Abstract
Progression through the various stages of skin tumorigenesis is correlated with an altered expression of the integrin α3β1, suggesting that it plays an important role in the tumorigenic process. Using epidermis-specific Itga3 KO mice subjected to the 7,12-dimethylbenzanthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate two-stage skin carcinogenesis protocol, we demonstrate that efficient tumor development is critically dependent on the presence of α3β1. In the absence of α3β1, tumor initiation is dramatically decreased because of increased epidermal turnover, leading to a loss of DMBA-initiated label-retaining keratinocytes. Lineage tracing revealed emigration of α3-deficient keratinocytes residing in the bulge of the hair follicle toward the interfollicular epidermis. Furthermore, tumor growth and cell proliferation were strongly reduced in mice with an epidermis-specific deletion of Itga3. However, the rate of progression of α3β1-null squamous cell carcinomas to undifferentiated, invasive carcinomas was increased. Therefore, α3β1 critically affects skin carcinogenesis with opposing effects early and late in tumorigenesis.
Keywords: skin cancer, cell adhesion, cell migration, laminin receptor, hair cycling
Skin cancer is the most common form of cancer among white populations, with basal cell carcinomas and squamous cell carcinomas (SSCs) being the most common subtypes. Although early detection and surgical resection can prevent most complications associated with this disease, SCCs frequently metastasize and then cannot be effectively treated. Understanding the molecular basis of skin tumorigenesis is a prerequisite for future prevention and therapy. The well-characterized 7,12-dimethylbenzanthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA) protocol models the multistep nature of human skin carcinogenesis in mice. Oncogenic mutations (e.g., Hras), induced by a single treatment with the carcinogen DMBA confer growth advantage to the initiated cells, which form benign papillomas under repetitive tumor-promoting treatments with the phorbol ester TPA. Subsequent progression to SCCs involves mutation of Trp53 and trisomization of chromosomes 6 and 7 (1–5).
Integrins are αβ heterodimeric adhesion receptors that play an important role in maintaining epithelial integrity. In the skin, the major integrins α2β1, α3β1, and α6β4 connect the cytoskeleton of basal keratinocytes to the underlying basement membrane (6). Besides their key function in skin physiology, these integrins also have been implicated in the development and progression of SCCs (7). Mouse models in which different integrins are either overexpressed in the suprabasal epidermis or mutated in the whole animal showed altered susceptibilities to chemically induced skin tumorigenesis (8–10). Increased expression of α2β1, α3β1, and α6β4 has been observed in hyperproliferating human cancers of the head and neck (11). Integrins thus seem to play a role in initiation and promotion of tumors. Surprisingly, the role of α3β1 in basal keratinocytes in skin tumorigenesis has not been investigated experimentally. To study its contribution to initiation, growth, and malignant progression of skin tumors, we subjected epidermis-specific Itga3 KO mice (Itga3 eKO) to chemically induced skin carcinogenesis.
Results
Reduced Two-Stage Skin Carcinogenesis in the Absence of Itga3.
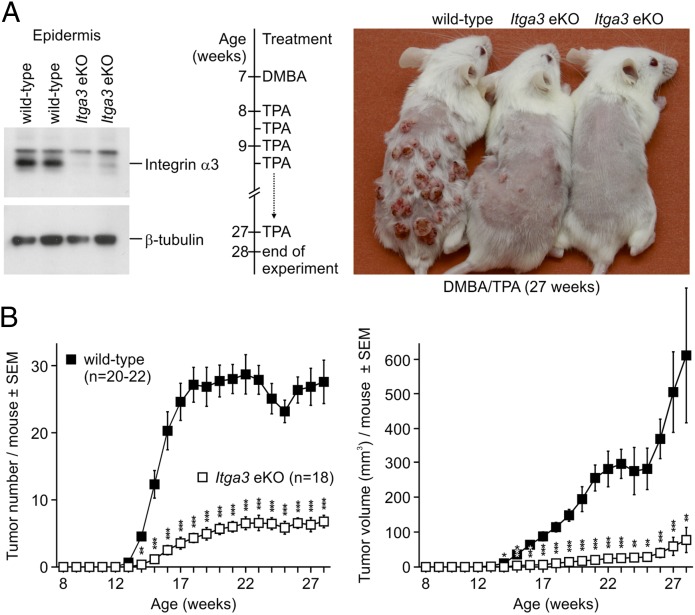
