Abstract
The circadian pacemaker in the hypothalamic suprachiasmatic nucleus (SCN) is a hierarchical multioscillator system in which neuronal networks play crucial roles in expressing coherent rhythms in physiology and behavior. However, our understanding of the neuronal network is still incomplete. Intracellular calcium mediates the input signals, such as phase-resetting stimuli, to the core molecular loop involving clock genes for circadian rhythm generation and the output signals from the loop to various cellular functions, including changes in neurotransmitter release. Using a unique large-scale calcium imaging method with genetically encoded calcium sensors, we visualized intracellular calcium from the entire surface of SCN slice in culture including the regions where autonomous clock gene expression was undetectable. We found circadian calcium rhythms at a single-cell level in the SCN, which were topologically specific with a larger amplitude and more delayed phase in the ventral region than the dorsal. The robustness of the rhythm was reduced but persisted even after blocking the neuronal firing with tetrodotoxin (TTX). Notably, TTX dissociated the circadian calcium rhythms between the dorsal and ventral SCN. In contrast, a blocker of gap junctions, carbenoxolone, had only a minor effect on the calcium rhythms at both the single-cell and network levels. These results reveal the topological specificity of the circadian calcium rhythm in the SCN and the presence of coupled regional pacemakers in the dorsal and ventral regions. Neuronal firings are not necessary for the persistence of the calcium rhythms but indispensable for the hierarchical organization of rhythmicity in the SCN.
Keywords: yellow cameleon 3.60, Nipkow-spinning disk confocal, adeno-associated virus, time-lapse imaging, acrophase map
In mammals, the master circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus and regulates various physiological functions and behaviors (1). The SCN comprises ∼20,000 neurons and generates coherent circadian rhythms via oscillatory coupling (2). Recent advances in molecular and cellular biology have proposed the molecular machinery for intracellular circadian rhythm generation is transcriptional/translational auto-feedback loops involving several clock genes and their protein products (3). The SCN is a hierarchical multioscillator system in which neuronal networks play a critical role in producing coherent circadian rhythms (2). Without neuronal networks as in dispersed SCN cell culture, the phase and period of the cellular circadian rhythm become more variable (4). The coupled SCN networks are resistant to genetic mutations (5) and temperature resetting (6), whereas the oscillatory coupling in the SCN is altered by light input, such as an abrupt shift of a light-dark cycle (7) and seasonal changes in day length (8, 9). These studies indicate that neuronal couplings and regional interactions play important roles in the hierarchy of the SCN circadian pacemaker.
Heterogeneous distribution of the circadian phase in SCN slices was shown by in situ hybridization and bioluminescence studies examining clock gene expression (e.g., Per1 or Per2) (10–14). Disruption of intercellular coupling by inhibiting neuronal firing with tetrodotoxin (TTX) or mechanical isolation of SCN subregions severely reduced clock gene expression and mutual synchronization (10, 11). In addition, SCN neurons are connected through gap junctions (15, 16), which were reported to synchronize neuronal firings under circadian regulation (17). Therefore, gap junctions may also play some roles in the expression of circadian rhythm at both single-cell and network levels. Previous studies have reported specific patterns in clock gene expression rhythm in the SCN (10, 11), and there are SCN subregions lacking autonomous clock gene rhythm (18), particularly in the regions where neurons receive major inputs from the retina. Thus, our understanding of the hierarchy of the SCN circadian pacemaker is still far from complete.
The cytosolic calcium concentration in the SCN is reported to fluctuate spontaneously in a circadian fashion and to change rapidly in response to the photic signals from the retina (19–21). The intracellular calcium is regulated by the input and output signals to or from the molecular feedback loop. To visualize the intracellular calcium throughout the SCN, we recently developed a large-scale time-lapse calcium imaging method using a genetically-encoded FRET-based calcium sensor (i.e., yellow cameleon 3.60, YC 3.60) and Nipkow spinning disk confocal system (22). In the present study, by taking advantage of this unique technology, we analyzed the topological and temporal specificities as well as hierarchical structures of the circadian calcium rhythm in the SCN.
Results
Detection of the Circadian Calcium Rhythm in the SCN Network.
By using recombinant adeno-associated virus (rAAV) and the neuron-specific promoter (i.e., human synapsin), YC 3.60 was expressed in the entire surface of cultured SCN slice (Fig. S1), including the subregion where PER2::LUC expression was not detected (Fig. S2 A–C). With specific labeling for neurons and glial cells (i.e., Nissl and GFAP), we confirmed that YC 3.60 expression was neuron-specific (Fig. S3) and absent in glial cells (Fig. S4). The transduction efficiency of YC 3.60 was 98.2 ± 0.4% and 97.7 ± 0.3% in the dorsal and ventral regions of SCN slice, respectively (Fig. S3C).
