Abstract
The Last Interglacial (LIG; ca. 125,000 y ago) resulted from rapid global warming and reached global mean temperatures exceeding those of today. The LIG thus offers the opportunity to study how life may respond to future global warming. Using global occurrence databases and applying sampling-standardization, we compared reef coral diversity and distributions between the LIG and modern. Latitudinal diversity patterns are characterized by a tropical plateau today but were characterized by a pronounced equatorial trough during the LIG. This trough is governed by substantial range shifts away from the equator. Range shifts affected both leading and trailing edges of species range limits and were much more pronounced in the Northern Hemisphere than south of the equator. We argue that interglacial warming was responsible for the loss of equatorial diversity. Hemispheric differences in insolation during the LIG may explain the asymmetrical response. The equatorial retractions are surprisingly strong given that only small temperature changes have been reported in the LIG tropics. Our results suggest that the poleward range expansions of reef corals occurring with intensified global warming today may soon be followed by equatorial range retractions.
Keywords: biodiversity, climate change, paleobiology
Current global warming triggers substantial range shifts in both terrestrial and marine ecosystems (1–3). Scleractinian reef corals, like many other marine organisms, are currently experiencing poleward range expansions (4–7), but there is little evidence for range retractions in low latitudes. Whether this trend will persist into the future is difficult to predict, partly because of the lack of well-studied examples on past responses to rapid global warming. Range expansions of fossil reef corals with climate warming have been observed in the early to mid-Holocene (10 to 6 ky ago) (7) and during the Last Interglacial (LIG; ca. 125 ky ago) (8, 9). These previous studies highlighted particular taxa and regions, but the generality of the observations remains uncertain. Moreover, the balance between leading-edge range expansions (defined as species ranges moving toward the poles) and trailing-edge contractions (defined as species ranges moving away from the equator) in these past warming phases is unexplored.
Here we compare latitudinal patterns of global reef coral distributions and diversity (number of species) from raised reef terraces of the LIG [Marine Isotope Stage 5 (MIS 5)] with modern-day distributions to highlight the impact of past global warming. During most of the Pleistocene, global temperatures were colder than today (10–12), but glacial episodes are not well documented in reef corals because coral reefs of those times are largely submerged today (but see ref. 13). Interglacial warming led to polar ice melting and sea levels substantially higher than today (12, 14–16), leaving fossil coral reefs accessible on land. Tectonic uplift on some Pacific islands may also leave a record outside the peak interglacials, but not of peak glacials. Although the interglacial record of reef corals extends through the entire Pleistocene, coral data are more limited in older interglacials (Fig. S1). Occurrence data of zooxanthellate reef corals were evaluated from two global datasets, the Paleobiology Database (PaleoDB) and the Ocean Biogeographic Information System (OBIS). Although detailed paleoecologic studies are available for some Pleistocene reefs (17, 18), analysis of occurrence data are currently the best compromise between detailed comparisons of community structure (13, 19, 20) and an assessment of range endpoints (9).
Although comprehensive, both occurrence datasets are incomplete in terms of taxonomic and geographic coverage. The Pleistocene record is especially degraded because, in addition to incomplete sampling, fossil preservation is limited by diagenesis, weathering, and burial. Any meaningful comparison between the Pleistocene and the Recent must involve rigorous sampling-standardization. We applied rarefaction within latitudinal bins to equalize occurrences across space. Simulations were used to assess remaining sampling issues.
Results
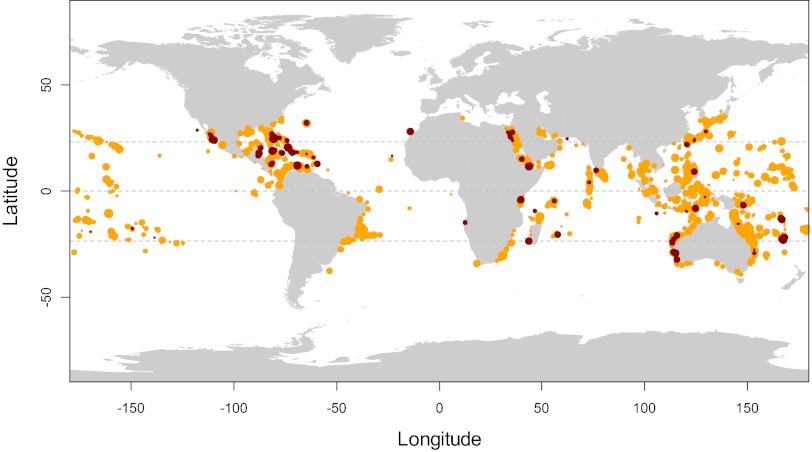
Species-level occurrence data of modern and LIG reef corals are pantropical (Figs. 1 and 2 A and B). Sampling of LIG reef corals is much less comprehensive than today but covers the same regions. Differences in preservation and sampling are most likely responsible for the considerably greater species richness of reef corals today (730 species) than in the LIG (269 species). The effect of sampling intensity on recorded Pleistocene richness is shown by the good correlation between the per-species occurrence counts now and then (Spearman rho = 0.56, P < 0.001). The less common a species is today, the less probable is its occurrence in the LIG. Importantly, the evenness (J) of species abundances within 5° latitudinal zones (a band across the globe extending over 5° latitude) are statistically indistinguishable between the LIG and today (two-sided Wilcoxon test: P = 0.40), justifying the use of rarefaction for sampling standardization.
