red symbols) under ambient light after being placed in the dark (period indicated in gray) and after return to ambient light.
The effect of light on islet function was further studied by performing i.p. glucose tolerance tests (GTTs). Glucose excursions were significantly larger in the same mice when the test was performed in the dark compared with ambient light, indicating that glucose tolerance was impaired by reducing illumination in recipients of intraocular islet grafts (Fig. 4A). Applying the muscarinic antagonist atropine to transplanted eyes in ambient light conditions significantly impaired glucose tolerance to levels similar to those obtained in darkness (Fig. 4C). These results indicate that the effects of changes in illumination were mediated by cholinergic input to the islet. In line with this notion, topical application of pilocarpine, a muscarinic agonist, improved glucose tolerance (Fig. 4D). Illumination or topical application of cholinergic drugs did not affect glucose tolerance in mice bearing islet grafts under the kidney capsule (Fig. 4B) or in nontransplanted mice (Fig. S3), indicating that local manipulation of the eye did not have systemic effects.
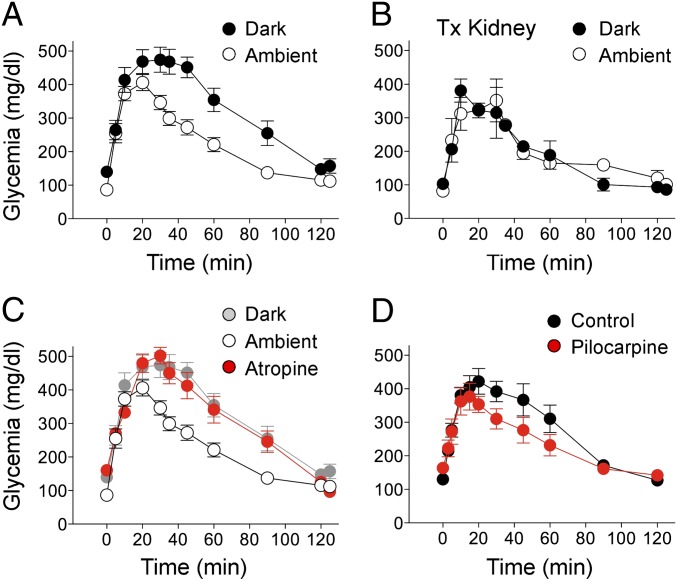
Fig. 4.
Noninvasive manipulation of the nervous input to intraocular islet grafts modulates islet function. (A and B) Glucose excursion during i.p. GTTs performed in the dark (solid symbols) or ambient light (open symbols) in mice transplanted in the eye (A) or under the kidney capsule (B). Values were obtained >2 mo after transplantation (n = 8 mice in A, n = 3 mice in B). Differences in glucose excursion were significant in A (repeated-measures ANOVA, F = 15.4, P < 0.001, followed by Tukey multiple comparison test, P < 0.05), but not in B. (C) Glucose excursion during i.p. GTTs performed in ambient light (black symbols) or in ambient light after topical application of the muscarinic receptor antagonist atropine (red symbols). Longitudinal results from the same group of mice are shown in A and C (glucose excursion in the dark is shown in light gray symbols). Differences in glucose excursion were significant in A (repeated-measures ANOVA, F = 15.4, P < 0.001, followed by Tukey multiple comparison test, P < 0.05). (D) Topical application of the muscarinic agonist pilocarpine reduces the glucose excursion during an i.p. GTT (red symbols). PBS solution was applied to the eyes of control mice (black symbols). Pilocarpine significantly reduced glucose excursion (n = 6 mice; paired Student t test, P < 0.05).
Noninvasive Manipulation of Parasympathetic Input Reveals Major Differences in Role of Innervation in Different Mouse Strains.
