Abstract
Cranial irradiation is widely used in cancer therapy, but it often causes cognitive defects in cancer survivors. Oxidative stress is considered a major cause of tissue injury from irradiation. However, in an earlier study mice deficient in the antioxidant enzyme extracellular superoxide dismutase (EC-SOD KO) showed reduced sensitivity to radiation-induced defects in hippocampal functions. To further dissect the role of EC-SOD in neurogenesis and in response to irradiation, we generated a bigenic EC-SOD mouse model (OE mice) that expressed high levels of EC-SOD in mature neurons in an otherwise EC-SOD–deficient environment. EC-SOD deficiency was associated with reduced progenitor cell proliferation in the subgranular zone of dentate gyrus in KO and OE mice. However, high levels of EC-SOD in the granule cell layer supported normal maturation of newborn neurons in OE mice. Following irradiation, wild-type mice showed reduced hippocampal neurogenesis, reduced dendritic spine densities, and defects in cognitive functions. OE and KO mice, on the other hand, were largely unaffected, and the mice performed normally in neurocognitive tests. Although the resulting hippocampal-related functions were similar in OE and KO mice following cranial irradiation, molecular analyses suggested that they may be governed by different mechanisms: whereas neurotrophic factors may influence radiation responses in OE mice, dendritic maintenance may be important in the KO environment. Taken together, our data suggest that EC-SOD plays an important role in all stages of hippocampal neurogenesis and its associated cognitive functions, and that high-level EC-SOD may provide protection against irradiation-related defects in hippocampal functions.
Cranial irradiation is widely used as a treatment modality for patients with primary or metastatic brain tumors (1–3), and is also used as a prophylactic treatment to prevent metastases of high-risk tumors to the nervous system (4). Although effective, cranial irradiation is associated with various complications or side effects in cancer survivors (1–3). One of the severe complications is neurocognitive impairment, which can include defects in executive functions and learning and memory (3). Neurocognitive impairments occur in both adults and children and are generally associated with higher doses and younger age (3). The pathogenesis of radiation-induced neurocognitive impairment is not completely understood, but recent studies suggest that suppressed hippocampal neurogenesis (5, 6), increased hippocampal neuronal apoptosis (7, 8), and reduced growth hormone secretion (9, 10) may be involved.
The production of reactive oxygen species (ROS) is considered a major cause of radiation-induced tissue damage (11). Ionizing irradiation not only results in the acute generation of short-lived ROS, it also results in a persistent state of oxidative stress that extends up to several months or even years after irradiation (12, 13). Accordingly, animal and cell models with altered antioxidant capacities have been used to investigate the biochemical pathways involved in radiation-induced tissue and cell injuries, and experimental antioxidant-based therapies have been designed to protect normal tissues during radiation treatments (14).
Hippocampal neurogenesis is important for hippocampal-dependent functions of learning and memory and the process is exquisitely sensitive to suppression by various stressors, including radiation and oxidative stress (5, 6, 15). To determine if altered redox balance in the hippocampal microenvironment affects hippocampal neurogenesis and the associated functions of learning and memory, we used a knockout mouse model (KO) deficient in the extracellular antioxidant enzyme, EC-superoxide dismutase (EC-SOD), in an earlier study with cranial irradiation (13, 16). When examined at 3–4 mo of age, EC-SOD deficiency was associated with a significant suppression of baseline neurogenesis and impaired hippocampal-dependent cognitive functions (13, 16). Unexpectedly, EC-SOD deficiency also rendered the process less sensitive to radiation-induced changes. The underlining mechanism for this paradoxical finding was not clear, but preliminary studies ruled out up-regulation of major antioxidant enzymes (13). The results suggested that the interaction between redox balance and irradiation and their effects on hippocampal functions can be complex, and understanding how these elements work in concert may be a key to identifying strategies for radioprotection.
To manipulate redox balance in the hippocampus, we generated a mouse model with inducible EC-SOD transgenes (17). In the current study, we combined the inducible transgenes with EC-SOD KO and generated a bigenic mouse model, designated as the overexpressor (OE), with high levels of EC-SOD expressed only in Ca/calmodulin-dependent protein kinase- (CaMKII) positive neurons in an otherwise EC-SOD–deficient environment (17). Hippocampal neurogenesis generates new granule cells that are functionally integrated into the hippocampal network (18). Because granule cells are CaMKII-positive neurons and are the principal excitatory neurons in the dentate gyrus, the manipulation leads to an estimated four- to fivefold increase in EC-SOD activity in the hippocampal formation in OE mice (17). The OE mice were used to investigate the effects of altered EC-SOD levels at different stages of hippocampal neurogenesis and the functional consequences of learning and memory. Comparison between WT, OE, and KO mice revealed the importance of EC-SOD in progenitor cell proliferation, dendritic development, and long-term survival of newborn neurons. The study results also suggested that maintenance of the dendritic system following cranial irradiation was important for preservation of neurocognitive functions.
Results
EC-SOD Level Affects Neurocognitive Functions.
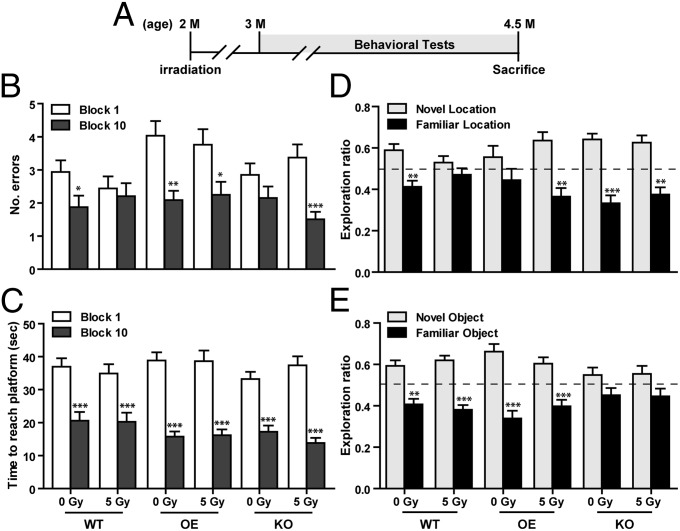
To determine if altered EC-SOD levels influenced neurocognitive functions, we carried out radial-arm water maze (RAWM) and novel location recognition (NLR) tests 1 mo after a single dose (5 Gy) of cranial irradiation (Fig. 1A). In these two studies, animals with normal spatial memory would make fewer mistakes by the end of the RAWM test and recognize objects in a novel location by increasing investigation time in the NLR test.