Fig. 1.
Distribution map of Recent (orange) and LIG (MIS 5; dark red) reef coral species occurrences. Size of circles is proportional to the logarithm of occurrence counts in unique coordinates or collections, respectively.
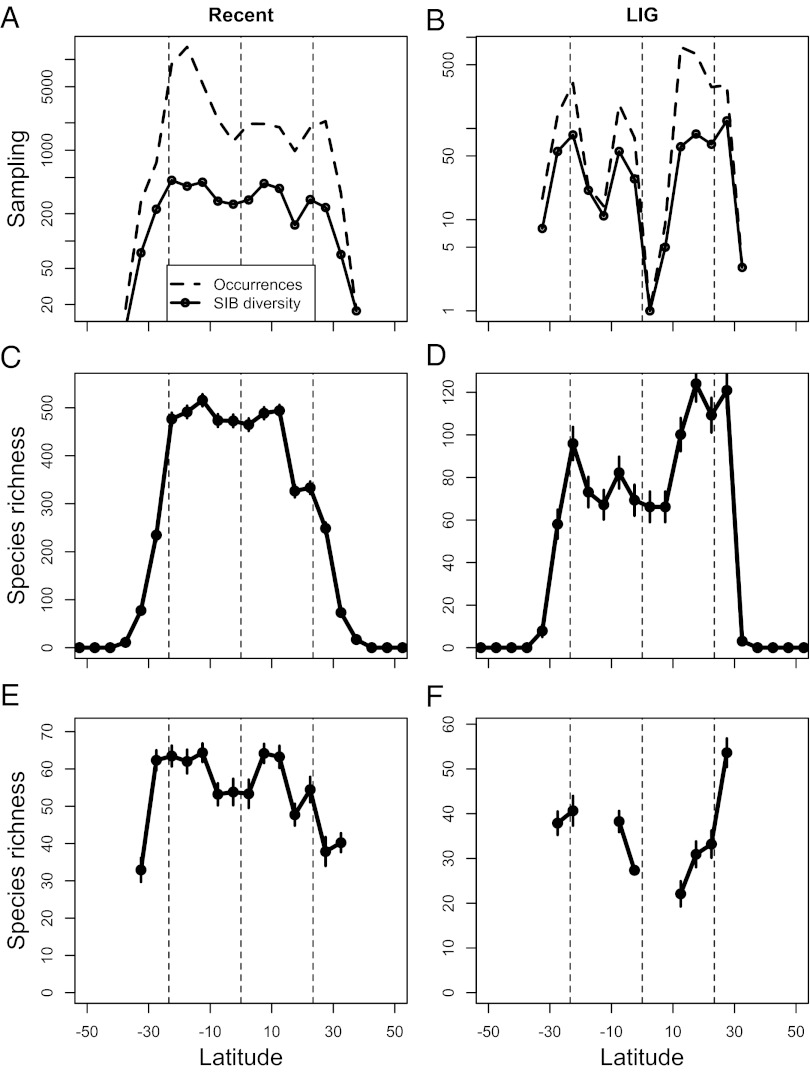
Fig. 2.
Latitudinal sampling and diversity patterns of reef corals binned by zones of 5° latitude. (A) Counts of recent occurrences and SIB species diversity. (B) Counts of LIG occurrences and SIB diversity. (C) Recent raw diversity based on latitudinal ranges (RT). (D) LIG raw diversity based on RT. (E) Recent SIB diversity based on a subsampling quota of 75 occurrences averaged across 1,000 trials. (F) Pleistocene subsampled SIB diversity with quota and trials as in E. Error bars are one SD in each direction based on 1,000 bootstrap replicates of ranges for raw data (C and D) and subsampling trials (E and F). Dashed vertical lines demarcate the equator and the tropics.