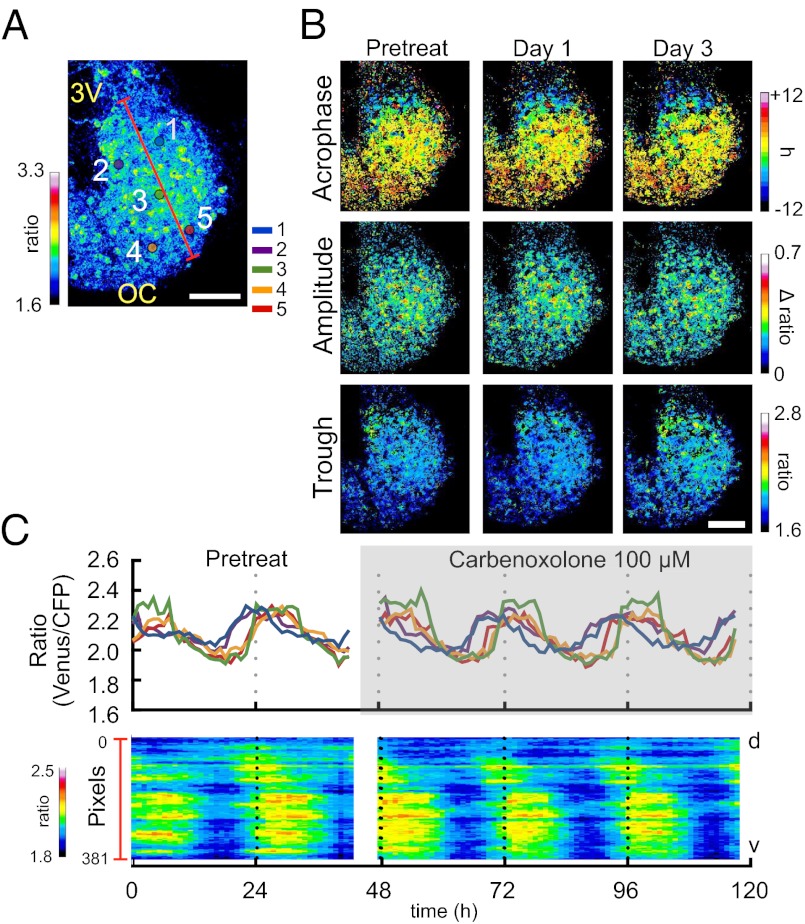
We successfully monitored the spatiotemporal dynamics of the intracellular calcium (Venus/CFP ratio) at 1-h intervals in all regions of the SCN for several days at single-cell resolution (Fig. 1 and Movie S1), and demonstrated that the SCN at the slice as well as single-cell levels exhibit robust circadian rhythms. Excitation/emission of FRET acceptor (Venus) alone did not show any circadian periodicity (Fig.S1D). The results indicate that circadian variation in FRET signals of YC 3.60 reflects primarily the circadian rhythms in intracellular calcium concentration (Fig. S1D). The circadian calcium rhythms in the dorsal region showed an advanced phase relative to the ventral one (Fig. 1 B and C, a–c). In contrast, no circadian calcium rhythm was detected in the subparaventricular zone (SPZ), indicating that a robust circadian calcium rhythm is a characteristic feature of the SCN (Fig. 1 B and C, d). Simultaneous recording of circadian calcium and PER2::LUC rhythms in the same SCN slices revealed that the former phase advanced by 5.67 ± 0.29 h compared with the later (n = 3) (Fig. S2D).
Fig. 1.
Topological characterization of the circadian calcium rhythm. (A) Pseudocolor image of YC 3.60 signals in the SCN slice. 3V, third ventricle; OC, optic chiasm. The image was taken near the peak phase of the rhythm. (B) Hourly montage of circadian signals in four representative areas. (Scale bar, 10 µm.) Left images show the locations of individual neurons. (C, Left) signal intensity of individual neurons over time (72 h). A horizontal bar indicates the time when the montage of images was collected. (Right) Raster plots of signal intensity across the dorsal tip to the end of ventral region as indicated by a red line in A. (D) Mapping of rhythm parameters: 1, Acrophase map: peak phase time is depicted and normalized relative to the mean phase of the whole slice; 2, period map; 3, amplitude map; 4, Trough map. (E) Bihourly images of the acrophase distribution. Black pixels indicate those with acrophase in this bin. Number on the upper margin of the top panel indicates the difference from the mean acrophase of the whole slice (h). Estimated borders of the SCN and the dorsal/ventral regions are shown as broken lines. (Scale bars, 100 µm all panels except B).