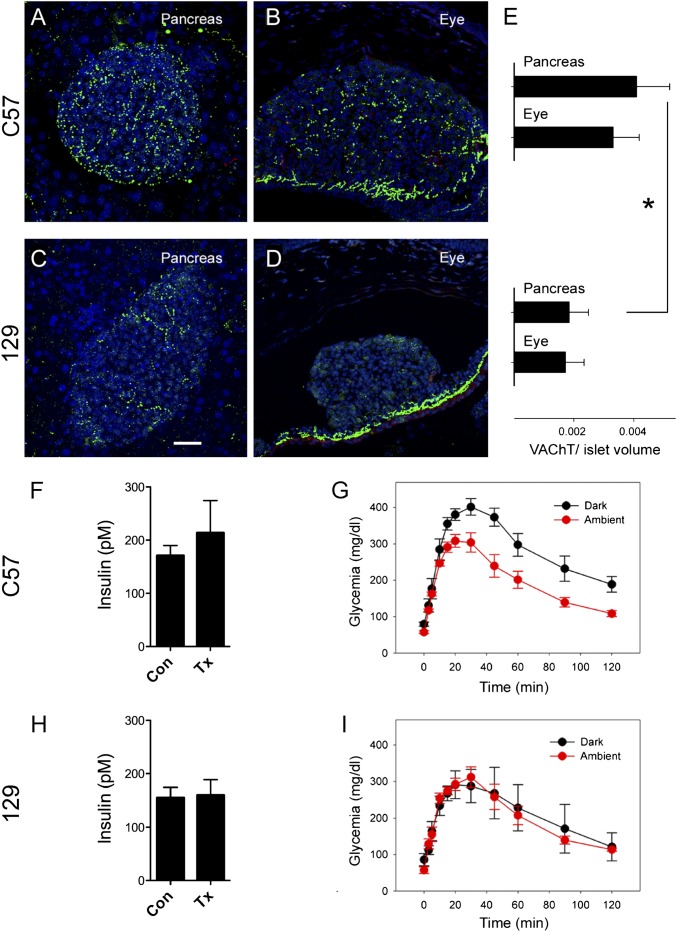
Insulin signaling and glucose homeostasis depend strongly on the genetic background of the mouse model (27, 28). In particular, the glucose threshold for the first phase of glucose-stimulated insulin secretion is higher in 129X1 mice than in C57BL/6 mice (27). We found that the innervation density of vAChT immunoreactive axons in islets of C57BL/6 mice was higher than that in islets of 129X1 mice (Fig. 5 A, C, and E), and, thus, differences in parasympathetic innervation and cholinergic input may contribute to the shift in the sensitivity to glucose. To test this hypothesis, we transplanted islets isolated from C57BL/6 or 129X1 mice into the anterior chamber of the eye of athymic Nude-Foxn1nu (nude) mice. Transplanted mice had insulin levels and glucose excursions during i.p. GTTs (dark light conditions) that were similar to those of the donor mice (Fig. 5 F and H and Fig. S4). C57BL/6 mice and nude mice transplanted with islets from C57BL/6 mice showed larger glucose excursions than 129X1 mice, and nude mice transplanted with islets from 129X1 mice (Fig. 5 G and I and Fig. S4). These data indicate that the differences in insulin levels and glucose excursion were transferred with the transplanted islets. The innervation densities of vAChT immunoreactive axons in the intraocular grafts also mirrored the differences observed in the pancreas of the donor mice (Fig. 5). Few axons could be seen in islets grafts from 129X1 mice (Fig. 5 B, D, and E). The glucose excursion decreased with enhanced illumination in mice transplanted with C57BL/6 islets but not in mice transplanted with 129X1 islets (Fig. 5 G and I). Because C57BL/6 and 129X1 islets respond similarly to 11 mM glucose in vitro (27), it is likely that lack of parasympathetic input diminishes the β-cell’s ability to respond adequately to glucose in 129X1 mice.
Fig. 5.
Manipulation of the nervous input to intraocular islet grafts reveals functional differences in the innervation of islets from C57BL/6 and 129X1 mice. (A–D) Z-stacks of confocal images (Z-depth, 40 µm; step size, 0.7 µm) of mouse pancreatic sections from C57BL/6 (A) and 129X1 mice (C) show different densities of parasympathetic islet innervation (vAChT; green). The differences in the density of vAChT innervation are maintained in fully engrafted and functional islets from C57BL/6 (B) and 129X1 mice (D) after transplantation into the eyes of nude mice (*P < 0.05, Student t test). (Scale bar: 20 μm.) (E) Quantification of vAChT immunostaining shows the densities of parasympathetic innervation of native islets and intraocular islet grafts in both mouse strains. (F and H) Insulin plasma concentrations (Con) in C57BL/6 (F) and 129X1 mice (H) and in nude mice transplanted (Tx) with intraocular islet grafts from C57BL/6 (F) or 129X1 mice (H). (G and I) Glucose excursion of nude mice transplanted with C57BL/6 (G) or 129X1 islets (I) during i.p. GTTs performed at ambient light (red) or in darkness (black).