Latitudinal transects of diversity based on the raw data roughly follow sampling, both for sampled-in-bin (SIB; species actually recorded in a 5° latitudinal zone) and range-through (RT; species inferred to be present in all zones between its range endpoints) data (Fig. 2 A–D). Not all latitudinal zones have sufficient data for a meaningful subsampling analysis of SIB diversity, but those that do suggest a slight depression near the equator today (Fig. 2E) and a dramatic decline north of the equator in the LIG (Fig. 2F). SIB diversity is least affected by the mid-domain effect, which holds that latitudinal diversity gradients can arise without physical gradients (21–24). Geometric constraints such as hard physical boundaries may lead to centered diversity peaks by overlapping ranges. That the equatorial diversity depression is robust when subsampling and RT are combined indicates that this depression is strong enough to mask the mid-domain effect (Fig. S2).
We tested potential physical controls of the modern raw RT latitudinal diversity pattern by multiple regressions, using three independent variables and their quadratic terms: multiannual averages of sea surface temperature (SST) and solar insolation (IN) and the potential habitat area for reef corals (HA), here defined as normal marine, shallow water area (0- to 50-m water depth) (Fig. S3). The best model based on the corrected Akaike information criterion (AICC; ref. 25) is a polynomial function of SST, suggesting that RT starts declining at high levels of SST (Table S1 and Fig. S4). Model averaging (26) of the top five models indicates that SST and SST2 are by far the most important predictor variables of RT (importance = 1.0 and 0.98, respectively) followed by HA (0.15), IN (0.11), HA2 (0.08), and IN2 (0.02). Only the effect sizes of SST2 and IN2 have a negative sign.
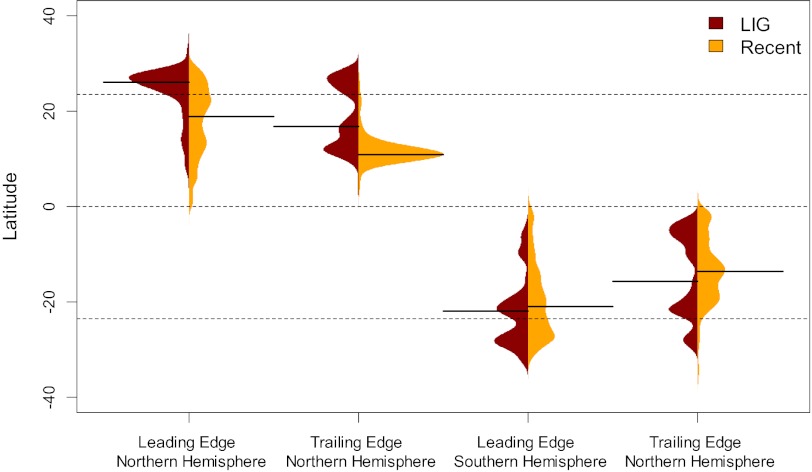
To assess changes in latitudinal positions of reef corals between the LIG and today, we performed a subsampling analysis of all occurrences, focusing on the poleward and equator-ward range limits of the 243 species that occur in both time intervals. The comparison of sampling-standardized range endpoints of all species indicates that Pleistocene reef corals tended to reach further poleward but also retracted further away from the equator than recent corals (Fig. 3). Pleistocene poleward (leading-edge) extensions were fully compensated by equatorial (trailing-edge) retractions. Range shifts are greatest in the Northern Hemisphere and generally significant, except for Southern Hemisphere equatorial retractions (Table 1). The changes in range limits vary substantially among species (Fig. S5).
Fig. 3.
Beanplots (49) of sampling-standardized range edges of reef corals in the LIG (left-hand beans; dark red) and today (right-hand beans; orange). The beanplots depict the estimated densities of species leading edge and trailing edge range limits on each hemisphere in the respective time intervals. Solid lines represent the medians. Dashed horizontal lines indicate the equator and the tropics.
Table 1.