To quantify and visualize the spatiotemporal profiles of the circadian calcium rhythm throughout the SCN, we developed a custom-made software program for analyzing essential parameters of the circadian rhythm (Fig. S5 and SI Materials and Methods). Briefly, in each pixel, the Venus/CFP signals were fitted to a cosine curve, and the acrophase (peak phase), period, amplitude (peak-trough difference), and trough level (the lowest value) of the best-fitted curve were obtained. Spatial profiles of the four parameters were generated and presented as pseudocolor maps; typical examples are presented in Fig. 1D. An acrophase map, constructed by normalizing phases to the mean of acrophase in pixel of entire SCN slice, revealed regional heterogeneity in the circadian calcium rhythm. In particular, the calcium rhythm was phase-advanced in the dorsal region near the third ventricle compared with the ventral region (Fig. 1 D, 1, and E). Similar results were obtained in all five slices examined, confirming that the topological pattern of the acrophase was stereotyped (Fig. S6). The other parameters (period, amplitude, and trough level) are also presented (Fig. 1 D, 2–4). Because these parameters in each pixel contained some degree of variance as a result of estimation error of fitting, we compared the parameters at region levels. Variance in the circadian period could well be within the range of fitting error, when the cellular rhythms are mutually synchronized. For quantitative analysis and regional comparisons, the SCN was divided into two regions, the dorsal and ventral, based on the neuropeptide distributions of arginine vasopressin (AVP) and vasoactive intestinal polypeptide (VIP), both of which are marker peptides of the dorsal and ventral SCN subregions, respectively (Fig. 1E). The SD of the phase distribution was used as an index of network synchronization and compared between the dorsal and ventral regions (Fig. 2A). The SD of the ventral region (2.0 ± 0.3 h) was significantly smaller than those of the dorsal region (3.2 ± 0.6 h) and of the whole SCN (3.2 ± 0.7 h). The period thus calculated in the ventral region (23.8 ± 0.4 h) was not different either from that in the dorsal region (23.5 ± 0.4 h) or in the whole SCN (23.6 ± 0.4 h) (Fig. 2B). The amplitude of the calcium rhythm was significantly higher in the ventral region (0.38 ± 0.04) than that in the dorsal region (0.29 ± 0.05) (Fig. 2C). The trough level of calcium rhythm was not different regionally (1.84 ± 0.05 and 1.83 ± 0.05 in the dorsal and ventral regions, respectively) (Fig. 2D). The nonrhythmic calcium level in the SPZ was 1.93 ± 0.01, which was slightly higher than the trough level in the SCN regions. Based on the calculation of the intracellular calcium concentrations (SI Materials and Methods), the amplitude and trough level were estimated to be 30.4 ± 1.6 nM and 84.9 ± 3.8 nM in the dorsal region and 39.7 ± 1.6 and 84.1 ± 4.2 nM in the ventral region, respectively.
Fig. 2.
Statistical comparison of circadian rhythm parameters in different SCN regions. (A) SD of phase distribution in the whole, dorsal, and ventral SCN. Period (B), amplitude (C), and trough level (D) in the dorsal and ventral SCN are demonstrated as the mean ± SD. The estimated calcium concentration is shown on the right of graph in C and D. *P < 0.05, **P < 0.01. n = 5 slices.
Fig. 4.
Statistical comparison of TTX effects. Effect of TTX on the (A) amplitude, (B) trough, and (C) distribution of phase expressed in SD are analyzed on the pretreat, first day, and third day of TTX treatment. Data are expressed as the mean ± SD *P < 0.05, **P < 0.01, ***P < 0.001. n = 4 slices. See legend of Fig. 2 for details.
TTX Disrupts Synchronization of the Circadian Calcium Rhythm.