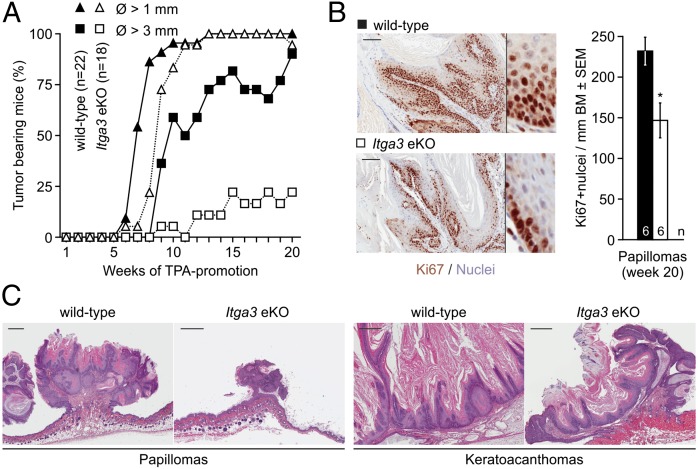
Skin carcinogenesis of epidermis-specific Itga3 KO mice (Itga3fl/fl; K14-Cre+, referred to as Itga3 eKO) and Cre-negative littermates (Itga3fl/fl; K14-Cre−, referred to as WT) was induced once with DMBA and promoted twice weekly with TPA (1) (Fig. 1A). Whereas WT mice readily developed tumors, Itga3 eKO mice showed a significant reduction in tumor number and volume (Fig. 1B; Fig. S1). The percentage of mice bearing tumors with a diameter of more than 1 mm was the same in the two groups, whereas tumors larger than 3 mm in diameter occurred almost exclusively in WT mice (Fig. 2A). Ki67 labeling showed reduced tumor cell proliferation in the DMBA/TPA-treated Itga3 eKO mice compared with that in WT littermates (Fig. 2B). Histological analysis revealed that most of the tumors formed in WT and Itga3 eKO mice were benign papillomas (>99%) and keratoacanthomas (Fig. 2C). As expected, the majority of papillomas tested carried the activating c.182A > T mutation in codon 61 of the Hras1 proto-oncogene (Fig. S2) (3). Malignant tumors such as SCCs or spindle cell carcinomas occurred at a frequency of <1% and were restricted to WT mice (Fig. S3). Malignant conversion in the Itga3 eKO group may have remained undetected because of the low overall total tumor number (<100). Together these data show that Itga3 eKO mice rarely develop tumors in response to DMBA/TPA, and if formed, proliferation is decreased.
Fig. 1.
Impaired skin carcinogenesis of Itga3 eKO mice. (A) From left to right: Western blot showing the absence of α3 in the epidermis of Itga3 eKO mice, timeline of the applied two-stage skin carcinogenesis protocol, and macroscopic image of three littermates at the end of the experiment. (B) Tumor number and volume are significantly diminished in Itga3 eKO mice compared with those in WT littermates after DMBA/TPA-induced skin carcinogenesis (*P < 0.05, **P < 0.01, ***P < 0.001).
Fig. 2.
Incidence and histology of benign tumors. (A) WT and Itga3 eKO mice develop at least one tumor with a diameter larger than 1 mm. In contrast, tumors with a diameter larger than 3 mm occur in virtually all WT but only in 25% of Itga3 eKO mice. (B) Proliferation is significantly diminished in papillomas of DMBA/TPA-treated Itga3 eKO mice compared with those of WT littermates. (Scale bars, 100 μm.) (Inset magnification, 5×; *P < 0.05.) (C) Histology of representative papillomas and keratoacanthomas. Tumors are histologically similar but are larger in WT mice than in Itga3 eKO mice. (Scale bars, 500 μm.)
Exit of Label-Retaining Cells from Their Niches and Increased Epidermal Turnover in Itga3 eKO Mice.
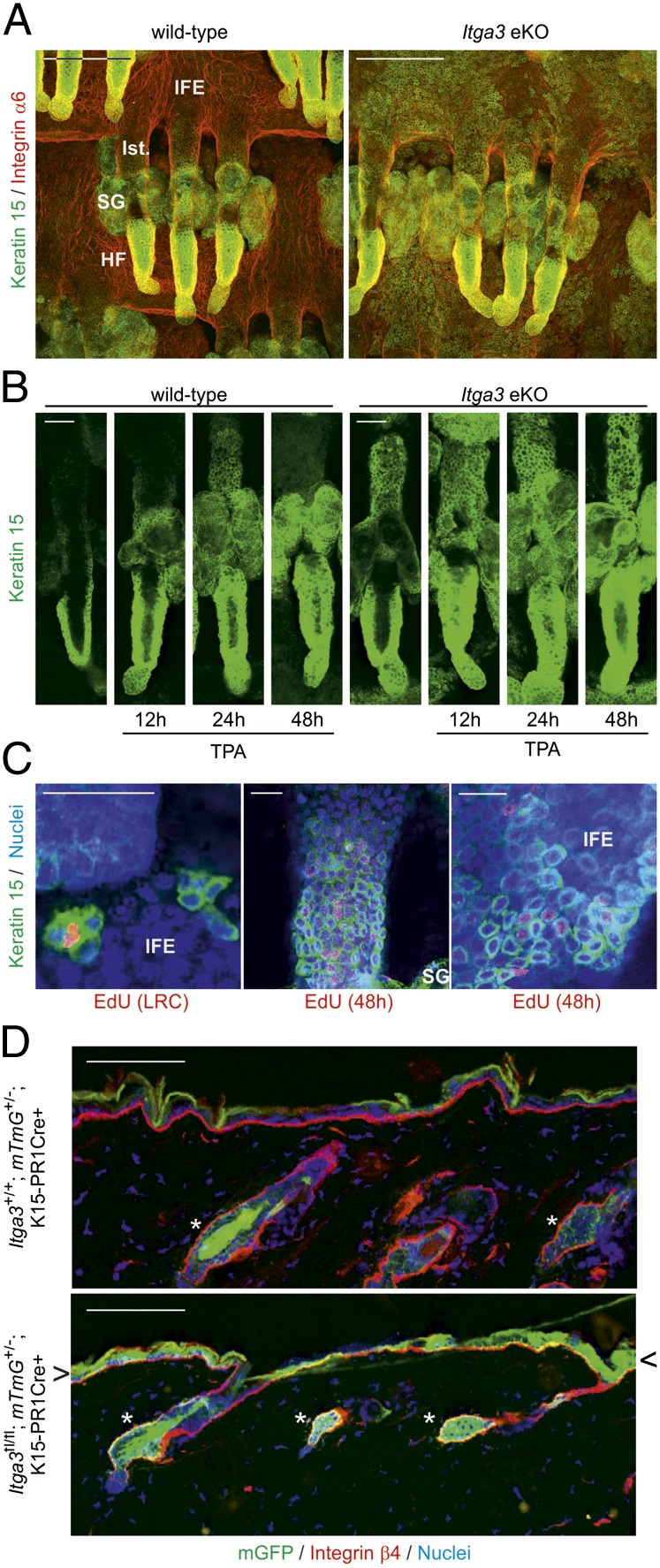
The efficacy of chemically induced skin carcinogenesis is critically dependent on the hair cycle phase in which the mice are treated with DMBA (12). We found no difference in the duration of the first two synchronized hair cycles between WT and Itga3 eKO mice. (Fig. S4 A and B). A second hypothesis that could explain the difference in tumor development concerns the fate of slow-cycling, label-retaining cells (LRCs) of hair follicles (HFs) and interfollicular epidermis (IFE). These cells are thought to be the primary source of chemically induced skin tumors, as DMBA-initiated cells persist and can efficiently advance to tumors even after extended periods of time (13, 14). LRCs have indeed been shown to retain mutagenic DMBA-DNA adducts (15) and