Discussion
Current methods to investigate the role of autonomic innervation in pancreatic islet function are cumbersome and invasive. Here we have introduced an approach that allows the study of the direct effects of autonomic nerves on islet physiology and glucose metabolism in vivo. In this model, syngeneic islets are transplanted into the anterior chamber of the eye, a site that is optimally suited to study the effects of autonomic innervation because local efferent axons can be monitored and manipulated noninvasively. Mice are examined 3 mo after having been rendered diabetic with streptozotocin and transplanted with islets, when the islet grafts have restored glucose homeostasis and become fully innervated. We were able to (i) visualize innervation intravitally, (ii) manipulate autonomic nervous input to islets noninvasively, (iii) intervene pharmacologically with topical drug application, and (iv) simultaneously record changes in glycemia and plasma hormone levels. Selective activation of nervous input to islet grafts as shown here can be coupled with noninvasive imaging of islet cell function (21, 22). Our model is easy to implement and to manipulate, and it will be relevant for studying pathophysiological changes occurring during diabetes. In addition, it can be readily extended to the study of other tissues.
Transplantation into the anterior chamber of the eye is well established to study the mechanisms of reinnervation of tissue grafts, including pancreas, and the behavior of intrinsic nerves (29–39). Studies have shown that iris sympathetic axons establish functional connections in intraocular brain grafts similar to the synapses normally formed in situ (40, 41). Islets are reinnervated after transplantation, both in the eye (present results and ref. 42) and elsewhere (43–46). Independently of the transplantation site, the innervation pattern of the islet grafts seems to differ only slightly from that in the native pancreas (43, 45). The density of innervation, however, varies with the transplantation site. Islets transplanted under the kidney capsule are more densely innervated than islet grafts in the spleen and liver (44), suggesting that the site shapes the reinnervation density of the islet grafts. The highly innervated iris is thus an ideal transplantation site for reinnervation of the islets, with the additional advantage that sympathetic and parasympathetic axons in the anterior chamber of the eye can be easily targeted pharmacologically with topical application of drugs, enabling noninvasive, longitudinal studies of the prolonged effects of nervous input to the islet.
Our results show that endocrine cells in intraocular islet grafts attract sympathetic and parasympathetic axons in patterns similar to those of islets in the pancreas (20). Parasympathetic axons innervate β- and α-cells as in the pancreas. In contrast to islets in the pancreas, sympathetic axons were not preferentially localized to the periphery of intraocular islet grafts (Fig. 1), but this is likely because their main targets, α-cells, are displaced from the periphery after transplantation. We also describe a mouse strain-specific pattern of parasympathetic innervation that is preserved after transplantation (Fig. 5). Therefore, our model allows studying the effects of nervous input in a system that mimics the local autonomic innervation pattern of islets. It is important to note, however, that intraocular islet grafts are not innervated by the same central autonomic nervous circuits that innervate islets in the pancreas. The sympathetic and parasympathetic axons in the eye do not receive input from the brain regions modulating islet function, but are part of the circuits regulating pupillary size. Intraocular islet grafts are nonetheless fully functional and restore glucose homeostasis, indicating that, as in the pancreas, the role of the autonomic nervous system is to modulate islet function, not to dictate it. Whereas autonomic nervous input to islets in the pancreas adapts hormonal secretion to the organism’s behavioral state, in the eye, investigators can now change ambient illumination to deliberately alter autonomic input, providing a powerful tool to investigate the role of islet innervation.
Our results show that parasympathetic axons influence islet function and glucose homeostasis. This is a direct demonstration of the notion that parasympathetic axons activate β-cells to control glycemia (3, 4). The degree of innervation and its effects, however, seem to vary between mouse strains. Our findings indicate that parasympathetic innervation may be important for islet function in C57BL/6 mice but less so in 129X1 mice. These differences within the same species, let alone other species, are striking. In the C57BL/6 mouse, which is among the least insulin sensitive mouse strains (27), a strong parasympathetic innervation potentiating glucose-stimulated insulin secretion may compensate for the increased insulin demand. Our findings further suggest that the factors leading to differences in innervation are intrinsic to the islet. Thus, manipulating the expression of axonal guidance molecules or trophic factors in islet cells and exploring their effects with our model could clarify how the innervation process is regulated.