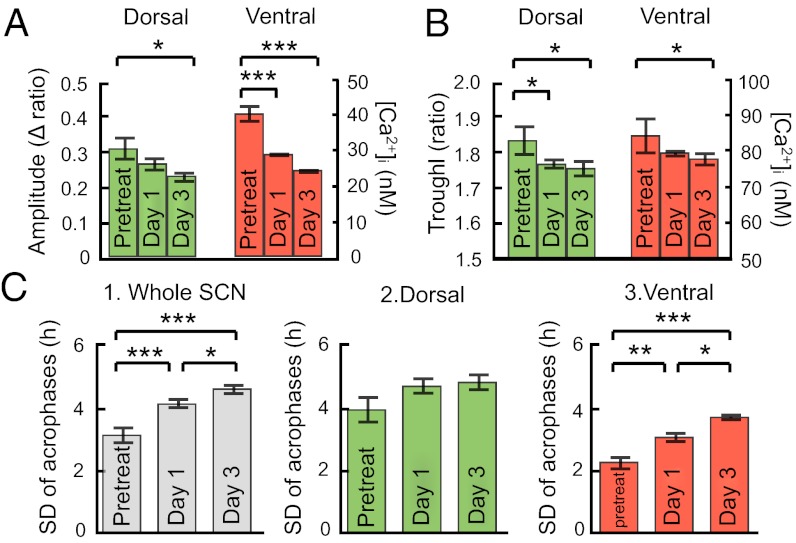
We applied 1 µM TTX to block sodium-dependent action potentials in SCN neurons and found that the calcium rhythm was TTX-sensitive in terms of the amplitude and coupling. However, cellular rhythms were still robust even on the third day of treatment (Fig. 3). Following TTX application, the amplitude of the calcium rhythm as well as the trough level in both the dorsal and ventral regions decreased, compared with the pretreat levels (Figs. 3C and 4 A and B, and Tables S1 and S2) (n = 4). The calcium level in the SPZ was also slightly decreased following TTX application (1.84 ± 0.01 at day 1, 1.80 ± 0.01 at day 3). Importantly, TTX application led to desynchronization of the SCN network (Figs. 3C and 4C). The SD of acrophases at pixel level in the whole SCN increased significantly (3.2 ± 0.3 h in the pretreat, 4.2 ± 0.2 h in TTX at day 1, 4.7 ± 0.2 h in TTX at day 3) (Fig. 4 C, 1, and Tables S1 and S2). Desynchronization significantly proceeded in the ventral region but not in the dorsal after TTX application (Fig. 4 C, 2 and 3, and Tables S1 and S2), indicating the heterogeneity of the TTX-sensitive mechanisms within SCN subregions. Rayleigh test indicated that TTX application reduced the average length of mean vector (r) significantly in the dorsal (Fig. S7B) as well as in the ventral regions (Fig. S7C), confirming that TTX desynchronized the SCN.
Fig. 3.
TTX affects the calcium rhythm and disrupts synchronization. (A) YC 3.60 signals in a representative SCN slice at the peak phase of calcium rhythm on the pretreat day. (B) Image of the SCN immunolabeled with AVP and VIP antibodies. (C, Upper) Changes in the signal intensity of YC 3.60 over time (142 h) in three individual neurons, the location of which is indicated in A. (Lower) Raster plots of calcium rhythm from the dorsal tip to the end of ventral region as indicated by a red line in A. The arrowhead is the approximate boundary between the two regions. (D) Mapping of the rhythm parameters before and during TTX application. (Scale bars, 100 µm.)
Notably, TTX enlarged the phase difference of the circadian calcium rhythms between the dorsal and ventral regions (Fig. 5). The distribution of acrophase in the entire SCN became flattened following TTX application (Fig. 5B). We generated histograms of acrophase in the dorsal (green) and ventral regions (red) separately in all four slices examined (Fig. 5C). Histogram shows a Gaussian-like distribution and the mean phase of respective distribution was calculated. The difference of two mean phases was enlarged by TTX application, which were 3.5 ± 0.2 h in the pretreat, 4.5 ± 0.2 h in TTX at day 1, and 4.4 ± 0.2 h in TTX at day 3. The intervals of two phases became significantly larger after the TTX application (P < 0.01). The dissociation was also seen in the raster plots across the dorsal to ventral regions (arrowhead in Fig. 3C). Furthermore, Rayleigh test showed that the phase angle difference between the two regions became larger following TTX application in all four slices (Fig. S7A). Medium change containing vehicle or extended long-term culturing did not affect any parameter of calcium rhythm (Fig. S8), indicating that the dissociation did not occur by vehicle treatment or in the course of culturing.
Fig. 5.
Enlargement of phase difference between the dorsal and ventral regions by TTX. (A) Bihourly images of the acrophase distribution before (pretreat) and during TTX application. The estimated borders of the SCN and the dorsal/ventral regions are shown as broken lines. Number on the upper margin of the Top panel indicates the difference from the mean acrophase (h). Data were obtained from the same SCN as displayed in Fig. 3. (Scale bar, 100 µm.) (B) Acrophase distribution expressed in histogram on the pretreat, first, and third day of TTX application. Histograms are normalized relative to the mean phase of the whole slice. (C) Histogram of the acrophase distribution within the dorsal and ventral regions in all four slices examined. The y axis is normalized to the peak of each region in a slice. Note that dispersion of two peaks is clear following TTX application (arrowheads).
Gap Junctions Do Not Contribute to the Circadian Calcium Rhythm and Network Synchronization.
