Abstract
Taxa within diverse lineages select and transport exogenous materials for the purposes of camouflage. This adaptive behavior also occurs in insects, most famously in green lacewing larvae who nestle the trash among setigerous cuticular processes, known as trash-carrying, rendering them nearly undetectable to predators and prey, as well as forming a defensive shield. We report an exceptional discovery of a green lacewing larva in Early Cretaceous amber from Spain with specialized cuticular processes forming a dorsal basket that carry a dense trash packet. The trash packet is composed of trichomes of gleicheniacean ferns, which highlight the presence of wildfires in this early forest ecosystem. This discovery provides direct evidence of an early acquisition of a sophisticated behavioral suite in stasis for over 110 million years and an ancient plant–insect interaction.
Keywords: paleoethology, paleoecology, behavioral stasis, paleoentomology, paleobotany
Covering the body with actively selected exogenous materials as camouflage is a protective strategy occurring in invertebrate lineages as diverse as sea urchins, gastropods, crabs, and immature stages of insects (1). Given the great availability of “trash” in the environment and the efficiency of such a defensive behavior, it be might expected that the origins of camouflage are ancient. However, like many behaviors, fossil evidence of such a complex defense is exceptionally scarce. The most famous examples of camouflage among insects are the specialized behaviors and associated morphologies in the larvae of green lacewings [family Chrysopidae and extinct allies (i.e., superfamily Chrysopoidea)]. Today Chrysopidae, with around 1,200 species, are the second largest neuropteran family, having an almost cosmopolitan distribution and particularly abundant in tropical and warm habitats (2). Their campodeiform larvae are voracious predators generally living on trees, shrubs, and plants in a wide variety of ecosystems, and have been extensively studied as potential biological control agents in pest management programs (3, 4). These immatures often exhibit camouflaging behavior, known as trash-carrying, in which they harvest plant materials or even detritus and arthropod remains and carry them on their backs, nestled among cuticular processes specialized for the entanglement and transport of such debris. This trash packet camouflages the larva, preventing detection by predators and prey and constituting a defensive shield in instances where the larva is attacked (5).
Although the lineage of chrysopids has a moderately good fossil record extending into the Jurassic, with about 60 extinct species described, they are largely known from isolated compressions of wings and, more infrequently, the full-body remains of adults (6, 7). By contrast, fossils of their immatures are extremely scarce and the earliest definitive occurrences were from the Cenozoic (SI Text). The discovery of a complete larva in Early Cretaceous amber from Spain is, therefore, of considerable significance. Remarkably, not only does the specimen have the specialized morphological structures for carrying debris, but its trash packet, comprising a dense cloud of fern trichomes, is intact, demonstrating that the antiquity of the trash-carrying behavior extends to at least the Early Cretaceous, confirming previous predictions (8). This specialized camouflage strategy implies certain morphological and behavioral adaptations that are discussed in this report and, more remarkably, highlights the long-term stasis of this behavior in green lacewings, stretching over at least 110 million years. The nature of the fern trichomes, which originate from early colonizers after wildfires, used in composing the trash packet reinforces previous paleoenvironmental interpretations of the resiniferous forests as experiencing seasonal fires and perhaps stimulating resin production.
Systematic Paleontology
The systematic paleontology is as follows: Order Neuroptera Linnaeus, 1758; Superfamily Chrysopoidea Schneider, 1851; Hallucinochrysa gen. nov. Pérez-de la Fuente, Delclòs, Peñalver & Engel. For additional information, see Figs. 1 and 2, Figs. S1 and S2, and Movie S1.