proliferate on TPA treatment (16). Using a BrdU label retention assay, we found the number of BrdU-positive LRCs to be strongly reduced in the HFs of Itga3 eKO mice compared with that in WT mice (Fig. 3A). No difference was observed immediately after BrdU injection (Fig. S5A). Although the number of LRCs in the IFE did not differ significantly between the two groups, LRCs were regularly found suprabasally in Itga3 eKO mice en route to terminal differentiation (Fig. 3A; Fig. S5A). Using double-label pulse-chase (5-ethynyl-2-deoxyuridine (EdU), 4 wk; BrdU, 2 h) experiments, we also showed that several LRCs in the IFE were mitotically active (Fig. 3B). Virtually no LRCs were found in whole-mounted HFs of Itga3 eKO mouse tails after a single dose of TPA (Fig. 3C). Based on these results, we hypothesized that epidermal turnover may be increased in the absence of Itga3 and found this indeed to be the case for the basal proliferative layer (14-d BrdU pulse-chase; Fig. 3D; Fig. S5B), as well as the uppermost cornified layer (dansyl chloride painting; Fig. 3E; Fig. S5C). Interfollicular epidermal width and proliferation were equal in Itga3 eKO and WT mice regardless of whether short-term DMBA/TPA treatments were applied (Fig. 3F). The number of apoptotic cells 24 h after DMBA was negligible and did not differ significantly between the two groups (Fig. 3F). In contrast, follicular proliferation was increased in the absence of α3 (Fig. 3G). To determine whether loss of LRCs has functional consequences on HF homeostasis, we treated the back skin of Itga3 eKO and WT mice on a C57BL/6 background semiweekly with TPA for 5 mo. Hair growth and HF density were diminished in Itga3 eKO mice (Fig. S4C).
Fig. 3.
Loss of label-retaining cells and increased epidermal turnover in Itga3 eKO mice. (A) The number of BrdU-LRCs is significantly reduced in back skin HFs of 8- and 14-wk-old Itga3 eKO mice compared with that in HFs of WT mice. LRCs occur almost twice less frequently in the IFE of Itga3 eKO mice (*P < 0.05). (B) LRCs are present in the IFE of Itga3 eKO mice and can proliferate (arrowheads) [nonspecific staining in the sebaceous glands (SGs)]. (Scale bar, 50 μm.) (C) Whole mounts of tail epidermis 24 h after a single application of TPA. Whereas a large number of LRCs are still present in the HF bulge of a WT mouse, very few LRCs remain in the HF of an Itga3 eKO littermate (nonspecific staining in SG). (Scale bars, 50 μm.) (D and E) Increased epidermal turnover and desquamation in Itga3 eKO mice is shown by fewer BrdU+ cells in the Itga3-null IFE 14 d after pulse and the accelerated loss of dansyl chloride from the epidermis of Itga3 eKO mice, respectively (*P < 0.05, **P < 0.01, ***P < 0.001). (Scale bar, 20 μm.) (F) Short-term treatment of mouse back skin with TPA induces hyperproliferation, which does not significantly differ between Itga3 eKO mice and WT littermates. DMBA treatment causes apoptosis in very few cells of the IFE of WT and Itga3 eKO mice. Treatments were a single dose of TPA, effect analyzed 24 h later; a single dose of DMBA followed by four doses of TPA spread evenly over 14 d, effect analyzed 24 h later; the respective vehicle controls (acetone); treated and analyzed in parallel; a single dose of DMBA, number of apoptotic cells (active caspase-3 IHC) analyzed 24 h later. (G) Increased proliferation in HFs of Itga3 eKO compared with that in HFs of WT mice (*P < 0.05). (Scale bar, 50 μm.)