Fig. 1.
Effects of EC-SOD and irradiation on hippocampal-dependent learning and memory. (A) Experimental timeline. Sham and irradiated (5 Gy) mice were subjected to various behavioral tests starting at 3 mo of age, and all tests were concluded by 4.5 mo of age. (B and C) Radial-arm water maze. Comparison of errors made in arm entry (B) [F(1,168) = 32.11, P < 0.0001] and time spent to reach the platform (C) [F(1,214) = 198, P < 0.0001)] in the beginning (block 1) and the end (block 10) of the test. (D and E) NLR and NOR tests. Comparison of exploration ratio for investigation of an object in a novel vs. familiar location (D) [F(1,174) = 78.50, P < 0.0001)] or a novel vs. a familiar object (E) [F(1,172) = 105.9, P < 0.0001]. The dashed line in D and E represents exploration by chance (i.e., exploration ratio of 0.5) between novel and familiar location or object. Data are presented as mean ± SEM. Two-way ANOVA with Bonferroni posttest was carried out. *P < 0.05, **P < 0.01, ***P < 0.001 for postanalysis comparing between block 1 and block 10 or between novel and familiar location or object within each genotype and treatment. n = 17–20 mice per genotype per treatment.
RAWM results showed that sham-irradiated WT and OE mice significantly reduced incorrect arm entries (i.e., errors, 36% and 48% reduction, respectively) at the end of the test, whereas sham-irradiated KO were not able to make significant improvement (Fig. 1B). Following irradiation, WT mice lost the ability to reduce errors, but OE mice maintained (two-way ANOVA, Bonferroni posttest, t = 2.89, P < 0.05) and KO mice improved (t = 3.56, P < 0.01) their ability to significantly reduce errors made at the end of the test (Fig. 1B). Despite differences in the number of errors made before reaching the platform, all mice significantly reduced the time spent reaching the target platform (Fig. 1C). There was also no significant difference in the number of errors made or time required to reach the platform among animals within the same treatment group.
In NLR tests, sham-irradiated WT (t = 3.71, P < 0.01) and KO (t = 4.76, P < 0.001) mice recognized the novel placement of the object and spent significantly more time investigating the object in its new location, but sham-irradiated OE mice were indifferent to the new location (Fig. 1D and Fig. S1). Following irradiation, WT mice failed to recognize the novel location, but OE (t = 5.64, P < 0.001) and KO (t = 4.92, P < 0.001) mice all spent significantly more time investigating the object in its new location (Fig. 1D). Taken together, these data suggested that both sham-irradiated OE and KO mice had some learning defects; however, following irradiation, these particular defects in hippocampal-dependent learning were largely corrected. Similar to RAWM findings, no significant difference in the exploration ratio for the novel location was observed across different cohorts.
Whereas NLR relies on hippocampal functions, novel object recognition (NOR) is independent of hippocampus (19). Despite differences in NLR results, both sham and irradiated WT and OE mice had no trouble recognizing a new object in the NOR test and spent significantly more time investigating the new object (Fig. 1E and Fig. S1). On the other hand, sham and irradiated KO mice were not able to significantly discern the difference between a novel and a familiar object (Fig. 1E and Fig. S1).
Additionally, open field and elevated zero maze tests were carried out to determine motor activities and anxiety levels. Although sham-irradiated OE mice spent significantly less amount of time in the center 50% area of an open field, no significant differences were observed in the elevated zero maze paradigm (Fig. S2).
In the behavioral field, contextual fear conditioning is commonly used to test hippocampal-dependent learning. However, preliminary studies with OE and KO mice showed both to be more sensitive to tactile and heat stimulation (Fig. S3). Although there was no direct correlation, increased tactile and heat sensitivity suggested that OE and KO mice might be more sensitive to electrical stimulation in the contextual fear-conditioning paradigm, which might result in enhanced freezing response and affect the data interpretation. Consequently, a contextual fear-conditioning test was not performed with these mice.
EC-SOD Affects Progenitor Cell Proliferation and Long-Term Survival.