Given that the innervation of human islets is quantitatively and qualitatively different from that of mouse islets (20), these studies could also be extended to investigate the role autonomic innervation plays in human islet cell function. Sympathetic axons preferentially innervate smooth muscle cells of the islet vasculature within human islets (20), and this innervation pattern is reproduced after transplantation into the eye. This suggests that the autonomic input changes vascular diameter to regulate blood flow locally, thus indirectly altering islet hormone secretion into the circulation. Because we can monitor blood flow in intraocular human islet grafts in real time, changes in vascular perfusion of the islet can be measured in response to activation of sympathetic axons in the eye. These studies will help determine the effector mechanisms used by autonomic axons to regulate human islet function.
Our study shows that intraocular islet transplantation is a versatile approach. In contrast to other approaches, our method enables noninvasive, local, and selective manipulation of the nervous input to transplanted islets while metabolic function is recorded locally or systemically in real time or longitudinally. Our approach can thus be used to investigate pathophysiological changes associated with diabetes and thereby clarify the molecular and cellular mechanisms leading to diabetic neuropathy.
Materials and Methods
Islet Isolation.
All animal procedures were performed under protocols approved by the University of Miami Institutional Animal Care and Use Committee. Donor C57BL/6 or 129X1 mice were humanely killed by exsanguination under general anesthesia. Mouse islet isolation was performed by using collagenase digestion followed by purification on density gradients (47).
Islet Transplantation into Anterior Chamber of Mouse Eye.
Thirty to 300 isolated islets were transferred from culture media to sterile PBS solution and aspirated into a 27-gauge eye cannula (27-gauge anterior chamber cannula; Katena) connected to a 1-mL Hamilton syringe (Hamilton) via 0.4-mm polyethylene tubing (Portex). C57BL/6 mice were anesthetized with ∼2% isoflurane (vol/vol). Eyes were kept humidified (ophthalmologic eye drops) to avoid drying of the cornea. Under a stereomicroscope, the cornea was punctured close to the sclera at the bottom part of the eye with a 31-gauge insulin needle. Using the needle, we made a small radial incision of approximately the size of the eye cannula (∼0.5 mm). For this incision, the needle was barely introduced into the anterior chamber, thus avoiding damage to the iris and bleeding. The blunt eye cannula was then gently inserted through this incision, first perpendicular to the surface of the cornea and then parallel to the cornea. When the cannula had been stably inserted into the anterior chamber, the islets were slowly injected in a 10-µL volume of sterile saline solution into the anterior chamber, where they settled on the iris. After injection, the cannula was carefully and slowly withdrawn (1 min) to avoid islets from flowing back through the incision. After awakening, mice were put back in the cages and monitored until full recovery, and observed daily thereafter. Analgesia was obtained after surgical procedures with buprenorphine (0.05–0.1 mg/kg s.c.).
Transplantation of Islets for Metabolic Studies.
Mouse pancreatic islets were isolated as described earlier. Diabetes was induced in recipient C57BL/6 and athymic nude mice with a single i.v. injection of streptozotocin (200 mg/kg; Sigma). Streptozotocin was freshly resuspended in citrate buffer at 10 to 20 mg/mL and administered as a single bolus via tail vein injection based on the weight of the animals, to a final dose of 200 mg/kg (22, 47, 48). Morbidity or mortality associated with streptozotocin toxicity was ∼5% and was generally observed within days after streptozotocin administration, possibly secondary to hepatic and renal toxicity in the setting or loss of insulin producing cells. Only animals in good conditions with nonfasting glycemic values >350 mg/dL received transplants. We transplanted 300 mouse islet equivalents into the anterior chamber of the right mouse eye. This provided an optimal β-cell mass. During the peritransplantation period, mice were supported with insulin pellets or insulin injections. After islet transplantation, normoglycemia helped maintain or increase body weight, suggesting full recovery from streptozotocin treatment. Manipulating and monitoring of the nervous input to islet grafts was started 3 mo after transplantation (i.e., long after any putative toxic effects of streptozotocin treatment could confound the results), when grafts were fully reinnervated. Comparable islet numbers were transplanted under the kidney capsule of control mice. Plasma glucose levels were monitored daily after transplantation. We considered transplantation successful and islet grafts functional when glycemic values had reached <200 mg/dL in recipient mice (22, 47, 49). After diabetes induction with streptozotocin, nonfasting glycemia is generally indicated by measurements of >350 mg/dL and, in response to glucose challenge, the measurements reach values >600 mg/dL (22). The cutoff of 200 mg/dL allows determining the efficiency of transplanted islets to restore and maintain tight metabolic control. Our success rate with ocular transplantation is >90%. Mice that did not revert to normoglycemia were not included in the studies.