Lineage Tracing of Keratin 15–Positive Cells.
Immunofluorescent analysis of Itga3 eKO skin revealed the presence of a substantial number of keratin 15–positive (Krt15+) keratinocytes in the infundibulum and IFE, whereas other HF markers were normally expressed (Fig. 4A; Fig. S6A). TPA treatment increased this number and caused a temporary efflux of Krt15+ keratinocytes from the HFs of WT littermates (Fig. 4B). In line with our previous data, we also found LRCs and transit amplifying cells among the Krt15+ IFE cell population (Fig. 4C). Microarray analysis showed identical Krt15 mRNA levels in the IFE of WT and Itga3 eKO mice (Fig. S7), making it unlikely that IFE keratinocytes expressed Krt15 de novo. The presence of Krt15+ keratinocytes in the adult IFE has thus far only been observed during wound healing (Fig. S8) (17, 18). Because adolescent Itga3 eKO mice occasionally display microblisters in the IFE, the loss of LRCs might be the result of a wound healing response (19, 20). To test this, we specifically deleted Itga3 in Krt15+ keratinocytes of telogen HFs and traced their progeny using a fluorescent Cre-based reporter system (21, 22). As shown in Fig. 4D, 10 d after Cre expression was induced in Krt15+ keratinocytes with the progesterone analog RU486, GFP+ cells were found exclusively in HFs of Itga3+/+; mTmG+/−; Krt1-15-CrePR1+ mice. In contrast, in similarly treated Itga3fl/fl; mTmG+/−; Krt1-15-CrePR1+ littermates, GFP+ cells were not only present in HFs but also in the IFE. Keratinocytes in the bulge of HFs lacking α3β1 can thus exit their niche and contribute to epidermal differentiation. Even though these cells remain positive for keratin 15 in the IFE, de novo expression of IFE-keratins 1, 5, and 10 and loss of HF-keratin 6 suggest that normal differentiation indeed occurs (Fig. S6B). In the context of skin carcinogenesis, it seems thus plausible that DMBA-initiated cells lacking α3β1 exit their compartment and terminally differentiate before they can acquire additional mutations that would lead to the onset of cancer.
Fig. 4.
Keratin 15–positive cells from the HF bulge exit their niche in the absence of Itga3. (A) HF and IFE of whole-mounted Itga3-null tails are Krt15+, whereas this cell population is confined to the HF bulge in WT littermates (Ist., isthmus). (Scale bar, 100 μm.) (B) Keratin 15 stainings of whole-mounted tail HFs at indicated time points after single TPA doses. Twenty-four hours after TPA, Krt15+ cells have migrated out of the bulge into the isthmus of WT HFs. In Itga3 eKO mice, basal efflux of Krt15+ cells increases further on TPA stimulation. (Scale bar, 50 μm.) (C) (Left) EdU+ LRC in IFE is Krt15+. (Center and Right) Forty-eight hours after pulse, EdU+ Krt15+ cells are present in the isthmus and IFE. (Scale bar, 50 μm.) (D) HF bulge cells from the back skin of Itga3fl/fl; mTmG+/−; K1-15-CrePR1+ mice leave their compartment (asterisk) and migrate into the IFE (arrowheads). They remain in the bulge region (asterisk) in WT littermates. (Scale bar, 100 μm.)
α3β1 Affects Migration, Adhesion, and Proliferation of (Transformed) Keratinocytes.
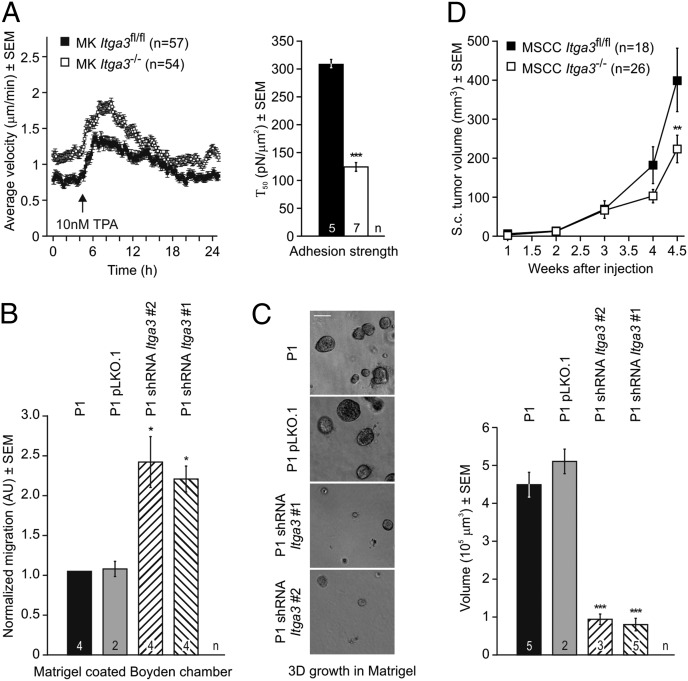
To directly assess the role of α3β1 in keratinocyte migration, we seeded normal mouse keratinocytes (MKs) ± Itga3 sparsely on laminin-332 and observed that the Itga3-null keratinocytes migrated faster throughout the experiment, with a temporary increase in the migration speed in response to TPA (Fig. 5A). As previously suggested (20), we noted a decrease in adhesion strength of keratinocytes lacking α3 (Fig. 5A). We confirmed the negative effect of α3β1 on migration in the precancerous papilloma cell line P1. On stable knockdown of Itga3 (Fig. S9), P1 cells migrated more efficiently through matrigel-coated filters (Fig. 5B). In addition, P1 cells lacking α3 formed smaller spheroids when grown in 3D matrigel (Fig. 5C). Similarly, mouse squamous carcinoma cells (MSCCs) lacking Itga3 generated 40% smaller tumors upon s.c. injection in mice than WT parental cells (Fig. 5D; Fig. S10F). As shown earlier, proliferation of untransformed keratinocytes is not affected by the absence of α3 (20) (Fig. 3F), indicating that only transformed keratinocytes require the presence of α3β1 for efficient proliferation.