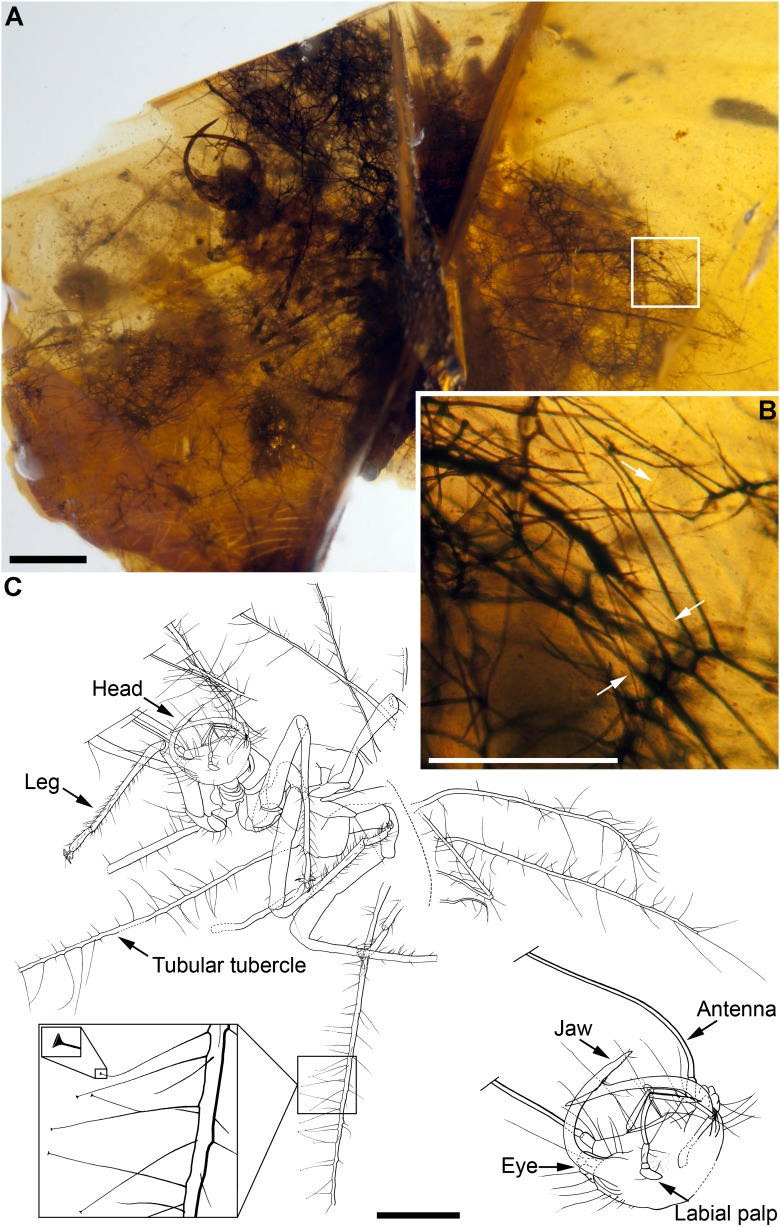
Fig. 1.
Green lacewing larva with its dense trash packet composed of fern trichomes from the Early Cretaceous of Spain. (A) Photograph of H. diogenesi gen. et sp. nov. (Neuroptera: Chrysopoidea) in ventrolateral view (holotype CES 418.1). (B) Close-up of box in A, showing enlargement of the apex of one abdominal tubular tubercle and some distal setae (arrows) trapping abundant trichomes. (C) Camera lucida drawing in the same view than A but with a slightly different inclination; the trash packet has been omitted for clarity, and several tubular tubercles are obscured by the multiple main fracture of the amber piece. The lower left inset notes the trumpet-shaped setal endings, and the lower right shows the head magnified. (Scale bars: A and C, 1 mm; B, 0.5 mm.)
Fig. 2.
Reconstruction of H. diogenesi gen. et sp. nov. (Neuroptera: Chrysopoidea) in dorsolateral view to show its life aspect. The trash packet was reconstructed at an initial stage of construction. Head and leg setation have been omitted for clarity. Length of antennae, body coloration, and number of abdominal tubular tubercle pairs are based on extant larvae. (Author: J. A. Peñas.)
Hallucinochrysa gen. nov. Pérez-de la Fuente, Delclòs, Peñalver & Engel.
Type species.
The type species has been designated Hallucinochrysa diogenesi sp. nov.
Etymology.
The generic name is from the Latin hallucinatus, “illusion of the mind,” after the bizarreness of the insect, and chrysa, a traditional ending of chrysopoid genus-group names, from Chrysopa (gender: feminine).
Diagnosis (immature).