Imaging of Islets in Mouse Eye.
For in vivo imaging, we transplanted islets from ChAT-GFP mice into ChAT-GFP mice. Imaging of islets in vivo in the anterior chamber of transplanted animals was performed as previously reported (22). Briefly, mice were anesthetized with ∼2% isoflurane air mixture and placed on a heating pad, and the head was restrained with a headholder. The eyelid was carefully pulled back and the eye gently supported. For fluorescence confocal imaging, an upright DMLFSA microscope equipped with a TCS-SP2-AOBS confocal scanner (Leica Microsystems) was used for imaging, together with long-distance water-dipping lenses (10 × 0.3 W, 20 × 0.5 W, and 40 × 0.8 W; HXC APO; Leica), by using Viscotears (Novartis) as an immersion liquid. GFP was excited at 488 nm (<43% acousto-optical tunable filter), and emission light was collected between 500 and 550 nm. Reflected light was imaged by illumination at 633 nm (<9% acousto-optical tunable filter) and collection between 630 and 639 nm. Postprocessing, analysis, and visualization of images were performed with Volocity (PerkinElmer) and Imaris software (Bitplane).
Imaging of Pancreatic Tissue Slices.
Pancreatic tissue slices of ChAT-GFP mice of 150-μm thickness were prepared as previously described (50). After slicing, the tissue was placed in a slice chamber on the stage of the confocal microscope, immerged in PBS solution, and imaged using the same parameters as described for in vivo imaging.
Assessment of Metabolic Function.
We monitored graft function by measuring nonfasting glycemia using a portable glucometer (OneTouch; LifeScan). Tail blood samples (20 µL) were taken for determination of plasma glucagon and insulin secretion in the nonfasting state in C57BL/6 and 129X1 mice and in diabetic nude mice transplanted with islets from C57BL/6 or 129X1 mice. Insulin and glucagon levels were also determined in C57BL/6 mice transplanted with intraocular C57BL/6 islets under ambient light conditions and in the dark. Hormone plasma levels were determined with the human or mouse Endocrine LINCOplex Kit (Linco) (51). The biomolecular assays were performed on a Bio-Plex protein array system (Bio-Rad). We performed GTTs after 12 to 16 h of fasting by measuring glycemic values on peripheral blood following i.p. injection of 2 g/kg glucose in saline solution. To exclude residual function of the native pancreas we removed the graft-bearing eye (enucleation) or kidney (nephrectomy) under general anesthesia (ketamine/xylazine). Atropine [1% atropine sulfate ophthalmic solution (wt/vol); Akorn], pilocarpine [1% pilocarpine hydrochloride ophthalmic solution (wt/vol); Akorn], or vehicle solution (PBS solution) was applied topically to the transplanted eye for 4 min, 30 min before the i.p. GTTs. To control for systemic effects, we applied atropine on one eye of nontransplanted mice. This treatment did not induce changes in glucose tolerance in these mice (Fig. S3).
Statistical Analyses.
The differences between experimental groups were examined with unpaired and paired Student t tests when comparing two groups of data or a repeated-measures ANOVA followed by a Tukey multiple comparison test when comparing data of longitudinal studies.
Supplementary Material
Acknowledgments
We thank the Diabetes Research Institute Cores (Histology, Imaging, Preclinical Cell Processing and Translational Models) and Kevin Johnson, Yelena Gadea, and Susana Villate for technical assistance. ChaT-GFP mice were a kind gift from Michael Kotlikoff (College of Veterinary Medicine, Cornell University). This work was funded by the Diabetes Research Institute Foundation; National Institutes of Health Grants R56DK084321 (to A.C.), R01DK084321 (to A.C.), 5U19AI050864-10 (to A.P.), F32DK083226 (to M.H.A.), and U-01 DK 089538 (to A.P. and P.-O.B.); the Juvenile Diabetes Research Foundation (Grant 3-2007-73 to S.S.); the European Foundation for the Study of Diabetes/Merck Sharpe and Dohme Basic Research program (S.S.); the Strategic Research Program in Diabetes at Karolinska Insitutet; Skandia Insurance Company, Ltd.; Berth von Kantzow's Foundation; the Knut and Alice Wallenberg Foundation; VIBRANT (FP7-228933-2); Swedish Research Council; Novo Nordisk Foundation; Swedish Diabetes Association; Family Erling–Persson Foundation; Söderbergs Foundation; Stichting af Jochnick Foundation; and World Class University Program through the National Research Foundation of Korea funded by Ministry of Education, Science and Technology Grant R31-2008-000-10105-0.