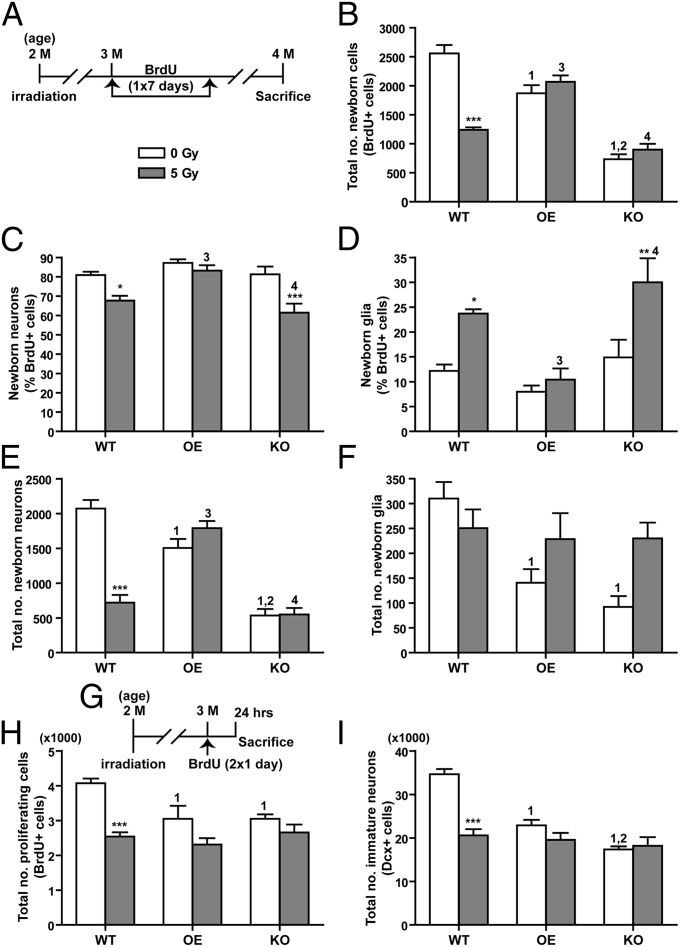
To determine if differences in neurocognitive functions were associated with changes in hippocampal neurogenesis, a 7-d BrdU injection protocol was carried out and the production and maturation of newborn cells in the subgranular zone (SGZ) of the dentate gyrus was assessed 4 wk after the first BrdU injection (Fig. 2A). Compared with sham-irradiated WT controls, there was an 81% reduction (t = 11.45, P < 0.001) in the total number of newly generated cells (BrdU+) in sham-irradiated KO mice (Fig. 2B). Reconstitution of high levels of EC-SOD in granule cells, on the other hand, substantially increased total number of BrdU+ cells in OE mice (OE vs. KO, t = 7.85, P < 0.001), although the level was still 27% lower than that in WT mice (t = 4.21, P < 0.001) (Fig. 2B). Irradiation led to a significant reduction in total BrdU+ cells in WT mice (51% reduction, t = 4.45, P < 0.001), but no significant changes were observed in irradiated OE or KO mice (Fig. 2B). Consequently, total BrdU+ cells in irradiated OE was 1.7-fold and 2.3-fold higher than that of irradiated WT and KO, respectively (Fig. 2B and Table S1).
Fig. 2.
Hippocampal neurogenesis following cranial irradiation. (A) Experimental timeline for identifying long-term survival of newly born cells. (B) Total number of mature BrdU+ cells in the SGZ of hippocampal dentate gyrus. There was a significant interaction between genotype and treatment [F(2,32) = 26.39, P < 0.0001]. Both irradiation [F(1,32) = 11.95, P = 0.0016] and genotype [F(2,32) = 70.74, P < 0.0001] played a significant role in the data variation. (C) Newly born neurons as percentage of BrdU+ cells in the SGZ. There was a significant interaction between genotype and treatment. Both irradiation [F(1,33) = 18.85, P = 0.0001] and genotype [F(2,33) = 8.82, P = 0.0009] played a significant role in the data variation. (D) Newly generated glia as percentage of BrdU+ cells in the SGZ. There was a significant interaction between genotype and treatment. Both irradiation [F(1,33) = 14.63, P = 0.0006] and genotype [F(2,33) = 9.38, P = 0.0006] played a significant role in the data variation. (E) Total number of newborn neurons (BrdU+/NeuN+ double-positive cells) in the SGZ. (F) Total number of newborn glia (BrdU+/GFAP+ cells) in the SGZ. (G) Experimental timeline for identifying proliferating cells with BrdU labeling. (H) Total number of BrdU+ cells, captured within a 24-h window, as a function of progenitor cell proliferation. There was a significant interaction between genotype and treatment [F(2,26) = 3.75, P = 0.037]. Both irradiation [F(1,26) = 26.45, P < 0.0001] and genotype [F(2,26) = 4.27, P = 0.0248] played a significant role in the data variation. (I) Total number of immature neurons (Dcx+ cells) in the SGZ at 1 mo following cranial irradiation. There was a significant interaction between genotype and treatment [F(2,24) = 26.58, P < 0.0001]. Genotype also played a significant role in the data variation [F(2,24) = 22.19, P < 0.0001]. Data are presented as mean ± SEM. Two-way ANOVA with Bonferroni posttest was carried out. *P < 0.05, ***P < 0.001 for comparison between 0 and 5 Gy within each genotype. 1, P < 0.05 compared with WT/0 Gy; 2, P < 0.05 compared with OE/0 Gy; 3, P < 0.05 compared with WT/5 Gy; 4, P < 0.05 compared with OE/5 Gy. n = 5–8 mice per genotype per treatment.
To determine the lineage preference of newborn BrdU+ cells, their identities as mature neurons (NeuN+) or astroglia (GFAP+) were examined. No significant difference in the percentage of BrdU+ cells that matured into neurons (BrdU+/NeuN+) or astroglia (BrdU+/GFAP+) was observed among the sham-irradiated groups (Fig. 2 C and D), suggesting that differences in EC-SOD levels did not affect lineage determination. Following irradiation, the percentage of BrdU+/NeuN+ cells declined significantly but the percentage of BrdU+/GFAP+ cells increased significantly in WT and KO mice (Fig. 2 C and D and Table S1). However, no significant changes were observed in irradiated OE mice (Fig. 2 C and D). After converting the percentage to total number of newborn neurons and glia, the profile of BrdU+/NeuN+ cell numbers (Fig. 2E) was similar to that of BrdU+ cells (Fig. 2B). On the other hand, the number of newborn astroglia in sham-irradiated WT was significantly higher than that in sham-irradiated OE and KO mice, but no significant difference in the number of BrdU+/GFAP+ cells was observed among the irradiated groups (Fig. 2F).
Depending on the activation state, activated microglia can be detrimental or beneficial to neurogenesis in adult brains (20–22). To know if the activated microglia population was altered by EC-SOD levels or irradiation, total number of CD68+ cells in the dorsal hippocampal area was determined. The number of CD68+ cells was comparable among all sham-irradiated groups and significant increases were observed in all three groups following irradiation, with OE mice showing the largest increase. Consequently, the number of CD68+ cells in irradiated OE mice was significantly higher than that in irradiated WT and KO mice (Fig. S4).