The diagnosis is as follows: cephalic capsule banana-shaped (i.e., very broad and short with frons strongly concave); presence of a coupling system at jaw apices; median labial palpomere without annulations; dorsum with pairs of setigerous, extremely elongated tubercles (i.e., tubular tubercles); and thorax with two pairs of tubular tubercles on each segment (lateral and laterodorsal pairs).
H. diogenesi sp. nov. Pérez-de la Fuente, Delclòs, Peñalver & Engel.
Etymology.
The species name is a patronym for the Greek philosopher Diogenes of Sinope, whose name has been applied to a human behavioral disorder characterized by compulsive hoarding of trash.
Diagnosis.
The diagnosis for the species is the same as for the genus.
Material.
The material is holotype CES 418.1, from the Albian El Soplao amber site (SI Text), housed at the laboratory of the institutional El Soplao collection in El Soplao cave, Celis, Cantabria (Northern Spain). The larva, most likely a third instar, is carrying a trash packet. Preservational and taphonomic characteristics of the sample are provided in SI Text.
Remarks.
H. diogenesi possesses a bizarre, unique morphology, because the characters shown in the diagnosis are unknown from the extant green lacewing diversity. As with most Neuroptera, all Recent trash-carrying green lacewings develop through three larval stages (9). Whereas first instars always show a low degree of setation and tubercle development, second and third instars reach the maximum tubercle length and setation (10). This suggests that H. diogenesi represents an advanced larval stage, likely a third instar, despite the fact that the relative size of legs would be more typical of early instars. The existence of Early Cretaceous adult chrysopoids much larger than extant ones, also in the Barremian of Las Hoyas and El Montsec in Spain (7), make it difficult to use the total size of H. diogenesi as an inference regarding its ontogenetic development. The fossil has abundant fern trichomes (Fig. 3) entangled among setae of extremely well-developed tubercles, forming a dense trash packet in the same fashion as modern trash-carrying chrysopids. A complete, detailed description and discussion of the systematic placement of the taxon are provided in the SI Text.
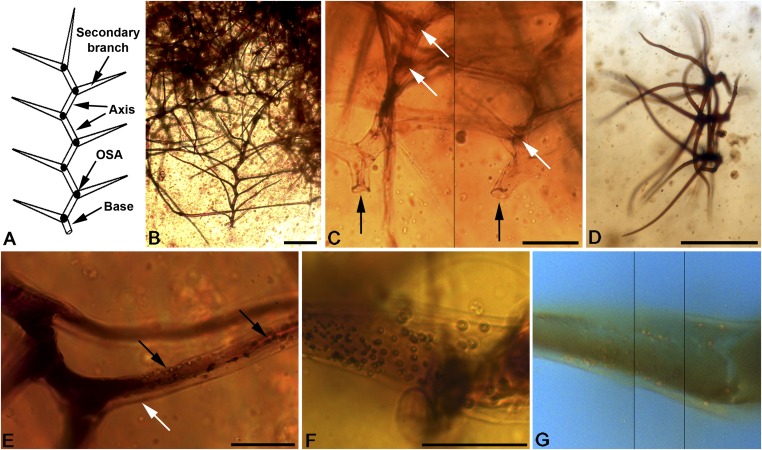
Fig. 3.
Morphological features of the trichomes of gleicheniacean fern affinity from the trash packet. (A) Morphological parts outlined within text. (B) Large trichome at the limits of the trash packet. (C) Basal regions of two contiguous trichomes, composed from two focal planes, showing trichome bases (black arrows) and OSAs (white arrows). (D) Cluster of three trichomes at the same stage of growth and orientation that were pulled out together by the larva. (E) Detail of the trichome cuticle and cell wall (white arrow) and micropapillae on the trichome surface (black arrows). (F) Detail of micropapillate trichome surface under transmitted light microscopy. (G) Same detail as F under fluorescence microscopy, composed from three focal planes (at the same scale that F). (Scale bars: B and D, 200 μm; C, 50 μm; E and F, 25 μm.)