Fig. 5.
Absence of α3 affects migration, adhesion, and proliferation in vitro. (A) Single cell migration (Left) and adhesion assay (Right) of untransformed MKs on laminin-332. MKs lacking α3 migrate faster than their parental cells, whereas both lines transiently increase their migration speed on TPA treatment. Adhesion to laminin-332 after 24 h is significantly diminished in the absence of α3 (Τ50 refers to the force at which 50% of cells detached, ***P < 0.001). (B and C) Stable knockdown of Itga3 increased migration of the papilloma cell line P1 through a matrigel-coated Boyden chamber and decreased spheroid formation in matrigel (cell lines characterized in Fig. S9; *P < 0.05, ***P < 0.001). (Scale bar, 50 μm.) (D) MSCCs lacking Itga3 (characterized in Fig. S10) develop smaller tumors than the parental Itga3fl/fl MSCC line when injected s.c. into nude mice.
Loss of α3 Potentiates Tumor Progression.
Because the incidence of tumor progression to SCCs after DMBA/TPA treatment was too low to deduce a function of α3β1, we subjected WT and Itga3 eKO mice to the complete carcinogenesis protocol of weekly DMBA applications. As in the DMBA/TPA model, we observed significantly fewer tumors in Itga3 eKO mice than in WT littermates (Fig. 6A), with the great majority of tumors being histologically classified as SCCs in both groups. However, the malignancy grade of tumors arising in Itga3 eKO mice was significantly higher (Fig. 6 B and C; Fig. S11). In cell lines representative of several stages of skin carcinogenesis (23, 24), we found α3 protein levels to be inversely correlated with the progression stage (Fig. 6D). Of note, in these cell lines, α3 was a more precise indicator of the malignancy grade than E-cadherin (Fig. 6D). SCCs of WT mice remained well differentiated until they reached a diameter of 5.0 ± 0.78 mm, whereas well-differentiated SCCs of Itga3 eKO mice only reached 2.1 ± 0.05 mm (P < 0.003, Student t test) before progressing. Dedifferentiation in the absence of α3 thus occurs fairly early during tumorigenesis. Our findings indicate a requirement for α3β1 in tumor initiation and early growth, whereas loss of α3β1 enhances progression of SCCs at later stages.
Fig. 6.
Loss of Itga3 results in fewer, but less differentiated, carcinomas after DMBA tumorigenesis. (A) Fewer tumors are formed in Itga3 eKO mice during a 25-wk regimen of DMBA-only carcinogenesis compared with WT littermates (*P < 0.05). (B) Pie chart of the tumor differentiation showing a high percentage of poorly differentiated Itga3-null carcinomas. The difference in malignancy grades of tumors in WT and Itga3 eKO mice is statistically significant (P < 0.001; χ2 test; see Fig. S11 for details). (C) (Upper) Representative histological examples of well, moderately, and poorly differentiated SCCs found in WT and Itga3 eKO mice after complete carcinogenesis. With the exception of larger well-differentiated tumors in WT mice, no differences were observed. (Lower) Histology of spindle cell and anaplastic carcinomas found in Itga3 eKO mice after a complete carcinogenesis regimen. (Scale bar, 100 μm.) (D) Western blot analysis showing high α3 protein levels in the papilloma cell line P1, moderate levels in the squamous carcinoma cell line B9, and virtually no α3 in the spindle carcinoma cell line A5. E-cadherin and β-tubulin are shown as controls.
Discussion
In this study, we investigated the role of α3β1 in a two-stage skin carcinogenesis model and show that mice lacking the epidermal integrin α3β1 are considerably less susceptible to skin carcinogenesis than WT mice. Whereas all mice developed benign tumors (papillomas and keratoacanthomas), the total number of tumors formed in the Itga3 eKO mice was significantly decreased. Furthermore, the few tumors formed in Itga3 eKO mice were much smaller than those formed in WT mice. These results suggest a role of α3β1 in both tumor initiation and proliferation of transformed cells.