Footnotes
Conflict of interest statement: P.-O.B. is the founder and member of the board of the biotech company Biocrine AB, which will use the anterior chamber of the eye as a commercial servicing platform. I.L. is involved in the commercialization of and A.C. holds a patent on this platform.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211659110/-/DCSupplemental.
References
- 1.Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia. 2000;43(4):393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 2.Havel PJ, Taborsky GJ., Jr The contribution of the autonomic nervous system to changes of glucagon and insulin secretion during hypoglycemic stress. Endocr Rev. 1989;10(3):332–350. doi: 10.1210/edrv-10-3-332. [DOI] [PubMed] [Google Scholar]
- 3.Daniel PM, Henderson JR. The effect of vagal stimulation on plasma insulin and glucose levels in the baboon. J Physiol. 1967;192(2):317–327. doi: 10.1113/jphysiol.1967.sp008302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunicardi FC, Shavelle DM, Andersen DK. Neural regulation of the endocrine pancreas. Int J Pancreatol. 1995;18(3):177–195. doi: 10.1007/BF02784941. [DOI] [PubMed] [Google Scholar]
- 5.Frohman LA, Ezdinli EZ, Javid R. Effect of vagotomy and vagal stimulation on insulin secretion. Diabetes. 1967;16(7):443–448. doi: 10.2337/diab.16.7.443. [DOI] [PubMed] [Google Scholar]
- 6.Kaneto A, Kosaka K, Nakao K. Effects of stimulation of the vagus nerve on insulin secretion. Endocrinology. 1967;80(3):530–536. doi: 10.1210/endo-80-3-530. [DOI] [PubMed] [Google Scholar]
- 7.Ahrén B, Taborsky GJ, Jr, Porte DJ., Jr Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia. 1986;29(12):827–836. doi: 10.1007/BF00870137. [DOI] [PubMed] [Google Scholar]
- 8.Healy JA, et al. Cholinergic augmentation of insulin release requires ankyrin-B. Sci Signal. 2010;3(113):ra19. doi: 10.1126/scisignal.2000771. [DOI] [PubMed] [Google Scholar]
- 9.Porte D, Jr, Williams RH. Inhibition of insulin release by norepinephrine in man. Science. 1966;152(3726):1248–1250. doi: 10.1126/science.152.3726.1248. [DOI] [PubMed] [Google Scholar]
- 10.Bloom SR, Edwards AV. Characteristics of the neuroendocrine responses to stimulation of the splanchnic nerves in bursts in the conscious calf. J Physiol. 1984;346:533–545. doi: 10.1113/jphysiol.1984.sp015039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahrén B, Veith RC, Taborsky GJ., Jr Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1). Effects on basal release of insulin and glucagon. Endocrinology. 1987;121(1):323–331. doi: 10.1210/endo-121-1-323. [DOI] [PubMed] [Google Scholar]
- 12.Kurose T, et al. Mechanism of sympathetic neural regulation of insulin, somatostatin, and glucagon secretion. Am J Physiol. 1990;258(1 Pt 1):E220–E227. doi: 10.1152/ajpendo.1990.258.1.E220. [DOI] [PubMed] [Google Scholar]
- 13.Gilliam LK, Palmer JP, Taborsky GJ., Jr Tyramine-mediated activation of sympathetic nerves inhibits insulin secretion in humans. J Clin Endocrinol Metab. 2007;92(10):4035–4038. doi: 10.1210/jc.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods SC, Porte DJ., Jr Neural control of the endocrine pancreas. Physiol Rev. 1974;54(3):596–619. doi: 10.1152/physrev.1974.54.3.596. [DOI] [PubMed] [Google Scholar]
- 15.Satin LS, Kinard TA. Neurotransmitters and their receptors in the islets of Langerhans of the pancreas: What messages do acetylcholine, glutamate, and GABA transmit? Endocrine. 1998;8(3):213–223. doi: 10.1385/ENDO:8:3:213. [DOI] [PubMed] [Google Scholar]
- 16.Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev. 2001;22(5):565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- 17.Taborsky GJ., Jr . Insulin and glucagon secretion in vivo and its neural control. In: Jefferson LS, Cherrington AD, editors. Handbook of Physiology. New York: Oxford Univ Press; 2001. pp. 153–176. [Google Scholar]