Discussion
In Chrysopidae, apart from the morphofunctional type of larvae that exhibit the trash-carrying behavior, there are the so-called “naked larvae,” along with a few cases of morphologically and ethologically intermediate stages (11). Trash-carrying extant larvae tend to possess morphological characters that increase the potential for ensnaring debris, as well as forming a defined space for the trash packet components (4). There is evidence suggesting that these adaptations evolved several times within Chrysopidae (12). Such adaptations are present in the fossil, as a gibbous (humped) body, adapted for moving while carrying great loads. However, two of these adaptations are strikingly peculiar. H. diogenesi has: (i) pairs of dorsal setigerous tubercles, but these are extremely elongated, tubule-shaped; and (ii) setation on the tubular tubercles, but representing a special system for ensnaring the trash packet components. In extant trash-carrying larvae, the lateral tubercles are often much more developed on the thorax than the abdomen, and the lengths of the thoracic tubercles never exceed the body width. By contrast, whereas those of the fossil are similarly developed on the thorax and abdomen, some of the preserved tubular tubercles not only exceed the body width but also the entire body length. Among the diversity of modified body setae observed in extant green lacewing larvae (4), setae with hooked ends or serrated along their rachis are the most specialized morphologies found for increasing the entanglement of the trash packet components. The morphology of the setae in the fossil represents yet another morphotype serving this same function. Similar setae occur in the neuropteran family Nymphidae (13). The trumpet-shaped setal endings (Fig. 1C, and Fig. S1 E and F, and SI Text) act as anchoring points among surfaces of tangled trichomes, whereas the extremely fine distal portions of the setae facilitate the setae to become flexible and to bend by gravity, enhancing their tangling and anchoring capacity. On the other hand, in extant larvae, the trash packet is constructed from successive loads gathered with the jaws and placed on the dorsum by arching the head backward while the thorax and abdomen are bowed forward (14, 15). The insect is also capable of reallocating the trash packet components with the jaws and abdominal peristaltic movements that cause movement from one set of bristles to contiguous sets (16). In the fossil trash packet, there are clusters constituted by trichomes with the same or very similar orientation (Fig. 3 C and D), indicating that they were pulled out together by the larva. The extreme lengths of the tubercles suggest that H. diogenesi had its own, unique set of stereotyped movements used to construct the trash packet.
The trash packet plays an eminently defensive role against attackers, including cannibalism (4). Predators such as true bugs, ladybirds, ants, and parasitoid wasps have been reported as natural enemies of larval green lacewings (3, 16, 17), and this was likely true during the Mesozoic as well. The trash packet defense is twofold (4, 15): first, its camouflaging properties prevent visual detection, also misleading predators by failing to properly recognize the larva during direct contact; and second, it acts as a physical shield. Hence, a plausible interpretation explaining the aberrant tubercle elongation of the present fossil is the function of special defense against predators with an elongated piercing/sucking proboscis, like true bugs, or parasitic wasps with elongated ovipositors.
Trash packets in extant larvae can be composed of a single debris source or various kinds of materials, and these can be of vegetal and/or animal origin. Animal trash packet components include invertebrate remains such as prey carcasses, insect exuviae (even their own), or snail shells and also waxy secretions from aphids and mealybugs (14, 15, 18, 19). Nonanimal elements include vegetal matter such as bark pieces, plant fibers, or leaf trichomes, as well as terrestrial algae, lichens, and other accidental elements like moss gametophytes, pollen, and fungal spores (16, 20, 21). H. diogenesi gathered a packet solely consisting of plant trichomes (Fig. 3 and Fig. S3). The trichomes are composed of an axis with a blunt base that corresponds to the trichome insertion and 8–14 single, gradually tapering secondary branches alternatingly inserted along a single plane (Fig. 3 A–C). Opaque spheroid areas (OSAs) are distributed within the main axis at the point of insertion of secondary branches, sometimes slightly displaced from nodes. Trichomes are oval in cross-section (Fig. 3C), with a maximum thickness 30 μm and have a micropapillate surface and a thick hyaline layer corresponding to cutinized cell wall (Fig. 3 E–G and Movie S2). Trichome morphology includes variation, as observed in extant individual plants, attributable, in part, to different stages of growth (SI Text). The overall structure corresponds to nonglandular, multicellular, branched, and dendritic trichomes (22, 23). We were able to positively identify the trichomes as of gleicheniacean ferns (Fig. S4 and SI Text), a lineage that comprises early colonizers in fire-prone environments (24). Ancient wildfires are promoters of resin production and accumulation, partially explaining the considerable abundance of amber in these coal deposits (25, 26) (SI Text). Interestingly, the particular branched morphology of the fern trichomes formed a more cohesive trash packet, hardly dislodgeable from the insect during movement. Additionally, trichomes also potentially provided the insect with a secondary, chemical defense against infectious agents, attributable to the presence of phenols on the cuticle surface, as has been detected by autofluorescence signals (SI Text), reflecting a possible complex chemical ecology already present in the Early Cretaceous.