Median difference of subsampled range edges of reef corals in the LIG relative to today
| Dataset | Shared species | Northern Hemisphere |
Southern Hemisphere |
||
| Leading edges | Trailing edges | Leading edges | Trailing edges | ||
| LIG, global | 243 | 7.1 (<0.001) | 6.0 (<0.001) | 0.9 (0.004) | 2.0 (0.10) |
| LIG, excl. Acropora | 200 | 8.9 (<0.001) | 5.8 (<0.001) | 2.5 (0.002) | −0.35 (0.53) |
| LIG, Indo-Pacific | 199 | 3.0 (<0.001) | 7.8 (<0.001) | −0.4 (0.08) | 2.1 (0.07) |
| LIG, W-Pacific | 136 | 13.0 (<0.001) | 8.1 (<0.001) | −2.9 (0.55) | 2.7 (0.006) |
| LIG, Caribbean | 39 | -0.2 (0.007) | 2.1 (<0.001) | NA | NA |
| MIS 5–11, global | 265 | 6.4 (<0.001) | 10.1 (<0.001) | 2.4 (0.001) | 1.5 (0.10) |
All differences are reported in degrees latitude. P values (in brackets) refer to a two-sided Wilcoxon test. Values in bold indicate significant differences. NA, not applicable.
Because the LIG is so much undersampled relative to the Recent, there is the risk of artificial range shifts created by large differences in sampling. We ran a simulation to explore the possibility that poor LIG sampling alone might explain the equatorial diversity depression and the dramatic range shifts. This simulation was based on a comparison of modern and surrogate LIG data that were created by downgrading recent data to Pleistocene levels without implying range shifts. There is no evidence for an equatorial depression in RT diversity (Fig. S6), and the maximum total summed range shift in 500 simulation runs is less than half of the 16° of latitude that we observed in the LIG data (compare sum of first row in Table 1 with Table S2). Although poor sampling alone is thus unlikely to cause the dramatic range shifts, they might still be exaggerated by taphonomy, the postmortem fate of organisms.
Pleistocene corals are usually sampled from outcrops, where they are exposed to weathering and diagenetic alteration. Much of the unexplained variance in the correlation of LIG and modern occurrence counts is likely to be due to these taphonomic factors rather than genuine ecological differences. The effect of preservation is best illustrated by the genus Acropora, the most speciose among recent corals. Species within this genus are hard to identify in fossil material because taphonomy has often obliterated species-diagnostic characters, many of which are found in the less robust radial corallites along the periphery of the branches. Indeed, the four most common species without a fossil record in the LIG belong to Acropora. The diversity of Acropora may thus be selectively depressed in the LIG, specifically in the wet tropics, where diagenetic overprint is especially strong. However, our main results are not affected by this potential bias. When Acropora is excluded, the poleward range expansion is even more substantial in both hemispheres, whereas the equatorial retractions become only marginally smaller (Table 1).
Finally, we asked whether the global patterns we observe for the LIG are also evident in particular regions (Indo-Pacific, west Pacific, and Caribbean) during the LIG (MIS 5) and for the combined interglacials of the last 400 ky (MIS 5–11) at global scales. Our main results show that, at least for the Northern Hemisphere, significant range shifts of leading and trailing edges are observed for all three geographic regions. Likewise, the range shifts are significant for the combined interglacials (Table 1).
Discussion
We find that the LIG was accompanied by substantial poleward range shifts of reef corals relative to today, for both trailing and leading edges. Range changes were more dramatic in the Northern Hemisphere than in the Southern Hemisphere. The trailing-edge movements explain the substantial drop in sampling-standardized diversity north of the interglacial equator. The maximum latitudinal range expansion of leading edges is ∼800 km. This finding may appear dramatic, but if we conservatively assume a duration of 2,000 y for the penultimate deglaciation (27), this finding translates into a range expansion of 400 m/y, whereas current range expansions of reef corals are reported to be up to 14 km/y (5). True migrations during the LIG may have been larger because reef corals had to expand from their glacial refugia, which are sometimes nearby (e.g., ref. 13) but not necessarily so. The true rates of latitudinal shifts may thus have been on the same order of magnitude as observed today.
At first glance, our results are in line with the effects of rapid global warming during the LIG. Temperature is clearly the dominant factor determining modern diversity patterns of reef corals (Table S1 and ref. 28), and thermal thresholds of reef corals could explain both the equatorial depression and the poleward expansion. Although thermal tolerances are indeed widely discussed as shaping diversity gradients on geologically short timescales (29, 30), there is an important issue with this scenario. Paleoclimatic proxy data and modeling suggest that LIG warming was more modest than suggested by temperature reconstructions from Arctic and Antarctic ice cores. The latter show that the LIG temperatures exceeded modern temperatures by 4–5 °C in these polar regions (12, 31). However, compilations of faunal and geochemical proxy data suggest that, although global average temperatures were 1.5 °C warmer than today (32), average SST may only have been 0.7 °C higher (16). Proxy data and climate models agree in suggesting smaller temperature change in the tropics and stronger warming in higher latitudes (16, 33, 34). LIG warming was also more uniform in higher northern latitudes (>30°) than in low latitudes, where several areas of cooler-than-today SST were observed (16).