To determine if increased production and maturation of new neurons was because of increased progenitor cell proliferation or commitment to the neuronal lineage, a short-term BrdU labeling (Fig. 2G) was carried out to identify proliferating cells. The number of immature neurons (doublecortin; Dcx+ cells) was also determined. In sham-irradiated mice, the total number of BrdU+ cells counted in the SGZ of OE (t = 3.39, P < 0.01) and KO (t = 3.76, P < 0.01) mice was 75% that of WT mice (Fig. 2H); the total Dcx+ cells in OE (t = 5.86, P < 0.001) and KO (t = 8.63, P < 0.001) mice were 66% and 50% that of WT controls (Fig. 2I), respectively. Following irradiation, total numbers of BrdU+ and Dcx+ cells in WT mice were reduced by 38% (t = 4.79, P < 0.001) and 41% (t = 7.02, P < 0.001), respectively. OE and KO mice, on the other hand, did not show significant reductions in total BrdU+ or Dcx+ cells from irradiation (Fig. 2 H and I). Consequently, no significant difference in total number of BrdU+ or Dcx+ cells was observed among irradiated WT, OE, and KO mice. Taken together, the results suggested that EC-SOD deficiency decreased progenitor cell proliferation and long-term survival of newborn cells in the SGZ, that reconstitution of high levels of EC-SOD in granule cells significantly enhanced long-term survival of newborn cells, and that EC-SOD OE and KO mice were not susceptible to radiation-induced suppression of neurogenesis.
EC-SOD and Irradiation Affect the Dendritic System.
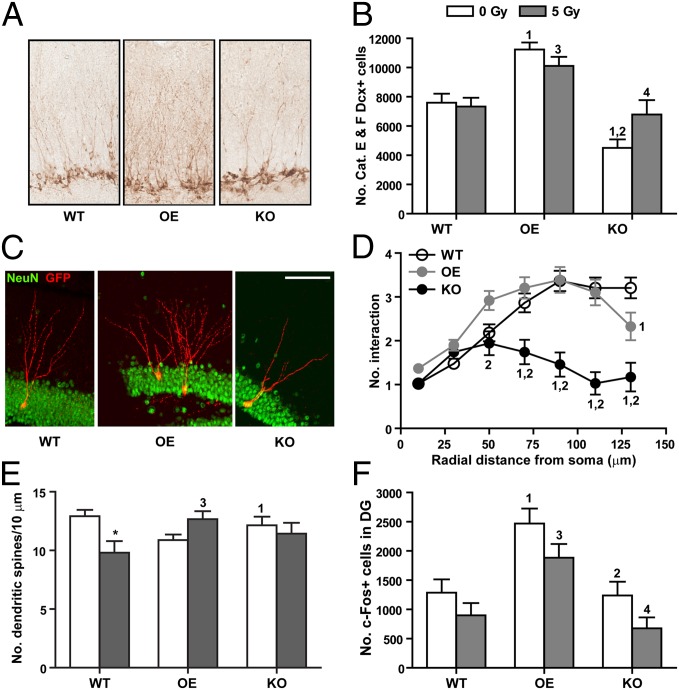
We noticed that Dcx+ cells from OE mice had a more elaborate pattern of dendritic arborization (Fig. 3A), suggesting that Dcx+ cells in OE mice may be more mature. Examination of Dcx+ cells with secondary dendritic branching (i.e., categories E and F cells) (23) showed the number of mature Dcx+ cells to be significantly higher in sham-irradiated OE (OE vs. WT, t = 3.89, P < 0.01) and significantly lower in sham-irradiated KO mice (KO vs. WT, t = 3.23, P < 0.01) (Fig. 3B), suggesting a positive association between the number of mature Dcx+ cells and EC-SOD levels. Irradiation did not lead to a significant change in the number of categories E and F Dcx+ cells in all three genotypes.
Fig. 3.
Dendritic system affected by EC-SOD levels and irradiation. (A) Representative immunohistochemical images showing the soma and the dendritic network of Dcx+ cells. (B) Total number of categories E and F Dcx+ cells (Dcx+ cells with more elaborate dendritic network). There was a significant interaction between genotype and treatment [F(2,25) = 3.53, P = 0.0445]. Genotype also played a significant role in the data variation [F(2,25) = 29.47, P < 0.0001]. (C) Representative images of dendritic arborizations in newly born neurons (labeled with GFP and NeuN). (Scale bar, 100 µm.) (D) Examination of the complexity of dendritic network by Sholl analysis. n = 44 (from three mice), 49 (from five mice), and 35 (from four mice) GFP+ cells for WT, OE, and KO, respectively. (E) Dendritic spine densities following behavioral studies (see experimental timeline in Fig. 1A). n = 6–8 mice each. There was a significant interaction between genotype and treatment [F(2,34) = 5.81, P = 0.0068]. (F) Total number of c-Fos+ cells in the hippocampal dentate gyrus. n = 5–8 mice each. Both irradiation [F(1,27) = 7.10, P = 0.0128] and genotype [F(2,27) = 6.42, P < 0.0001] played a significant role in the data variation. All data are presented as mean ± SEM. Two-way ANOVA (B, E, and F) and two-way repeated-measures ANOVA (D) with Bonferroni posttest were used for data analysis. *P < 0.05 compared with the sham-irradiated counterpart; 1, P < 0.05 compared with WT/0 Gy; 2, P < 0.05 compared with OE/0 Gy; 3, P < 0.05 compared with WT/5 Gy; 4, P < 0.05 compared with OE/5 Gy.