We next investigated whether gap junctions contributed to circadian calcium rhythm generation and synchronization in the SCN network. After application of a widely used specific gap-junction blocker, carbenoxolone (CBX), the circadian parameters of calcium rhythm were analyzed (n = 3) (Fig. 6). CBX application did not cause any notable change in the rhythm parameters. Individual neurons were able to sustain a robust circadian calcium rhythm with only a slight reduction in amplitude in the dorsal region on day 3 of the treatment (Figs. 6 B and C and 7 A and B, and Tables S3 and S4). The calcium levels in the SPZ did not change following CBX application (1.92 ± 0.01 at day 1, 1.91 ± 0.01 at day 3). Similarly, the SD of acrophases did not show any change in the whole SCN as well as the dorsal and ventral regions (Fig. 7C, and Tables S3 and S4).
Fig. 6.
Effect of calbenoxolone on the calcium rhythm and network synchronization. (A) YC 3.60 signals in a representative SCN slice near the peak phase of calcium rhythm on the pretreat. (B) Maps of the circadian rhythm parameters before and during CBX application in an SCN. (C, Upper) Circadian calcium rhythms before (pretreat) and during CBX treatment (shadowed area). Circadian calcium rhythms of four individual cells, indicated in A, demonstrate that gap-junction blocking has no significant effect on any circadian parameter of calcium rhythms. (Lower) Raster plots of calcium rhythm from the dorsal tip to the end of ventral region as indicated by a red line in A. See legend of Fig. 1 for details. (Scale bars, 100 µm.)
Fig. 7.
Statistical comparison of calbenoxolone effects. Effect of CBX on amplitude (A), trough (B), and the SD of acrophases (C) on the pretreat, first, and third day of CBX treatment are shown for the whole, dorsal, and ventral SCN. Data are expressed as the mean ± SD of three SCN slices. *P < 0.05.
Discussion
Topological Characterization of the SCN Network.
Topological specificity of the SCN network has been characterized by the patterns of clock gene expression using in situ hybridization and bioluminescence imaging (2, 10–14). However, the autonomous expression of a clock gene is either absent or undetectable in certain regions, particularly those where neurons receive major light information from the retina (18). Furthermore, the reporter of clock gene (i.e., GFP) and its protein product (PER1) do not colocalize in certain neurons (18). These findings indicate the limitation of SCN network analysis only by clock gene expressions. In contrast, the calcium probes used in this study are widely expressed in the entire surface of the SCN slice and allowed the topological patterns of the calcium rhythm as well as regional interactions to be characterized. We found that the circadian calcium rhythm in the ventral region had a larger amplitude than the dorsal. In addition, the circadian phase in the ventral region was more delayed and less variable than in the dorsal region. TTX experiments revealed that the circadian rhythms in the dorsal and ventral regions were coupled by neuronal firing-mediated mechanisms.
Our rhythm analysis at pixel level indicated that the dorsal and ventral regions of the SCN showed substantial difference in circadian calcium rhythms. Previously, the differential induction as well as localization of Per1 and Per2 was shown in the core and shell regions of the SCN (23). Recent reports indicate that the circadian peaks of Per1 or Per2 appear first in the dorsal region and flow progressively toward the ventral region like a “wave” (10–14). However, the temporal order of the circadian peak detected in different SCN areas does not necessarily mean that the circadian signals flow and transfer along this direction via neuronal couplings, because neuronal interactions via synaptic (e.g., GABA) or diffusible factors (e.g., AVP, VIP or GRP) are more rapid processes compared with the spread of “wave” in the SCN. Instead, the appearance of the circadian peak at different times of the day may result from differences in the intrinsic circadian period among subregions under mutually synchronized condition. The circadian period was reported to be shorter in the dorsal region than that in the ventral one (24–26). These results are well consistent with the present findings.
In the present study, we observed the topological specificity of circadian calcium rhythm. Previous studies using biolistic particle delivery of YC 2.1 with a gene gun (27, 28) showed that only 60–65% of YC 2.1-expressing neurons exhibited circadian calcium rhythm. In contrast, virtually all neurons in the surface of the SCN slice showed circadian calcium rhythm in the present study. Our success to find the topological specificity is presumably because of high transduction efficiency and less invasive delivery of transgenes in SCN slices.
The amplitude of the calcium rhythm is larger in the ventral than in the dorsal SCN. In contrast, Per1 and Per2 expressions have been reported to show a larger amplitude in the dorsal than in the ventral SCN (10, 11). The amplitude of bioluminescence signals (e.g., Per1 and Per2) is generally regarded to reflect the oscillator amplitude. In contrast, the amplitude of calcium rhythm is involved in both the circadian rhythms of the input and output signals. Therefore, the difference in amplitude could reflect the different roles in circadian oscillation between clock gene and calcium. Alternatively, the calcium rhythms of high amplitude were detected in the region where autonomous clock gene expression is not evident, which could explain the discrepancy between clock genes and cellular calcium. In addition, the results suggest that clock mechanisms other than those involving PER1 or PER2 exist in the region where clock gene expression is undetectable, because the calcium rhythms in this region continued even after the shut down of neural inputs by TTX from other areas where autonomous clock gene expression exists.