In sum, given current paleoclimatic knowledge of the LIG, our results would even fit a scenario of polar range expansions due to warming and equatorial retractions driven by cooling. We reject the equatorial cooling hypothesis for two reasons. First, one of the regions where tectonic uplift made coral reefs accessible on land outside the peak interglacials is Huon Peninsula of Papua New Guinea (13, 35). This region, situated just south of the equator (ca. 6°S), records coral reef terraces from times when SST in the LIG was substantially lower than today (10). However, coral diversity was not depressed, neither in those reefs (35) nor in the latitudinal zone to which those terraces contributed most data (Fig. 2). Second, proxy data are often contradictory (i.e., faunal proxies give cooler temperatures than geochemical proxies; refs. 16 and 36) or are at odds with modeling results such that they cannot be taken at face value at the resolution relevant for this study. Recent climate modeling does confirm the MIS 5 was among the warmest Pleistocene interglacials and indicates temperature rise near the equator due to increased insolation and greenhouse gas warming (34).
Spatial temperature gradients (measured in °C/km) are shallower in the tropics than in midlatitudes such that even modest temperature change (measured in °C/y) may lead to high rates of isotherm migration and hence velocities of climate change (measured in km/y) (37, 38). Similar to the LIG, recent ocean warming is greatest in mid to high northern latitudes, yet the velocity is highest near the equator where it is also greater than on land (38). Thus, the strong equatorial retractions of reef corals in the LIG northern hemisphere may well be due to <1 °C of SST warming. The asymmetric range shifts are probably due to greater warming in the Northern Hemisphere. Changes in Northern Hemisphere summer insolation are thought to be a key driver of glacial–interglacial cycles, particularly during the LIG (39) when Northern Hemisphere summer insolation was 11% greater than today (15).
Given the small direct influence of HA and IN on modern RT diversity, these factors are unlikely to have contributed substantially to the observed range shifts. Increases of HA due to rising sea levels and higher IN may have added marginally to LIG range expansions, but not to the equatorial retractions. Similarly, ocean acidification may have occurred during deglaciation but probably at a much smaller scale than already occurring today (40). In addition, acidification through increases in atmospheric CO2 concentrations should affect higher latitudes first (41) and thus lead to contractions rather than expansions of leading edges.
In conclusion, our results suggest that temperature rise during the LIG caused dramatic range shifts of reef corals. The data are in good agreement with paleoclimatic data and modeling results for the LIG, which has several attributes indicative of an analog for current and future global warming (34, 42). The leading-edge expansions we observed in the LIG relative to today are similar to present-day expansions of reef corals, but the strong trailing-edge retractions have no modern analog. Warning signs of future trailing-edge range retractions are known from terrestrial records (43, 44), and the fossil record of reef corals informs us that global warming may lead to equatorial retractions of the same magnitude as polar expansions. Hence temperature shelter in high latitudes may not be sufficient to counteract the loss of equatorial diversity in a warming world. These inferences are conservative because they only relate to the effects of warming and do not consider anthropogenic impact or ocean acidification.
Data and Methods
Data.
We assessed occurrence data of zooxanthellate corals from two independent databases with global scope: OBIS (www.iobis.org) for modern corals and the PaleoDB (paleodb.org) for fossil corals. OBIS data comprised 418,115 taxonomic occurrences of scleractinian corals when downloaded on July 7, 2012. PaleoDB yielded 5,807 Pleistocene coral occurrences with reliable genus identifications in the download from July 19, 2012.
Occurrence data were filtered to exclude azooxanthellate corals and apozooxanthellate corals and occurrences not identified to the species level. To improve credibility of species identifications, we dropped all occurrences from rapid assessment surveys and checked all species names for synonymies (SI Data and Methods). The fossil data were additionally filtered to only comprise corals that can reliably be assigned to the LIG. We also dropped repeated occurrences of the same species from exactly the same coordinates (OBIS) or collections (PaleoDB). The final dataset has 43,348 occurrences occupying 3,308 unique coordinates for recent corals and 2,802 occurrences from 466 collections for LIG corals.