- 18.Purves D. Neuroscience. 4th Ed. Sunderland, MA: Sinauer; 2008. [Google Scholar]
- 19.Gautam D, et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3(6):449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Diaz R, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14(1):45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speier S, et al. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat Protoc. 2008;3(8):1278–1286. doi: 10.1038/nprot.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speier S, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008;14(5):574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor D, Seiger A, Freedman R, Olson L, Hoffer B. Electrophysiological analysis reinnervation of transplants in the anterior chamber of the eye by the autonomic ground plexus of the iris. Proc Natl Acad Sci USA. 1978;75(2):1009–1012. doi: 10.1073/pnas.75.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tucker DC, Gist R. Sympathetic innervation alters growth and intrinsic heart rate of fetal rat atria maturing in oculo. Circ Res. 1986;59(5):534–544. doi: 10.1161/01.res.59.5.534. [DOI] [PubMed] [Google Scholar]
- 25.Tallini YN, et al. BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons. Physiol Genomics. 2006;27(3):391–397. doi: 10.1152/physiolgenomics.00092.2006. [DOI] [PubMed] [Google Scholar]
- 26.Lowenstein O, Loewenfeld IE. Role of sympathetic and parasympathetic systems in reflex dilation of the pupil; pupillographic studies. Arch Neurol Psychiatry. 1950;64(3):313–340. doi: 10.1001/archneurpsyc.1950.02310270002001. [DOI] [PubMed] [Google Scholar]
- 27.Goren HJ, Kulkarni RN, Kahn CR. Glucose homeostasis and tissue transcript content of insulin signaling intermediates in four inbred strains of mice: C57BL/6, C57BLKS/6, DBA/2, and 129X1. Endocrinology. 2004;145(7):3307–3323. doi: 10.1210/en.2003-1400. [DOI] [PubMed] [Google Scholar]
- 28.Berglund ED, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57(7):1790–1799. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerhardt GA, et al. Adrenergic transmission in hippocampus-locus coeruleus double grafts in oculo: Demonstration by in vivo electrochemical detection. Brain Res. 1984;306(1-2):319–325. doi: 10.1016/0006-8993(84)90381-0. [DOI] [PubMed] [Google Scholar]
- 30.Bickford-Wimer P, et al. Human fetal cerebellar and cortical tissue transplanted to the anterior eye chamber of athymic rats: Electrophysiological and structural studies. Proc Natl Acad Sci USA. 1987;84(16):5957–5961. doi: 10.1073/pnas.84.16.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donáth T, Adeghate E. Re-innervation of pancreatic tissue implants in normal and in sympathetically denervated eyes of rats. Acta Morphol Hung. 1988;36(3-4):147–154. [PubMed] [Google Scholar]
- 32.Granholm AC, et al. Morphological and electrophysiological studies of human hippocampal transplants in the anterior eye chamber of athymic nude rats. Exp Neurol. 1989;104(2):162–171. doi: 10.1016/s0014-4886(89)80010-x. [DOI] [PubMed] [Google Scholar]
- 33.Adeghate E, Donáth T. Morphological findings in long-term pancreatic tissue transplants in the anterior eye chamber of rats. Pancreas. 1990;5(3):298–305. doi: 10.1097/00006676-199005000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Adeghate E, Donáth T. Dopamine-beta-hydroxylase-positive nerves in normal and transplanted pancreatic tissue in the anterior eye-chamber of rats. J Chem Neuroanat. 1991;4(3):223–227. doi: 10.1016/0891-0618(91)90004-v. [DOI] [PubMed] [Google Scholar]
- 35.Adeghate E, Donáth T. Ultrastructural cytochemistry of acetylcholinesterase enzyme activity in pancreatic tissue transplants in rats. Cell Transplant. 1994;3(2):171–177. doi: 10.1177/096368979400300205. [DOI] [PubMed] [Google Scholar]