Neuronal Firings Reinforce Circadian Calcium Rhythm and Network Synchronization.
Previously, Ikeda et al. (27) demonstrated that the circadian calcium rhythm was insensitive to TTX at single-cell levels. They also found that the calcium rhythms preceded the neuronal firing rhythms by ∼4 h, indicating the independence of the calcium rhythms from the neural firing rhythms. The findings were further elaborated by showing three types of neurons responding differently to TTX (no change, gain, and loss of the calcium rhythms) (28). These previous studies used a biolistic particle delivery method of YC2.1 to neurons. On the other hand, other studies reported that TTX blocked neuronal firing and eliminated the day-night difference in the intracellular calcium concentration using a synthetic calcium dye, fura-2 (20, 21, 29). All these results are different from our present findings that the calcium rhythms persisted with reduced amplitude and circadian phases were dissociated between the dorsal and ventral SCN after TTX treatment. The discrepancy may be caused by the differences in experimental methods. First, the dynamic range of intracellular calcium estimation was wider in YC3.60 than in YC2.1 (30), which allowed us to estimate more precise evaluation of intracellular calcium. Actually, the cellular calcium concentration was estimated as 80–120 nM in the present study, which was similar to the estimation by fura-2 (20, 21, 29) but lower than those in the studies of Ikeda et al., (120–440 nM) (27). Second, the number of analyzed neurons in the SCN was different among studies. rAAV in the present study labeled the entire surface of SCN slices, but biolistic delivery labeled randomly a relatively small number of SCN neurons (27, 28). The former method enabled us to analyze calcium rhythms more systematically in the SCN.
The persistence of circadian rhythm with reduced amplitude by TTX is consistent with the idea of reinforcement of circadian rhythms by mutual synchronization through neuronal networks (2). By TTX application, the neuronal connection was shut down and the reinforcing effect of networks did not work, resulting in the reduction of circadian amplitude in constituent neurons. The mechanism of reinforcement is not well understood but could well be calcium influx through voltage-gated calcium channels that are opened by action potentials or synaptic/paracrine input from other neurons (20, 21). Calcium rhythm phase-leads the PER2 by ∼5 h, suggesting that calcium is involved in the input to the molecular feedback loop. Theoretically, intracellular calcium is also involved in the output from the loop to the mechanisms of, for example, neurotransmitter release (e.g., GABA) (31, 32) or paracrine signaling (e.g., AVP, VIP, or GRP) (33). TTX shut down the input signals from other neurons and reduced the amplitude of intracellular calcium rhythm by approximately 30%, which may confirm the contribution of calcium in the input pathway to the rhythmicity, but also indicate the involvement of calcium in the output pathway. In this respect, it is interesting to note that amplitude reduction by TTX was more pronounced in the ventral SCN than in the dorsal. The ventral SCN receives major input signals from outside the SCN. Therefore, the contribution of calcium rhythm in the input pathway is more important in the ventral SCN than in the dorsal, which could reflect the differential response of amplitude to TTX application. In the present study, the trough level was also suppressed by TTX, indicating that the TTX-sensitive and nonrhythmic components present in the intracellular calcium concentration. The TTX-insensitive component could arise from calcium released from ryanodine-sensitive internal store (27).
In addition to the reduction of reinforcement through shut down of neuronal networks, TTX affected the hierarchical structure of the SCN circadian system. Previously, two distinct regional pacemakers were found in the SCN by demonstrating that the AVP and VIP release showed independent circadian rhythms in organotypic slice cultures (34). Dissociation of the regional pacemakers in the dorsal and ventral SCN was also reported to occur after an abrupt shift of a light-dark cycle (7), photoperiodic change (35, 36), and temperature change (6). In the present study, TTX enlarged the phase difference between the population rhythms in the dorsal and ventral SCN. This finding indicates that TTX sensitive mechanism is involved in the coupling of the regional pacemakers. Furthermore, TTX affected differentially the synchrony of population rhythms constituting the regional pacemakers. Desynchrony was more pronounced in the ventral pacemaker than in the dorsal one. This finding suggests differential network structures in the regional pacemakers, which could relate to the fact that neurons in the dorsal region are more tightly packed than those in the ventral (37). Previously, a loss of synchronization among cellular circadian rhythms was reported in Per1 bioluminescence by TTX application for 7 d (10). However, the regional specificity was not examined in this study.
Minor Role of Gap Junctions.