To determine if differences in dendritic arborization persisted as newborn neurons matured, intracranial injection of retrovirus was carried out to label new neurons with GFP. Morphological analysis was carried out 4 wk later to allow time for maturation. Study results showed that GFP+/NeuN+ cells in WT mice were well integrated into the granule cell layer, with secondary and tertiary dendrites branching into the molecular layer (Fig. 3C). In comparison, GFP+/NeuN+ cells in OE mice started branching earlier and some cells even had multiple primary dendrites. These newborn neurons also showed more complex dendritic arborization (Fig. 3D), but the dendrites did not reach as far into the molecular layer as WT mice. Consequently, total dendritic lengths and number of branches were not significantly different between WT and OE (Fig. S5). KO mice, on the other hand, had minimal dendritic arborization (Fig. 3D), which resulted in significantly fewer dendritic branches and shorter dendritic lengths (Fig. S5).
To further ascertain the effects of EC-SOD and irradiation on the dendritic system, spine densities were analyzed. No significant differences were observed among the sham-irradiated groups (Fig. 3E). Following irradiation, significant decreases in spine densities were observed in WT mice (t = 3.05, P < 0.05); however, no significant changes were observed in OE and KO mice (Fig. 3E and Table S1).
To determine if alterations in the dendritic complexity was associated with changes in neuronal activity, expression of the immediate early gene c-Fos was determined 2 mo following irradiation (see timeline in Fig. 2A). Sham-irradiated OE mice had significantly higher numbers of c-Fos+ granule cells in the dentate gyrus (Fig. 3F). Irradiation resulted in reductions in c-Fos+ cells across all genotypes; however, because of large biological variations the extent of reduction did not reach a statistically significant level. Total number of c-Fos+ cells in irradiated OE mice remained significantly higher than that in irradiated WT and KO mice (Fig. 3F). Results from quantitative RT-PCR (qRT-PCR) analysis of c-Fos message levels in the hippocampal formation were consistent with the profile of c-Fos+ cells among different cohorts (Fig. S6A).
EC-SOD and Irradiation Affect Expression of Neurotrophic Factors and Molecules Controlling Axon/Dendrite Maintenance.
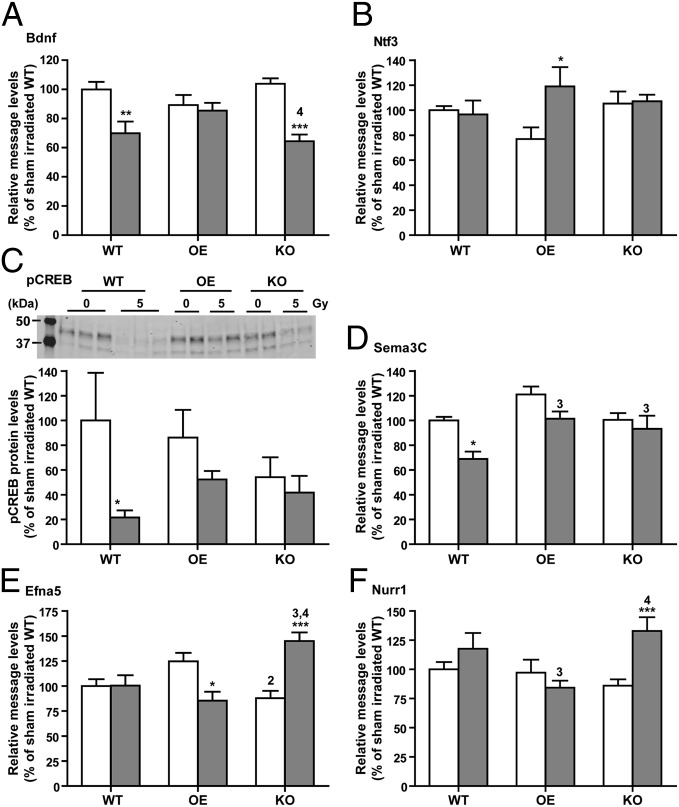
To identify the molecular and biochemical pathways involved in alterations in neurogenesis, dendritic arborization, and spine density because of differences in EC-SOD levels or as a result of radiation treatment, gene array studies were performed. Based on identified candidate genes associated with neurogenesis and neuroplasticity, qRT-PCR (Table S2) and Western blot analyses were then carried out. Two neurotrophic factors, brain-derived neurotrophic factor (Bdnf) and neurotrophin 3 (Ntf3), showed expression profiles that were favorable to OE mice in terms of long-term survival of newborn neurons and improved neuronal plasticity: (i) irradiated OE mice were able to maintain the same level of Bdnf expression when more than 30% reduction was observed in WT (t = 3.63, P < 0.01) and KO (t = 4.97, P < 0.001) mice following irradiation (Fig. 4A); (ii) expression levels of Ntf3 were significantly increased in irradiated OE (55% increase, t = 2.99, P < 0.05), but stayed the same in irradiated WT and KO mice (Fig. 4B).
Fig. 4.
Neurotrophic factors, axon/dendrite guidance, and transcription factors affected by EC-SOD and irradiation. (A and B) Relative message levels of Bdnf and Ntf3, respectively in the hippocampus. (C) pCREB protein levels in the hippocampus. (D–F) Relative message levels of Sema3C, Efna5, and Nurr1, respectively in the hippocampus. n = 8–15 mice each for message quantification; n = 5–10 each for pCREB analysis. All data are presented as mean ± SEM. All message levels were normalized to that of sham irradiated WT controls. Two-way ANOVA with Bonferroni posttest was carried out. *P < 0.05; **P < 0.01; ***P < 0.001 for comparison between 0 and 5 Gy within each genotype. 2, P < 0.05 compared with OE/0 Gy; 3, P < 0.05 compared with WT/5 Gy; 4, P < 0.05 compared with OE/5 Gy.