The fossil specimen is evidence of a plant–insect interaction in which the structures produced by the plant to protect itself from herbivores are taken advantage of by a predatory insect to protect itself from predators. The trash packet’s singular composition suggests that Hallucinochrysa larvae specifically harvested trichomes from gleicheniacean ferns and, thus, preyed on phytophagous arthropods living in these ferns (SI Text), but further evidence would be needed to consider this interaction as a result of a symbiotic relationship. Today, the immatures of a chrysopid species have been reported exclusively constructing its trash packet with trichomes from the Arizona sycamore tree (16). Despite the dominance of gymnosperms in the arboreal composition of the paleoenvironment, the fern affinity of the trichomes preserved in the trash packet harvested by H. diogenesi suggests that these insects actively sought plant materials from the understory rather than the trees most likely secreting the resin, which subsequently entombed them.
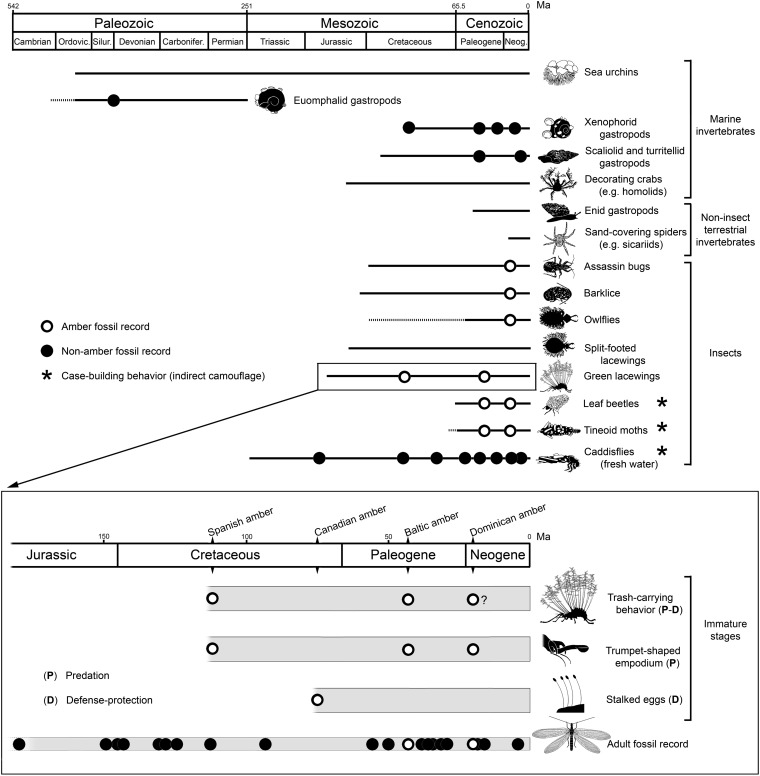
In conclusion, we interpret the present fossil as a highly specialized, probably derived, trash-carrying morphotype, rather than a primitive condition for modern green lacewings. The fossil represents some of the oldest direct evidence of camouflaging behavior in the fossil record and the earliest known testimony of trash-carrying behavior for insects, greatly reinforcing the idea that Mesozoic green lacewing immature stages responded to similar adaptive pressures as did their Cenozoic counterparts (Fig. 4). The latter was first suggested when Cretaceous green lacewing eggs were found built upon stalks, a specialized strategy for avoiding ant predation or parasitoids known in living species (27). On the other hand, it is clear that green lacewing immatures, like most holometabolan insect lineages, experience different selective pressures than their adults. For the green lacewings this would be particularly true during changes in vegetation. Some decisive changes occurred in the terrestrial paleoecology during the Early Cretaceous, when H. diogenesi lived, mainly attributable to the incipient diversification of the angiosperms (28, 29). Extant green lacewing larvae mainly occur on gymnosperms and angiosperms (3, 4), which have a comparatively larger suite of phytophagous arthropods (30), but our finding suggests that, before the angiosperm radiation, ferns had a leading role in the evolution of trash-carrying in these insects.