As an underlying cause for the effect of α3β1 on tumor initiation, we excluded altered hair cycling in Itga3 eKO mice and subsequently focused on the fate of slow-cycling LRCs as the proposed target of DMBA and on the cell of origin of the tumors. In the absence of epidermal α3β1, the number of LRCs was significantly reduced, and LRCs were regularly found suprabasally. After short-term treatment (48 h) with TPA, LRCs were virtually absent in Itga3 eKO mice. We therefore suggest that the DMBA-initiated cells lacking α3β1 leave their compartment and terminally differentiate, reducing the probability to accumulate the necessary mutations required for tumor onset. Indeed, we found many Krt15+ keratinocytes outside their normal niche (the HF bulge) in Itga3 eKO mice, as shown by lineage tracing experiments of α3-deficient Krt15+ HF keratinocytes. The increased mobility and subsequent loss of Krt15+ label-retaining HF cells lacking α3 led to a decrease in HF density with age, which was aggravated after long-term TPA treatment. Notably, TPA was able to induce Krt15+ cell efflux from HFs in WT mice, possibly because of hemidesmosome instability following phosphorylation of the β4 subunit of integrin α6β4 leading to decreased adhesion strength and increased migration (25, 26). TPA indeed temporarily increased the velocity of WT keratinocytes in vitro. In the absence of α3β1, both the efflux of Krt15+ cells in vivo and their migration speed in vitro were increased under all conditions. In line with previously published data, we provide evidence that the increased migration speed of Itga3 KO keratinocytes is caused by decreased adhesion strength of these cells to the underlying matrix (20). Together these findings emphasize the importance of α3β1 in epidermal turnover and the long-term maintenance of Krt15+ LRCs keratinocytes in the HF bulge.
The fact that efficient formation of large tumors depends on α3β1 indicates that the integrin confers a proliferative growth advantage to transformed keratinocytes. Indeed, Itga3 KO papillomas contained significantly fewer proliferating Ki67+ keratinocytes than WT papillomas. Furthermore, we found that SCCs lacking α3β1 give rise to significantly smaller tumors when injected s.c. into nude mice. The positive influence of α3β1 on tumor growth is most likely direct, because efficient spheroid formation of pure papilloma cell populations in vitro critically depends on the presence of α3β1. A similar effect on proliferation of skin tumors has been observed in β1 mutant mice (8). A role of α3β1 in supporting cell proliferation has already been suggested in previous reports. For example, it was shown that the expression of α3β1 in suprabasal keratinocytes increases during hyperproliferative pathological conditions (6, 11). Furthermore, engineered suprabasal expression of α3β1 in mice increased BrdU incorporation on TPA treatment (9), whereas deletion of α3β1 from transformed keratinocytes caused reduced tumor growth (27). Although the exact mechanism responsible for the contribution of α3β1 to tumor cell proliferation remains to be determined, the importance of integrins and their associated signaling pathways in skin carcinogenesis is further demonstrated by the fact that FAK and Src, two kinases that are activated downstream of integrin engagement, critically contribute to experimental mouse skin carcinogenesis (8, 28). Another example of transformed keratinocytes being critically dependent on integrin signaling is that the laminin-binding integrin α6β4 confers proliferative advantage to Ras-transformed keratinocytes (29), whereas the lack of β4 renders Ras-IκBα–transformed keratinocytes resistant to tumor formation (30).
Unfortunately we could not deduce a function of α3β1 during tumor progression from the results of the initial DMBA/TPA experiment because the rate of progression to malignant SCCs was too low. We were, however, able to investigate the role of α3β1 in the development and progression of SCCs using the full carcinogenesis protocol of DMBA-only treatments. Tumor number was again significantly reduced in Itga3 eKO mice, which we attribute to a loss of DMBA-initiated LRCs. The fact that the difference in the number of tumors is less pronounced than after DMBA/TPA treatments is consistent with the finding that the efflux of Krt15+ cells lacking α3β1 following TPA treatment is increased. We found that the absence of α3β1 strongly promotes the malignant progression of SCCs. The few well-differentiated Itga3-null SCCs were much smaller than those in WT mice, indicating that dedifferentiation occurs at an earlier time point during tumorigenesis. In cell lines representative of several stages of skin carcinogenesis (23, 24), α3 protein levels are inversely correlated with progression stage. We postulate that α3β1, as a constitutively expressed basal keratinocyte integrin, allows transformed keratinocytes in SCCs of WT mice to maintain their relative integrity and permit the formation of larger clusters of more differentiated cells compared with SCC keratinocytes of Itga3 eKO mice, whose adhesion to the basement membrane is destabilized. Altogether, our studies identify α3β1 as a critical factor for the initiation and progression of chemically induced skin tumors.
Materials and Methods
Animal Experiments.
According to Mouse Genome Informatics (Jackson Laboratory), the names of epidermal Itga3 KO mice are Itga3tm1Son/tm1Son; Krt14tm1(cre)Wbm on FVB(N6) or C57BL/6(N10) (31, 32). Krt15+ cell-specific Itga3 KO mice carrying the mTmG reporter were Itga3tm1Son/tm1Son; mTmG+/−; Tg(Krt1-15-cre/PGR)22Cot on FVB(N3) (21, 22). For DMBA/TPA-induced carcinogenesis, the backs of 7-wk-old mice were shaved and treated with a single dose of DMBA (30 μg in 200 μL acetone; Sigma) followed by semiweekly applications of TPA (12.34 μg in 200 μL acetone; Sigma) for 20 wk. For DMBA-only carcinogenesis, the backs of 7-wk-old mice were shaved and treated with weekly doses of DMBA (30 μg in 200 μL acetone) for up to 25 wk. For LRC tracing, mice were injected i.p. with 6 × 50 μg BrdU or EdU every 12 h from postnatal day 3 and chased for up to 14 wk (33). Detailed descriptions of all other animal experiments can be found in SI Materials and Methods.