Because activated/phosphorylated cAMP-response element binding protein (pCREB) is known to be associated with Bdnf and Ntf3 and is important for learning and memory (24–27), the level of pCREB was also determined. A significant reduction in pCREB was observed in irradiated WT mice (80% reduction, t = 2.62, P < 0.05), implying a diminished level of pCREB-mediated neuronal function in this cohort. In contrast, no significant changes were identified in irradiated OE and KO mice. A similar profile was observed in the expression of the axon guidance molecule semaphorin 3C (Sema3C) with a significant reduction in irradiated WT (31% reduction, t = 2.97, P < 0.05) (Fig. 4D). Collectively, the data implicated a change in neurotrophic factors and guidance molecules with negative impact on neurogenesis and maintenance of the dendritic system in irradiated WT mice.
Ephrin A5 (Efna5), a ligand for the Eph-related tyrosine kinase receptor, and the nuclear receptor related protein 1 (Nurr1) were also differentially regulated by EC-SOD and irradiation: Efna5 expression levels were significantly elevated in irradiated KO (65% increase, t = 4.67, P < 0.001), but the levels were either not changed or reduced in irradiated WT and OE mice (Fig. 4E). Similarly, Nurr1 expression levels were significantly increased in irradiated KO mice (54% increase, t = 3.98, P < 0.001), but remained unchanged in WT and OE mice following irradiation (Fig. 4F). Other neurogenesis-related genes differentially regulated in irradiated KO mice include nNOS, Etv1, and Bcl2l1 (Fig. S6 B–D).
Discussion
In this study, we showed that: (i) EC-SOD deficiency had a negative impact on progenitor cell proliferation and long-term survival of newborn neurons in the dentate gyrus of hippocampus; (ii) high levels of EC-SOD in granule cells supported dendritic development and long-term survival of newborn neurons; (iii) compared with WT mice, OE and KO mice were less sensitive to irradiation-induced changes in hippocampal neurogenesis and the associated cognitive functions; and (iv) neurotrophic factors and molecules controlling axon/dendrite maintenance were differentially affected by EC-SOD levels and by irradiation.
Hippocampal neurogenesis and synaptic activities can be influenced by redox balance in the local microenvironment. Although EC-SOD is secreted into the extracellular environment, the enzyme is bound locally to extracellular matrix and only a small percentage is released as a circulating form in the CNS (17, 28). Therefore, by reconstituting EC-SOD expression to only mature neurons, we are able to generate a mouse model (OE mice) in which an EC-SOD–deficient SGZ is adjacent to an EC-SOD–rich granule cell layer in the hippocampal dentate gyrus (Fig. S7A). The manipulation did not result in changes in other SODs and major peroxidases in the hippocampal formation of OE mice (Fig. S7B).
Comparing the “hybrid” environment in OE mice to that with ubiquitous EC-SOD expression in WT or ubiquitous deficiency in KO mice, we showed that proliferation of neuronal progenitor cells was suppressed to the same extent in sham irradiated OE and KO mice (Fig. 2H), suggesting a negative effect of the EC-SOD–deficient neurogenic environment on progenitor cell proliferation. Moreover, because a small percentage of EC-SOD is expected to be released from granule cells and diffuse into the SGZ in OE mice, it is reasonable to expect low levels of EC-SOD in the neurogenic environment in OE mice (17). Consequently, the data imply that to maintain normal progenitor cell proliferation, it may be critical for neuronal progenitor cells per se to produce EC-SOD. The number of immature neurons (Dcx+ cell) in sham-irradiated OE and KO mice showed a similar profiles to that of BrdU+ numbers (Fig. 2I), suggesting that EC-SOD deficiency did not affect the initial differentiation toward the neuronal lineage and that the low number of Dcx+ cells in sham-irradiated OE and KO mice probably stemmed from changes in the proliferation of neuronal progenitor cells.
There appeared to be a positive association between EC-SOD levels in the granule cell layer and the number of immature neurons with more elaborate dendritic development, which was also reflected in mature newborn neurons with more extensive dendritic arborization (Fig. 3 A–D). The elaborate dendritic system likely provided more synaptic connections that had been reported to be important for the survival and functional integration of newborn neurons (29). Consequently, the extent of neuronal activity, based on the number of c-Fos+ cells in the dentate gyrus, was significantly higher in sham-irradiated OE mice compared with sham-irradiated WT and KO mice (Fig. 3F). It was possible that the redox environment in the EC-SOD–rich granule cell layer promoted dendritic development in newborn neurons in OE mice. How this was accomplished was not entirely clear. EC-SOD had been shown to control the bioavailability of nitric oxide (NO) within the vascular wall and the lung (30, 31). Although similar work had not been performed in the nervous system, it was reasonable to assume that more NO would be available in the OE mice to facilitate dendrite outgrowth (32, 33). Because the molecular layer of dentate gyrus was expected to be devoid of EC-SOD (other than the circulating form originating from the granule cells) in OE mice, the observation that the dendrites in OE mice did not reach as far into the molecular layer as that in WT mice (Fig. 3 C and D) also supported a role of EC-SOD in dendritic arborization and maintenance.
Sham-irradiated OE mice appeared to make more mistakes in the beginning of RAWM training and were not able to discern novel placement of an object in the NLR tests (Fig. 1 B and D, and Fig. S1). It was possible that the more elaborate dendritic network in OE mice resulted in more synaptic connections that were not necessarily beneficial for synaptic transmission. Alternatively, an earlier study using an independent strain of transgenic mice with ubiquitous overexpression of EC-SOD showed similar cognitive deficits and suggested that normal levels of superoxide radicals may be important for hippocampal-dependent learning and that high levels of EC-SOD can affect learning by reducing extracellular superoxide needed for NMDA receptor activation (34).