Fig. 4.
Insect and other invertebrate groups in which the camouflaging behavior by actively harvesting and carrying exogenous materials is known, and their geological range. Direct fossil evidence of such behavior, when reported, has been marked with dots. The lower box details the fossil evidence of the adaptations for predation and/or defense–protection present in the immature stages of green lacewings [family Chrysopidae and extinct allies (i.e., superfamily Chrysopoidea], and the geological range of the adult fossil record. The data used for this figure are provided in SI Text.
Methods
The holotype was isolated within a small piece of transparent amber (13 × 9 × 5 mm) and embedded in a regular prism (23 × 15 × 5 mm) of epoxy resin (EPO-TEK 301) for optimal viewing and curation (31). An Olympus BX51 transmitted-light microscope was used to study the insect in dorsal, ventral, and lateral views. Photography of the specimen used both ColorView IIIu digital camera attached to an Olympus BX51 and Nikon D1X digital camera attached to an Infinity K-2 long-distance microscope lens. Nomenclature used for the insect description follows that of C. A. Tauber and coworkers (9, 32). Its reconstruction was undertaken to depict its aspect in life. The tridimensional model lacking setation was performed with LightWave 3D computer graphics program (NewTek). Movement patterns, body coloration, antennal length, and number of abdominal tubular tubercle pairs were reconstructed as in Chrysopidae representatives. Setation of tubular tubercles and several trichome clusters simulating an initial stage of a trash packet construction were added in the reconstruction image (Fig. 2). The trichomes were studied using a Zeiss Axio Imager D1 bright field/fluorescence microscope equipped with EC Plan-Neofluar 20×/0.50, Plan-Apochromat 40×/0.90 oil/glyc/water-immersion, and Plan-Apochromat 63×/1.40 oil-immersion objectives and using transmitted light with Köhler illumination. A CCD AxiocamHRc Rev 2 Zeiss camera and Carl Zeiss Axiovision 4.7 software were used to capture images of the trichomes. The autofluorescence signals from the trichome samples were observed with a Zeiss Axio Imager D1 microscope in the epifluorescence mode using EC Plan-Neofluar 20×/0.50 and Plan-Apochromat 40×/0.90 oil/glyc/water-immersion objectives. The microscope was equipped with a mercury lamp and specific filters for DAPI [Zeiss Filter Set 49; excitation/emission (Ex/Em): 365/420–470 nm], eGFP (Zeiss Filter Set 38; Ex/Em: 450–490/500–550 nm), and rhodamine (Zeiss Filter Set 20; Ex/Em: 540–552/567–647 nm).
Supplementary Material
Acknowledgments
We thank the staff of the El Soplao cave in Cantabria (Spain) for curation and access to the specimen. R. López del Valle prepared the specimen, J. A. Peñas did the figure and movie reconstructions, and E. Barrón provided paleobotanical data and two photographs. We thank the Government of Cantabria. This study was supported by Instituto Geológico y Minero de España Project 491-CANOA 35015; Spanish Ministry of Economy and Competitiveness Projects CGL2008-00550, CGL2010-16004, and CGL2011-23948; and United States National Science Foundation Project DEB-0542909 (to M.S.E.). R.P.-d.l.F. is supported by an Ajuts de Personal Investigador en Formació grant from the University of Barcelona, and M.S. is a Junta de Ampliación de Estudios para Doctores from Consejo Superior de Investigaciones Científicas–Fondo Social Europeo contract holder.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213775110/-/DCSupplemental.