Cell Lines.
All cell lines were grown at 37 °C in a humidified atmosphere of 5% CO2 in air. Migration and adhesion assays of MK Itga3fl/fl and MK Itga3−/− (6) on a laminin-332 rich matrix deposited by Rac11-P cells were performed as previously described (20, 34–36). Murine skin cancer cell lines P1, B9, and A5 were cultured as described (23, 24) (SI Materials and Methods). Stable Itga3 knockdown P1 cells were generated by lentiviral transduction of short RNA hairpins cloned into pLKO.1 vectors (clone 1: TRCN0000065998; clone 2: TRCN0000066002; Thermo Scientific Dharmacon RNAi Technologies) and sorted three times for lack of cell surface α3. Standard matrigel (BD) spheroid formation and Boyden chamber assays were performed as described in SI Materials and Methods. MSCC Itga3fl/fl were isolated from the sentinel lymph node of a SCC-bearing Itga3fl/fl mouse, treated with DMBA/TPA by mechanical disruption followed by collagenase digestion, and cultured in DMEM containing 10% FCS (Gibco). α3 was deleted using Adeno-Cre (37) to generate MSCC Itga3−/−.
Immunohistochemistry, Immunofluorescence, Immunoblotting, and FACS.
We applied a standard methodology that can be found in SI Materials and Methods and Table S1.
Supporting Information.
SI Materials and Methods includes detailed materials and methods. Fig. S1 shows most DMBA/TPA-treated WT and Itga3 eKO mice at the end of the carcinogenic protocol. Fig. S2 shows Hras1 status of papillomas. Fig. S3 depicts histological representations of progressed tumors following DMBA/TPA treatment of WT mice. Fig. S4 illustrates the synchronized hair cycle of WT and Itga3 eKO mice and the decrease of HF density in the absence of Itga3 after long-term TPA treatment. Fig. S5 shows histological examples of BrdU pulse-chase and dansyl chloride desquamation experiments. Fig. S6 shows immunofluorescent analysis of HF markers in WT and Itga3 eKO mice, as well as differentiation markers in GFP+ cells from Itga3fl/fl; mTmG+/−; Krt1-15-CrePR1+ mice. Fig. S7 shows the MA plot of a microarray comparing the IFE of WT and Itga3 eKO mice. Fig. S8 depicts the contribution of Krt15+ HF bulge cells to reepithelialization after wounding. Figs. S9 and S10 show characterization of P1 cells and MSCCs ± Itga3. Fig. S11 lists the detailed number of malignancies after the complete carcinogenesis protocol.
Supplementary Material
Acknowledgments
We thank Drs. W. Birchmeier, G. Cotsarelis, and L. Luo for providing mouse strains. We also thank all staff members of the facilities for animal maintenance, histology, digital microscopy, and flow cytometry at The Netherlands Cancer Institute for excellent technical assistance; and Drs. A. Berns and M. Ports for critically reading the manuscript. This work was supported by a grant from the Dutch Cancer Society.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204614110/-/DCSupplemental.
References
- 1.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: Fundamentals and applications. Nat Protoc. 2009;4(9):1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldaz CM, Trono D, Larcher F, Slaga TJ, Conti CJ. Sequential trisomization of chromosomes 6 and 7 in mouse skin premalignant lesions. Mol Carcinog. 1989;2(1):22–26. doi: 10.1002/mc.2940020104. [DOI] [PubMed] [Google Scholar]
- 3.Brown K, Buchmann A, Balmain A. Carcinogen-induced mutations in the mouse c-Ha-ras gene provide evidence of multiple pathways for tumor progression. Proc Natl Acad Sci USA. 1990;87(2):538–542. doi: 10.1073/pnas.87.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggeri B, et al. Alterations of the p53 tumor suppressor gene during mouse skin tumor progression. Cancer Res. 1991;51(24):6615–6621. [PubMed] [Google Scholar]
- 5.Scribner JD, Süss R. Tumor initiation and promotion. Int Rev Exp Pathol. 1978;18:137–198. [PubMed] [Google Scholar]
- 6.Margadant C, Charafeddine RA, Sonnenberg A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010;24(11):4133–4152. doi: 10.1096/fj.09-151449. [DOI] [PubMed] [Google Scholar]
- 7.Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer. 2006;6(3):175–183. doi: 10.1038/nrc1817. [DOI] [PubMed] [Google Scholar]
- 8.Meves A, et al. Beta1 integrin cytoplasmic tyrosines promote skin tumorigenesis independent of their phosphorylation. Proc Natl Acad Sci USA. 2011;108(37):15213–15218. doi: 10.1073/pnas.1105689108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owens DM, Watt FM. Influence of beta1 integrins on epidermal squamous cell carcinoma formation in a transgenic mouse model: alpha3beta1, but not alpha2beta1, suppresses malignant conversion. Cancer Res. 2001;61(13):5248–5254. [PubMed] [Google Scholar]
- 10.Owens DM, Romero MR, Gardner C, Watt FM. Suprabasal alpha6beta4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGFbeta signalling. J Cell Sci. 2003;116(Pt 18):3783–3791. doi: 10.1242/jcs.00725. [DOI] [PubMed] [Google Scholar]
- 11.Van Waes C, et al. Increase in suprabasilar integrin adhesion molecule expression in human epidermal neoplasms accompanies increased proliferation occurring with immortalization and tumor progression. Cancer Res. 1995;55(22):5434–5444. [PubMed] [Google Scholar]