Dendritic arborization and integration into the existing network are tightly linked to the survival of newborn neurons (29). Although increased dendritic arborization did not affect long-term survival of newborn neurons in sham-irradiated OE mice, reduced dendritic networks in sham-irradiated KO mice (Fig. 3 C and D) may be, in part, responsible for reduced long-term survival of newborn neurons. Thus, comparison between the number of newborn neurons (BrdU+/NeuN+ cells) (Fig. 2E) and the number of immature neurons (Dcx+ cells) (Fig. 2I) showed that, whereas 6% and 6.6% immature neurons in WT and OE mice, respectively, became mature neurons 4 wk later, only 3.1% in KO mice made it that far (Table S1). The defect in KO mice was not just limited to the hippocampus because behavioral studies showed learning deficits in sham-irradiated KO mice in the hippocampal-independent NOR task as well (Fig. 1 B and E, and Fig. S1).
Following a single dose of cranial irradiation, hippocampal neurogenesis in WT mice decreased by 50%, but no significant reduction was observed in irradiated OE or KO mice (Fig. 2 B and C). Consequently, the percentage of immature neurons that became mature remained at 3% in irradiated KO mice, but it went from 6% to 3.5% in irradiated WT mice. Interestingly, the survival was enhanced to 9.2% in irradiated OE mice (Table S1). This result may be partly because of enhanced expression of Ntf3 and the ability to maintain normal levels of Bdnf expression in the postirradiation environment in OE mice (Fig. 4 A and B). Together with normal pCREB activation (Fig. 4C) and normal dendritic spine density (Fig. 3E), the data were consistent with the observation that irradiated OE mice were able to maintain the same performance level in the RAWM task and improve in the NLR task (Fig. 1 B and D).
Although no reduction in neurogenesis was seen in irradiated KO mice, the number of newborn neurons remained low compared with irradiated WT and OE mice. However, irradiated KO mice were able to improve performance in the RAWM task (Fig. 1B). The dissociation between neurogenesis and cognitive performance in this cohort suggested that factors other than neurogenesis probably played a prominent role. We showed that irradiated KO mice were able to maintain normal dendritic spine density (Fig. 3E), which was important for normal synaptic transmission. In addition, irradiated KO mice were able to maintain normal Sema3C expression and up-regulate Efna5 and Nurr1 (Fig. 4 D–F). Sema3C had been shown to be important for axon guidance and neuritogenesis (35, 36); Efna5 was shown to enhance survival of adult born neurons and increase normal synaptic transmission in the hippocampus (37); and Nurr1 expression was important for normal cognitive processes (38, 39). Collectively, the transcriptional profile would have supported a more robust cognitive learning in irradiated KO mice. How irradiated KO mice maintained Sema3C and up-regulated Efna5 and Nurr1 expression in the hippocampus was not clear, but some of these messages may be regulated by pCREB through activation of the redox-sensitive ERK1/2 (40, 41).
Taking these data together, it is possible that normal cognitive performance in irradiated OE and KO mice are supported by different mechanisms with the OE environment maintained by neurotrophic factors and the downstream pCREB signaling pathway and the KO environment enhanced by dendritic maintenance and cognitive processes to support survival of newborn neurons and normal cognitive learning. Additionally, the study results revealed the importance of EC-SOD in neuronal progenitor cell proliferation, dendritic development, and protection against irradiation. In the future, it will be reasonable to test, individually and in combination of, small synthetic molecules with SOD-like property or with an ability to mimic the function of neurotrophic factors to provide an environment that supports all stages of hippocampal neurogenesis and synaptic connections to preserve neurocognitive functions following cranial irradiation.
Methods
Mouse models used in the study have been described (13, 17). Neurogenesis and behavioral studies follow previously established procedures (13, 15, 16). Detailed descriptions for all experimental procedures are provided in SI Methods.
Supplementary Material
Acknowledgments
We thank Xinli Wang for excellent animal care; Sunny Jeong for technical assistance; the Stanford Neuroscience Gene Vector and Virus Core (supported by National Institute of Neurological Disorders and Stroke P30 NS069375) for producing the MLV-CAG-GFP used in this study; and Drs. Stefan Marklund and James Crapo for making extracellular-superoxide dismutase KO mice available. This work was supported by funding from the National Institutes of Health Grants NS046051 and NS072143 (to J.R.F. and D.J.C.); a Veteran’s Affairs Merit review (T.-T.H.); a Department of Neurology and Neurological Sciences start-up fund (to T.-T.H.); a Palo Alto Institute for Research and Education residual fund (to T.-T.H.); and the resources and facilities at the Veteran’s Affairs Palo Alto Health Care System.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216913110/-/DCSupplemental.