The present study showed that gap junctions have only a minor effect on the circadian calcium rhythm both at the single-cell and network levels. Previously, the gap junction antagonists, octanol and halothane, were shown to reduce the amplitude of the circadian rhythm of AVP and VIP release in the SCN (16). However, these drugs are known to have poor selectivity and considerable side effects. In the present study, a most widely used gap-junction blocker of high selectivity, CBX, slightly suppressed the amplitude of the circadian calcium rhythm only in the dorsal region, but other parameters remained unchanged. Mice lacking Connexin36, a structural component of gap junction in the SCN, displayed altered circadian behaviors (17), whereas, a few “miniature” gap junctions mediating weak electrotonic coupling were recently demonstrated between limited numbers of neuron pairs, suggesting only minor roles of gap junctions in the SCN network (38). The electrical synapses via gap junctions may contribute to SCN coupling under a certain condition, but our data showed that chemical interactions (e.g., synaptic or paracrine) are more important than electrotonic signal transduction.
Possible Role of the Circadian Calcium Rhythm.
We report here the topological characterization of the circadian calcium rhythm in the SCN and the oscillatory coupling between the dorsal and ventral SCN. However, very little is known about the physiological importance of circadian calcium rhythm in the SCN. Calcium influx has been reported to be essential for generation of the circadian rhythm in the SCN (39). In addition, calcium-activated potassium channels are highly expressed in the SCN and regulate behavioral rhythms as well as neuronal firing (40). High intracellular calcium level in the subjective day is hypothesized to prevent light-induced phase shifts of the SCN cellular rhythms because the already high calcium level does not allow further increase (41). This process may be the cellular/network mechanism of the “gating” in which light input to the circadian clock is shut down depending on the circadian phase. Applying the same logic to the interaction of dorsal-ventral SCN, the dorsal pacemaker may be highly sensitive to the phase-shifting signals from the ventral when the circadian calcium rhythm reaches the trough level.
Materials and Methods
The experiments in this study were ethically approved by Animal Research Committee of Hokkaido University (approval number 0800277) and performed following the Guide for the Care and Use of Laboratory Animals at Hokkaido University. Neonate mice were decapitated, and SCN slices were cultured as described previously with minor modifications (42). More information is available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Takeharu Nagai (Osaka University) for valuable advice on calcium imaging; Harold Gainer (National Institutes of Health/National Institute of Neurological Disorders and Stroke) for providing anti-mouse arginine vasopressin monoclonal antibody; and Joseph S. Takahashi (University of Texas Southwestern Medical Center) for providing us with the PER2::LUC mice. This work was supported in part by Japan Society for the Promotion of Science KAKENHI Grants 218900 and 23790268 (to R.E.), 21390064 and 24390055 (to S.H.), and 20249010 (to K.H.). This work was also supported by the Research Foundation for Opto-Science and Technology (R.E.); the Fritz Thyssen Stiftung and the Max Planck Society (M.T.H.); and the Cooperative Research Project for Advanced Photonic Bioimaging and by the Project for Developing Innovation Systems of the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214415110/-/DCSupplemental.
References
- 1.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 2.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 4.Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci USA. 2009;106(38):16493–16498. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu AC, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129(3):605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330(6002):379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagano M, et al. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J Neurosci. 2003;23(14):6141–6151. doi: 10.1523/JNEUROSCI.23-14-06141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazlerigg DG, Ebling FJ, Johnston JD. Photoperiod differentially regulates gene expression rhythms in the rostral and caudal SCN. Curr Biol. 2005;15(12):R449–R450. doi: 10.1016/j.cub.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki N, Honma S, Ono D, Tanahashi Y, Honma K. Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc Natl Acad Sci USA. 2007;104(18):7664–7669. doi: 10.1073/pnas.0607713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi S, et al. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302(5649):1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- 11.Maywood ES, et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16(6):599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Evans JA, Leise TL, Castanon-Cervantes O, Davidson AJ. Intrinsic regulation of spatiotemporal organization within the suprachiasmatic nucleus. PLoS ONE. 2011;6(1):e15869. doi: 10.1371/journal.pone.0015869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda H, Tokuda I, Hashimoto S, Hayasaka N. Quantitative analysis of phase wave of gene expression in the mammalian central circadian clock network. PLoS ONE. 2011;6(8):e23568. doi: 10.1371/journal.pone.0023568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foley NC, et al. Characterization of orderly spatiotemporal patterns of clock gene activation in mammalian suprachiasmatic nucleus. Eur J Neurosci. 2011;33(10):1851–1865. doi: 10.1111/j.1460-9568.2011.07682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colwell CS. Rhythmic coupling among cells in the suprachiasmatic nucleus. J Neurobiol. 2000;43(4):379–388. doi: 10.1002/1097-4695(20000615)43:4<379::aid-neu6>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinohara K, Funabashi T, Mitushima D, Kimura F. Effects of gap junction blocker on vasopressin and vasoactive intestinal polypeptide rhythms in the rat suprachiasmatic nucleus in vitro. Neurosci Res. 2000;38(1):43–47. doi: 10.1016/s0168-0102(00)00141-3. [DOI] [PubMed] [Google Scholar]