- 12.Miller SJ, et al. Mouse skin is particularly susceptible to tumor initiation during early anagen of the hair cycle: Possible involvement of hair follicle stem cells. J Invest Dermatol. 1993;101(4):591–594. doi: 10.1111/1523-1747.ep12366045. [DOI] [PubMed] [Google Scholar]
- 13.Berenblum I, Shubik P. The persistence of latent tumour cells induced in the mouse’s skin by a single application of 9:10-dimethyl-1:2-benzanthracene. Br J Cancer. 1949;3(3):384–386. doi: 10.1038/bjc.1949.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stenbäck F, Peto R, Shubik P. Initiation and promotion at different ages and doses in 2200 mice. I. Methods, and the apparent persistence of initiated cells. Br J Cancer. 1981;44(1):1–14. doi: 10.1038/bjc.1981.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris RJ, Fischer SM, Slaga TJ. Evidence that the centrally and peripherally located cells in the murine epidermal proliferative unit are two distinct cell populations. J Invest Dermatol. 1985;84(4):277–281. doi: 10.1111/1523-1747.ep12265358. [DOI] [PubMed] [Google Scholar]
- 16.Morris RJ, Fischer SM, Slaga TJ. Evidence that a slowly cycling subpopulation of adult murine epidermal cells retains carcinogen. Cancer Res. 1986;46(6):3061–3066. [PubMed] [Google Scholar]
- 17.Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11(12):1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 18.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102(4):451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 19.DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137(3):729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margadant C, et al. Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009;122(Pt 2):278–288. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- 21.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22(4):411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 22.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 23.Burns PA, et al. Loss of heterozygosity and mutational alterations of the p53 gene in skin tumours of interspecific hybrid mice. Oncogene. 1991;6(12):2363–2369. [PubMed] [Google Scholar]
- 24.Haddow S, Fowlis DJ, Parkinson K, Akhurst RJ, Balmain A. Loss of growth control by TGF-beta occurs at a late stage of mouse skin carcinogenesis and is independent of ras gene activation. Oncogene. 1991;6(8):1465–1470. [PubMed] [Google Scholar]
- 25.Frijns E, Sachs N, Kreft M, Wilhelmsen K, Sonnenberg A. EGF-induced MAPK signaling inhibits hemidesmosome formation through phosphorylation of the integrin beta4. J Biol Chem. 2010;285(48):37650–37662. doi: 10.1074/jbc.M110.138818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Germain EC, Santos TM, Rabinovitz I. Phosphorylation of a novel site on the beta4 integrin at the trailing edge of migrating cells promotes hemidesmosome disassembly. Mol Biol Cell. 2009;20(1):56–67. doi: 10.1091/mbc.E08-06-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamar JM, Pumiglia KM, DiPersio CM. An immortalization-dependent switch in integrin function up-regulates MMP-9 to enhance tumor cell invasion. Cancer Res. 2008;68(18):7371–7379. doi: 10.1158/0008-5472.CAN-08-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLean GW, et al. Decreased focal adhesion kinase suppresses papilloma formation during experimental mouse skin carcinogenesis. Cancer Res. 2001;61(23):8385–8389. [PubMed] [Google Scholar]
- 29.Raymond K, Kreft M, Song JY, Janssen H, Sonnenberg A. Dual role of alpha6beta4 integrin in epidermal tumor growth: Tumor-suppressive versus tumor-promoting function. Mol Biol Cell. 2007;18(11):4210–4221. doi: 10.1091/mbc.E06-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dajee M, et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421(6923):639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- 31.Sachs N, et al. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006;175(1):33–39. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105(4):533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 33.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61(7):1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 34.Boettiger D. Quantitative measurements of integrin-mediated adhesion to extracellular matrix. Methods Enzymol. 2007;426:1–25. doi: 10.1016/S0076-6879(07)26001-X. [DOI] [PubMed] [Google Scholar]
- 35.Loerke D, et al. Quantitative imaging of epithelial cell scattering identifies specific inhibitors of cell motility and cell-cell dissociation. Sci Signal. 2012;5(231):rs5. doi: 10.1126/scisignal.2002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachs N, et al. Blood pressure influences end-stage renal disease of Cd151 knockout mice. J Clin Invest. 2012;122(1):348–358. doi: 10.1172/JCI58878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anton M, Graham FL. Site-specific recombination mediated by an adenovirus vector expressing the Cre recombinase protein: A molecular switch for control of gene expression. J Virol. 1995;69(8):4600–4606. doi: 10.1128/jvi.69.8.4600-4606.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.