References
- 1.Laack NN, Brown PD. Cognitive sequelae of brain radiation in adults. Semin Oncol. 2004;31(5):702–713. doi: 10.1053/j.seminoncol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Sarkissian V. The sequelae of cranial irradiation on human cognition. Neurosci Lett. 2005;382(1-2):118–123. doi: 10.1016/j.neulet.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 3.Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97(3):370–376. doi: 10.1016/j.radonc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bovi JA, White J. Radiation therapy in the prevention of brain metastases. Curr Oncol Rep. 2012;14(1):55–62. doi: 10.1007/s11912-011-0208-6. [DOI] [PubMed] [Google Scholar]
- 5.Mizumatsu S, et al. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–4027. [PubMed] [Google Scholar]
- 6.Raber J, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 7.Kim JS, et al. Comparison of the dose-response relationship of radiation-induced apoptosis in the hippocampal dentate gyrus and intestinal crypt of adult mice. Radiat Prot Dosimetry. 2012;148(4):492–497. doi: 10.1093/rpd/ncr191. [DOI] [PubMed] [Google Scholar]
- 8.Motomura K, Ogura M, Natsume A, Yokoyama H, Wakabayashi T. A free-radical scavenger protects the neural progenitor cells in the dentate subgranular zone of the hippocampus from cell death after X-irradiation. Neurosci Lett. 2010;485(1):65–70. doi: 10.1016/j.neulet.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 9.Quik EH, et al. Reduced growth hormone secretion after cranial irradiation contributes to neurocognitive dysfunction. Growth Horm IGF Res. 2012;22(1):42–47. doi: 10.1016/j.ghir.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Quik EH, et al. Cognitive performance in older males is associated with growth hormone secretion. Neurobiol Aging. 2012;33(3):582–587. doi: 10.1016/j.neurobiolaging.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Riley PA. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65(1):27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 12.Panagiotakos G, et al. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS ONE. 2007;2(7):e588. doi: 10.1371/journal.pone.0000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rola R, et al. Lack of extracellular superoxide dismutase (EC-SOD) in the microenvironment impacts radiation-induced changes in neurogenesis. Free Radic Biol Med. 2007;42(8):1133–1145. doi: 10.1016/j.freeradbiomed.2007.01.020. discussion 1131–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberger JS, Epperly MW. Review. Antioxidant gene therapeutic approaches to normal tissue radioprotection and tumor radiosensitization. In Vivo. 2007;21(2):141–146. [PubMed] [Google Scholar]
- 15.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 16.Raber J, et al. Irradiation enhances hippocampus-dependent cognition in mice deficient in extracellular superoxide dismutase. Hippocampus. 2011;21(1):72–80. doi: 10.1002/hipo.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Y, Chen CH, Fike JR, Huang TT. A new mouse model for temporal- and tissue-specific control of extracellular superoxide dismutase. Genesis. 2009;47(3):142–154. doi: 10.1002/dvg.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31(29):10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biscaro B, Lindvall O, Tesco G, Ekdahl CT, Nitsch RM. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer’s disease. Neurodegener Dis. 2012;9(4):187–198. doi: 10.1159/000330363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekdahl CT. Microglial activation—Tuning and pruning adult neurogenesis. Front Pharmacol. 2012;3:41. doi: 10.3389/fphar.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thored P, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57(8):835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 23.Plümpe T, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bender RA, Lauterborn JC, Gall CM, Cariaga W, Baram TZ. Enhanced CREB phosphorylation in immature dentate gyrus granule cells precedes neurotrophin expression and indicates a specific role of CREB in granule cell differentiation. Eur J Neurosci. 2001;13(4):679–686. doi: 10.1046/j.1460-9568.2001.01432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jagasia R, et al. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29(25):7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merz K, Herold S, Lie DC. CREB in adult neurogenesis—Master and partner in the development of adult-born neurons? Eur J Neurosci. 2011;33(6):1078–1086. doi: 10.1111/j.1460-9568.2011.07606.x. [DOI] [PubMed] [Google Scholar]
- 27.Herold S, Jagasia R, Merz K, Wassmer K, Lie DC. CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol Cell Neurosci. 2011;46(1):79–88. doi: 10.1016/j.mcn.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson K, Sandström J, Edlund A, Marklund SL. Turnover of extracellular-superoxide dismutase in tissues. Lab Invest. 1994;70(5):705–710. [PubMed] [Google Scholar]
- 29.Bergami M, Berninger B. A fight for survival: The challenges faced by a newborn neuron integrating in the adult hippocampus. Dev Neurobiol. 2012;72(7):1016–1031. doi: 10.1002/dneu.22025. [DOI] [PubMed] [Google Scholar]
- 30.Jung O, et al. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: In vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93(7):622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed MN, Codipilly C, Hogg N, Auten RL. The protective effect of overexpression of extracellular superoxide dismutase on nitric oxide bioavailability in the lung after exposure to hyperoxia stress. Exp Lung Res. 2011;37(1):10–17. doi: 10.3109/01902148.2010.497893. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, et al. N-cadherin mediates nitric oxide-induced neurogenesis in young and retired breeder neurospheres. Neuroscience. 2006;140(2):377–388. doi: 10.1016/j.neuroscience.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morales-Medina JC, Mejorada A, Romero-Curiel A, Flores G. Alterations in dendritic morphology of hippocampal neurons in adult rats after neonatal administration of N-omega-nitro-L-arginine. Synapse. 2007;61(9):785–789. doi: 10.1002/syn.20406. [DOI] [PubMed] [Google Scholar]
- 34.Thiels E, et al. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20(20):7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno-Flores MT, et al. Semaphorin 3C preserves survival and induces neuritogenesis of cerebellar granule neurons in culture. J Neurochem. 2003;87(4):879–890. doi: 10.1046/j.1471-4159.2003.02051.x. [DOI] [PubMed] [Google Scholar]
- 36.Steup A, et al. Sema3C and netrin-1 differentially affect axon growth in the hippocampal formation. Mol Cell Neurosci. 2000;15(2):141–155. doi: 10.1006/mcne.1999.0818. [DOI] [PubMed] [Google Scholar]
- 37.Hara Y, Nomura T, Yoshizaki K, Frisén J, Osumi N. Impaired hippocampal neurogenesis and vascular formation in ephrin-A5-deficient mice. Stem Cells. 2010;28(5):974–983. doi: 10.1002/stem.427. [DOI] [PubMed] [Google Scholar]
- 38.Colón-Cesario WI, et al. Knockdown of Nurr1 in the rat hippocampus: Implications to spatial discrimination learning and memory. Learn Mem. 2006;13(6):734–744. doi: 10.1101/lm.407706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peña de Ortiz S, Maldonado-Vlaar CS, Carrasquillo Y. Hippocampal expression of the orphan nuclear receptor gene hzf-3/nurr1 during spatial discrimination learning. Neurobiol Learn Mem. 2000;74(2):161–178. doi: 10.1006/nlme.1999.3952. [DOI] [PubMed] [Google Scholar]
- 40.Kim SY, et al. The dopamine D2 receptor regulates the development of dopaminergic neurons via extracellular signal-regulated kinase and Nurr1 activation. J Neurosci. 2006;26(17):4567–4576. doi: 10.1523/JNEUROSCI.5236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darragh J, et al. MSKs are required for the transcription of the nuclear orphan receptors Nur77, Nurr1 and Nor1 downstream of MAPK signalling. Biochem J. 2005;390(Pt 3):749–759. doi: 10.1042/BJ20050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.