- 17.Long MA, Jutras MJ, Connors BW, Burwell RD. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci. 2005;8(1):61–66. doi: 10.1038/nn1361. [DOI] [PubMed] [Google Scholar]
- 18.LeSauter J, et al. A short half-life GFP mouse model for analysis of suprachiasmatic nucleus organization. Brain Res. 2003;964(2):279–287. doi: 10.1016/s0006-8993(02)04084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honma S, Honma K. The biological clock: Ca2+ links the pendulum to the hands. Trends Neurosci. 2003;26(12):650–653. doi: 10.1016/j.tins.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Irwin RP, Allen CN. Calcium response to retinohypothalamic tract synaptic transmission in suprachiasmatic nucleus neurons. J Neurosci. 2007;27(43):11748–11757. doi: 10.1523/JNEUROSCI.1840-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irwin RP, Allen CN. Neuropeptide-mediated calcium signaling in the suprachiasmatic nucleus network. Eur J Neurosci. 2010;32(9):1497–1506. doi: 10.1111/j.1460-9568.2010.07411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enoki R, Ono D, Hasan MT, Honma S, Honma K. Single-cell resolution fluorescence imaging of circadian rhythms detected with a Nipkow spinning disk confocal system. J Neurosci Methods. 2012;207(1):72–79. doi: 10.1016/j.jneumeth.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci. 2002;16(8):1531–1540. doi: 10.1046/j.1460-9568.2002.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura W, Honma S, Shirakawa T, Honma K. Regional pacemakers composed of multiple oscillator neurons in the rat suprachiasmatic nucleus. Eur J Neurosci. 2001;14(4):666–674. doi: 10.1046/j.0953-816x.2001.01684.x. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi T, Watanabe K. Regional differences in circadian period within the suprachiasmatic nucleus. Brain Res. 2008;1239:119–126. doi: 10.1016/j.brainres.2008.08.082. [DOI] [PubMed] [Google Scholar]
- 26.Noguchi T, Watanabe K, Ogura A, Yamaoka S. The clock in the dorsal suprachiasmatic nucleus runs faster than that in the ventral. Eur J Neurosci. 2004;20(11):3199–3202. doi: 10.1111/j.1460-9568.2004.03784.x. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda M, et al. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron. 2003;38(2):253–263. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 28.Hong JH, Jeong B, Min CH, Lee KJ. Circadian waves of cytosolic calcium concentration and long-range network connections in rat suprachiasmatic nucleus. Eur J Neurosci. 2012;35(9):1417–1425. doi: 10.1111/j.1460-9568.2012.08069.x. [DOI] [PubMed] [Google Scholar]
- 29.Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci. 2000;12(2):571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA. 2004;101(29):10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol. 2005;15(10):886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 32.Han S, et al. Na(V)1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms. Proc Natl Acad Sci USA. 2012;109(6):E368–E377. doi: 10.1073/pnas.1115729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci USA. 2011;108(34):14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. Two distinct oscillators in the rat suprachiasmatic nucleus in vitro. Proc Natl Acad Sci USA. 1995;92(16):7396–7400. doi: 10.1073/pnas.92.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de la Iglesia HO, Cambras T, Schwartz WJ, Díez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr Biol. 2004;14(9):796–800. doi: 10.1016/j.cub.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 36.VanderLeest HT, et al. Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol. 2007;17(5):468–473. doi: 10.1016/j.cub.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 37.Moore RY, Leak RK. In: Handbook of Behavioral Neurobiology. Takahashi JS, Turek FW, Moore RY, editors. New York: Kluwer Academic/Plenum; 2001. pp. 141–179. [Google Scholar]
- 38.Rash JE, et al. Connexin36 vs. connexin32, “miniature” neuronal gap junctions, and limited electrotonic coupling in rodent suprachiasmatic nucleus. Neuroscience. 2007;149(2):350–371. doi: 10.1016/j.neuroscience.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci. 2005;25(33):7682–7686. doi: 10.1523/JNEUROSCI.2211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meredith AL, et al. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9(8):1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12(10):553–569. doi: 10.1038/nrn3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishide SY, Honma S, Honma K. The circadian pacemaker in the cultured suprachiasmatic nucleus from pup mice is highly sensitive to external perturbation. Eur J Neurosci. 2008;27(10):2686–2690. doi: 10.1111/j.1460-9568.2008.06231.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.