Data for SST, IN, and HA were gathered from spatially resolved online resources. For IN, we downloaded the Release 6.0 Data Set from NASA (http://eosweb.larc.nasa.gov/sse/), which reports 22-y monthly averages of incident insolation in 1° grid cells (July 1983–June 2005). These data were filtered to only incorporate insolation over the ocean and averaged over the year. SSTs were downloaded and averaged from the Hadley Centre (http://badc.nerc.ac.uk/view/badc.nerc.ac.uk__ATOM__dataent_hadisst) for the same 22-y period. Bathymetric data were extracted from the ETOPO-2 dataset in the geophysical data system GEODAS (www.ngdc.noaa.gov/mgg/geodas/). We measured potential HA for reef corals as fully marine areas between 0 and 50 m water depth. All data were averaged (SST, IN) or summed (HA) across 5° latitudinal zones.
Sampling Standardization.
We applied by-occurrence subsampling (rarefaction) to account for the large differences in sampling between the Recent and the LIG among latitudinal bins. For diversity gradients, we kept the number of occurrences drawn from each bin and time constant at 75 occurrences. To explore latitudinal range shifts, we first limited the dataset to shared species, omitting all species that presumably became extinct in the Pleistocene or are extant and have no fossil record in the Pleistocene. Subsampling was performed by randomly drawing the same number of occurrences from each latitudinal bin and time interval. To maximize sample size, the number of occurrences subsampled (quota) was allowed to vary among bins. The quota for each bin was dictated by the Pleistocene data, because sampling is lower than today for all bins. To allow for some variability among subsampling trials, the quota was chosen to be 10% lower than the minimum sample size in each bin and time. All subsampling results are reported as averages over 1,000 trials.
Analyses and Sensitivity Tests.
Latitudinal range shifts were assessed globally for both time intervals, focusing on polar and equatorial range edges. By convention, we speak of range expansion if the leading edges of LIG coral species occurred in a more poleward position than their recent counterparts. Likewise, we speak of equatorial retraction if the trailing edges of LIG corals occurred further away from the equator than today. Because data are usually not normally distributed, we rely on nonparametric statistics for hypothesis testing. For testing physical controls on latitudinal diversity, we focused on factors that are commonly thought to control reef coral diversity: HA (45), SST (28), and IN (46). We first logged the raw data and removed autocorrelations by generalized differencing (47, 48). We then applied multiple polynomial regressions using modern raw RT diversity as the dependent variable. Finally, model sets were generated, and model averaging was performed following the guidelines of Grueber et al. (26). All analyses were carried out in the R programming environment (http://r-project.org).
Sensitivity tests were performed based on (i) geographic subsets, (ii) by extending the scope of analysis to all interglacials after the Mid-Brunhes event (ca. 430 ky ago), and (iii) by a numeric simulation. To simulate the effect of undersampling in the Pleistocene on perceived range shifts, we downgraded the recent data in two steps and analyzed the outcome as surrogates of LIG data. The first step filtered the recent data to exclude all species that are not recorded in the LIG. In the second step, we randomly drew from each latitudinal band the same number of occurrences that has been recorded in the LIG. The data were then treated in the same way as true LIG data. This simulation was repeated 500 times.
Supplementary Material
Acknowledgments
We thank U. Merkel for data entry; A. Hendy and M. Kosnik for contributions to the dataset of Pleistocene corals; M. Burrows for helping with the acquisition of SST data; M. Aberhan, S. Connolly, S. Keith, D. Lazarus, M. Mihaljevic, and D. Tittensor for discussions; anonymous reviewers for insightful reviews; and all OBIS data providers for making modern coral occurrence data accessible. This work was supported by the Deutscher Akademischer Austauschdienst, the Group of Eight Australian Universities, the VolkswagenStiftung, and the Australian Research Council Centre of Excellence for Coral Reef Studies. This is Paleobiology Database Publication No. 172.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214037110/-/DCSupplemental.