- 36.Adeghate E, Donáth T. Transplantation of tissue grafts into the anterior eye chamber: A method to study intrinsic neurons. Brain Res Brain Res Protoc. 2000;6(1-2):33–39. doi: 10.1016/s1385-299x(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 37.Törnqvist N, Björklund L, Strömberg I. Evidence for target-specific nerve fiber outgrowth from subpopulations of grafted dopaminergic neurons: A retrograde tracing study using in oculo and intracranial grafting. Exp Neurol. 2001;169(2):329–339. doi: 10.1006/exnr.2001.7658. [DOI] [PubMed] [Google Scholar]
- 38.Granholm AC, Gerhardt GA, Bygdeman M, Strömberg I. Human fetal xenografts of brainstem tissue containing locus coeruleus neurons: Functional and structural studies of intraocular grafts in athymic nude rats. Exp Neurol. 1992;118(1):7–17. doi: 10.1016/0014-4886(92)90017-k. [DOI] [PubMed] [Google Scholar]
- 39.Tucker DC, Torres A. Adrenal hormones interact with sympathetic innervation to modulate growth of embryonic heart in oculo. Am J Physiol. 1992;262(2 pt 2):H318–H325. doi: 10.1152/ajpheart.1992.262.2.H318. [DOI] [PubMed] [Google Scholar]
- 40.Hoffer B, Seiger A, Ljungberg T, Olson L. Electrophysiological and cytological studies of brain homografts in the anterior chamber of the eye: Maturation of cerebellar cortex in oculo. Brain Res. 1974;79(2):165–184. doi: 10.1016/0006-8993(74)90409-0. [DOI] [PubMed] [Google Scholar]
- 41.Palmer M, Björklund H, Olson L, Hoffer B. Trophic effects of brain areas on the developing cerebral cortex: II. Electrophysiology of intraocular grafts. Brain Res. 1983;282(2):141–148. doi: 10.1016/0165-3806(83)90092-5. [DOI] [PubMed] [Google Scholar]
- 42.Adeghate E. Pancreatic tissue grafts are reinnervated by neuro-peptidergic and cholinergic nerves within five days of transplantation. Transpl Immunol. 2002;10(1):73–80. doi: 10.1016/s0966-3274(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 43.Korsgren O, Andersson A, Jansson L, Sundler F. Reinnervation of syngeneic mouse pancreatic islets transplanted into renal subcapsular space. Diabetes. 1992;41(2):130–135. doi: 10.2337/diab.41.2.130. [DOI] [PubMed] [Google Scholar]
- 44.Korsgren O, Jansson L, Andersson A, Sundler F. Reinnervation of transplanted pancreatic islets. A comparison among islets implanted into the kidney, spleen, and liver. Transplantation. 1993;56(1):138–143. [PubMed] [Google Scholar]
- 45.Houwing H, et al. Noradrenergic and cholinergic reinnervation of islet grafts in diabetic rats. Cell Transplant. 1996;5(1):21–30. doi: 10.1177/096368979600500106. [DOI] [PubMed] [Google Scholar]
- 46.Persson-Sjögren S, Forsgren S, Täljedal IB. Expression of tyrosine hydroxylase, calcitonin gene-related peptide, substance P and protein gene product 9.5 in mouse islets transplanted under the kidney capsule. Neuropeptides. 1998;32(4):307–318. doi: 10.1016/s0143-4179(98)90053-1. [DOI] [PubMed] [Google Scholar]
- 47.Pileggi A, et al. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes. 2001;50(9):1983–1991. doi: 10.2337/diabetes.50.9.1983. [DOI] [PubMed] [Google Scholar]
- 48.Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman HJ. The streptozotocin-induced diabetic nude mouse model: Differences between animals from different sources. Comp Med. 2011;61(4):356–360. [PMC free article] [PubMed] [Google Scholar]
- 49.Nyqvist D, et al. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes. 2011;60(10):2571–2577. doi: 10.2337/db10-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Speier S, Rupnik M. A novel approach to in situ characterization of pancreatic beta-cells. Pflugers Arch. 2003;446(5):553–558. doi: 10.1007/s00424-003-1097-9. [DOI] [PubMed] [Google Scholar]
- 51.Cabrera O, et al. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell Transplant. 2008;16(10):1039–1048. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.