References
- 1.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421(6918):37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 2.Sorte CJB, Williams SL, Carlton JT. Marine range shifts and species introductions: Comparative spread rates and community impacts. Glob Ecol Biogeogr. 2010;19(3):303–316. [Google Scholar]
- 3.Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333(6045):1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 4.Marsh LM. Proceedings of the Seventh International Coral Reef Symposium. Vol 2. UOG Station, Guam: Univ of Guam Press; 1992. The occurrence and growth of Acropora in extra-tropical waters off Perth, Western Australia; pp. 1233–1238. [Google Scholar]
- 5.Yamano H, Sugihara K, Nomura K. Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophys Res Lett. 2011;38:L04601. [Google Scholar]
- 6.Vargas-Ángel B, Thomas JD, Hoke SM. High-latitude Acropora cervicornis thickets off Fort Lauderdale, Florida, USA. Coral Reefs. 2003;22(4):465–473. [Google Scholar]
- 7.Precht WF, Aronson RB. Climate flickers and range shifts of reef corals. Front Ecol Environ. 2004;2:307–314. [Google Scholar]
- 8.Muhs DR, Simmons KR, Steinke B. Timing and warmth of the Last Interglacial period: New U-series evidence from Hawaii and Bermuda and a new fossil compilation for North America. Quat Sci Rev. 2002;21(12-13):1355–1383. [Google Scholar]
- 9.Greenstein BJ, Pandolfi JM. Escaping the heat: Range shifts of reef coral taxa in coastal Western Australia. Glob Change Biol. 2008;14(3):513–528. [Google Scholar]
- 10.McCulloch MT, et al. Coral record of equatorial sea-surface temperatures during the penultimate deglaciation at Huon Peninsula. Science. 1999;283(5399):202–204. doi: 10.1126/science.283.5399.202. [DOI] [PubMed] [Google Scholar]
- 11.Bintanja R, van de Wal RSW, Oerlemans J. Modelled atmospheric temperatures and global sea levels over the past million years. Nature. 2005;437(7055):125–128. doi: 10.1038/nature03975. [DOI] [PubMed] [Google Scholar]
- 12.Jouzel J, et al. Orbital and millennial Antarctic climate variability over the past 800,000 years. Science. 2007;317(5839):793–796. doi: 10.1126/science.1141038. [DOI] [PubMed] [Google Scholar]
- 13.Tager D, et al. Community dynamics of Pleistocene coral reefs during alternative climatic regimes. Ecology. 2010;91(1):191–200. doi: 10.1890/08-0422.1. [DOI] [PubMed] [Google Scholar]
- 14.Lisiecki LE, Raymo ME. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography. 2005;20:PA1003. [Google Scholar]
- 15.Anderson P, et al. Last Interglacial Arctic warmth confirms polar amplification of climate change. Quat Sci Rev. 2006;25:1383–1400. [Google Scholar]
- 16.McKay NP, Overpeck JT, Otto-Bliesner BL. The role of ocean thermal expansion in Last Interglacial sea level rise. Geophys Res Lett. 2011;38:L14605. [Google Scholar]
- 17.Humblet M, Iryu Y, Nakamori T. Variations in Pleistocene coral assemblages in space and time in southern and northern Central Ryukyu Islands, Japan. Mar Geol. 2009;259:1–20. [Google Scholar]
- 18.Pandolfi JM, Jackson JBC. Community structure of Pleistocene coral reefs of Curaçao, Netherlands Antilles. Ecol Monogr. 2001;71(1):49–67. [Google Scholar]
- 19.Pandolfi JM. Response of Pleistocene coral reefs to environmental change over long temporal scales. Am Zool. 1999;39:113–130. [Google Scholar]
- 20.Pandolfi JM. Coral community dynamics at multiple scales. Coral Reefs. 2002;21(1):13–23. [Google Scholar]
- 21.Colwell RK, Lees DC. The mid-domain effect: Geometric constraints on the geography of species richness. Trends Ecol Evol. 2000;15(2):70–76. doi: 10.1016/s0169-5347(99)01767-x. [DOI] [PubMed] [Google Scholar]
- 22.Koleff P, Gaston KJ. Latitudinal gradients in diversity: Real patterns and random models. Ecography. 2001;24(3):341–351. [Google Scholar]
- 23.Connolly SR, Bellwood DR, Hughes TP. Indo-Pacific biodiversity of coral reefs: Deviations from a mid-domain model. Ecology. 2003;84(8):2178–2190. [Google Scholar]
- 24.Arita HT, Vázquez-Domínguez E. The tropics: Cradle, museum or casino? A dynamic null model for latitudinal gradients of species diversity. Ecol Lett. 2008;11(7):653–663. doi: 10.1111/j.1461-0248.2008.01197.x. [DOI] [PubMed] [Google Scholar]
- 25.Hurvich CM, Tsai C-L. Regression and time series model selection in small samples. Biometrika. 1989;76(2):297–307. [Google Scholar]
- 26.Grueber CE, Nakagawa S, Laws RJ, Jamieson IG. Multimodel inference in ecology and evolution: Challenges and solutions. J Evol Biol. 2011;24(4):699–711. doi: 10.1111/j.1420-9101.2010.02210.x. [DOI] [PubMed] [Google Scholar]
- 27.Kukla GJ, et al. Last Interglacial climates. Quat Res. 2002;58(1):2–13. [Google Scholar]
- 28.Tittensor DP, et al. Global patterns and predictors of marine biodiversity across taxa. Nature. 2010;466(7310):1098–1101. doi: 10.1038/nature09329. [DOI] [PubMed] [Google Scholar]
- 29.Yasuhara M, Hunt G, Dowsett HJ, Robinson MM, Stoll DK. Latitudinal species diversity gradient of marine zooplankton for the last three million years. Ecol Lett. 2012;15(10):1174–1179. doi: 10.1111/j.1461-0248.2012.01828.x. [DOI] [PubMed] [Google Scholar]
- 30.Sunday JM, Bates AE, Dulvy NK. Thermal tolerance and the global redistribution of animals. Nat Climate Change. 2012;2(9):686–690. [Google Scholar]
- 31.Andersen KK, et al. North Greenland Ice Core Project members High-resolution record of Northern Hemisphere climate extending into the last interglacial period. Nature. 2004;431(7005):147–151. doi: 10.1038/nature02805. [DOI] [PubMed] [Google Scholar]
- 32.Turney CSM, Jones RT. Does the Agulhas Current amplify global temperatures during super-interglacials? J Quaternary Sci. 2010;25(6):839–843. [Google Scholar]
- 33.Yin QZ, Berger A. Insolation and CO2 contribution to the interglacial climate before and after the Mid-Brunhes Event. Nat Geosci. 2010;3(4):243–246. [Google Scholar]
- 34.Yin QZ, Berger A. Individual contribution of insolation and CO2 to the interglacial climates of the past 800,000 years. Clim Dyn. 2012;38(3):709–724. [Google Scholar]
- 35.Pandolfi JM. Limited membership in Pleistocene reef coral assemblages from the Huon Peninsula, Papua New Guinea: Constancy during global change. Paleobiology. 1996;22(2):152–176. [Google Scholar]
- 36.Visser K, Thunell R, Stott L. Magnitude and timing of temperature change in the Indo-Pacific warm pool during deglaciation. Nature. 2003;421(6919):152–155. doi: 10.1038/nature01297. [DOI] [PubMed] [Google Scholar]
- 37.Loarie SR, et al. The velocity of climate change. Nature. 2009;462(7276):1052–1055. doi: 10.1038/nature08649. [DOI] [PubMed] [Google Scholar]
- 38.Burrows MT, et al. The pace of shifting climate in marine and terrestrial ecosystems. Science. 2011;334(6056):652–655. doi: 10.1126/science.1210288. [DOI] [PubMed] [Google Scholar]
- 39.Yuan D, et al. Timing, duration, and transitions of the last interglacial Asian monsoon. Science. 2004;304(5670):575–578. doi: 10.1126/science.1091220. [DOI] [PubMed] [Google Scholar]
- 40.Hönisch B, et al. The geological record of ocean acidification. Science. 2012;335(6072):1058–1063. doi: 10.1126/science.1208277. [DOI] [PubMed] [Google Scholar]
- 41.Hoegh-Guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318(5857):1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 42.Muhs DR, Simmons KR, Schumann RR, Halley RB. Sea-level history of the past two interglacial periods: New evidence from U-series dating of reef corals from south Florida. Quat Sci Rev. 2011;30(5-6):570–590. [Google Scholar]
- 43.Thomas CD, Franco AMA, Hill JK. Range retractions and extinction in the face of climate warming. Trends Ecol Evol. 2006;21(8):415–416. doi: 10.1016/j.tree.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Doak DF, Morris WF. Demographic compensation and tipping points in climate-induced range shifts. Nature. 2010;467(7318):959–962. doi: 10.1038/nature09439. [DOI] [PubMed] [Google Scholar]
- 45.Bellwood DR, Hughes TP. Regional-scale assembly rules and biodiversity of coral reefs. Science. 2001;292(5521):1532–1535. doi: 10.1126/science.1058635. [DOI] [PubMed] [Google Scholar]
- 46.Gattuso JP, et al. Light availability in the coastal ocean: Impact on the distribution of benthic photosynthetic organisms and their contribution to primary production. Biogeosciences. 2006;3:489–513. [Google Scholar]
- 47.McKinney ML, Oyen CW. Causation and nonrandomness in biological and geological time series: Temperature as a proximal control of extinction and diversity. Palaios. 1989;4:3–15. [Google Scholar]
- 48.Kiessling W. Long-term relationships between ecological stability and biodiversity in Phanerozoic reefs. Nature. 2005;433(7024):410–413. doi: 10.1038/nature03152. [DOI] [PubMed] [Google Scholar]
- 49.Kampstra P. Beanplot: A boxplot alternative for visual comparison of distributions. J Stat Software. 2008;28(1):1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.