References
- 1.Berke SK, Miller M, Woodin SA. Modelling the energy-mortality trade-offs of invertebrate decorating behaviour. Evol Ecol Res. 2006;8(8):1409–1425. [Google Scholar]
- 2.Brooks SJ, Barnard PC. The green lacewings of the world: A generic review (Neuroptera: Chrysopidae) Bull Br Mus Nat Hist Ent. 1990;59(2):117–286. [Google Scholar]
- 3.Canard M, Séméria Y, New TR. Biology of Chrysopidae. The Hague: Dr. W. Junk Publishers; 1984. [Google Scholar]
- 4.McEwen PK, New TR, Whittington AE. Lacewings in the Crop Environment. Cambridge, UK: Cambridge Univ Press; 2001. [Google Scholar]
- 5.Eisner T, Hicks K, Eisner M, Robson DS. “Wolf-in-sheep’s-clothing” strategy of a predaceous insect larva. Science. 1978;199(4330):790–794. doi: 10.1126/science.199.4330.790. [DOI] [PubMed] [Google Scholar]
- 6.Grimaldi D, Engel MS. Evolution of the Insects. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 7.Nel A, Delclòs X, Hutin A. Mesozoic chrysopid-like Planipennia: A phylogenetic approach (Insecta: Neuroptera) Ann Soc Entomol Fr. 2005;41(1):29–69. [Google Scholar]
- 8.Engel MS, Grimaldi DA. The neuropterid fauna of Dominican and Mexican amber (Neuropterida: Megaloptera, Neuroptera) Am Mus Novit. 2007;3587:1–58. [Google Scholar]
- 9.Tauber CA. Systematics of North American chrysopid larvae: Chrysopa carnea group (Neuroptera) Can Entomol. 1974;106(11):1133–1153. [Google Scholar]
- 10.Díaz-Aranda LM, Monserrat VJ. Aphidophagous predator diagnosis: Key to genera of European chrysopid larvae (Neur.: Chrysopidae) Entomophaga. 1995;40(2):169–181. [Google Scholar]
- 11.Tauber CA, Tauber MJ, Albuquerque GS. Plesiochrysa brasiliensis (Neuroptera: Chrysopidae): Larval stages, biology, and taxonomic relationships. Ann Entomol Soc Am. 2001;94(6):858–865. [Google Scholar]
- 12.Tauber CA. Larval characteristics and taxonomic position of the lacewing genus Suarius. Ann Entomol Soc Am. 1975;68(4):695–700. [Google Scholar]
- 13.New TR. The larva of Nymphes Leach (Neuroptera: Nymphidae) Neurop Int. 1982;2(2):79–84. [Google Scholar]
- 14.Jones DT. Further notes on the snail-collecting aphis-lion larva (Neuroptera: Chrysopidae) Entomol News. 1941;52:39–44. [Google Scholar]
- 15.Eisner T, Silberglied RE. A chrysopid larva that cloaks itself in mealybug wax. Psyche (Stuttg) 1988;95:15–19. [Google Scholar]
- 16.Eisner T, Carrel JE, Van Tassel E, Hoebeke ER, Eisner M. Construction of a defensive trash packet from sycamore leaf trichomes by a chrysopid larva (Neuroptera: Chrysopidae) Proc Entomol Soc Wash. 2002;104(2):437–446. [Google Scholar]
- 17.Nakahira K, Arakawa R. Defensive functions of the trash-package of a green lacewing, Mallada desjardinsi (Neuroptera: Chrysopidae), against a ladybird, Harmonia axyridis (Coleoptera: Coccinellidae) Appl Entomol Zool (Jpn) 2006;41(1):111–115. [Google Scholar]
- 18.Millbrath LR, Tauber MJ, Tauber CA. Prey specificity in Chrysopa: An interspecific comparison of larval feeding and defensive behaviour. Ecology. 1993;74(5):1384–1393. [Google Scholar]
- 19.Tauber CA. A systematic review of the genus Leucochrysa (Neuroptera: Chrysopidae) in the United States. Ann Entomol Soc Am. 2004;97(6):1129–1158. [Google Scholar]
- 20.Skorepa AC, Sharp AJ. Lichens in “packets” of lacewing larvae (Chrysopidae) The Bryol. 1971;74(3):363–364. [Google Scholar]
- 21.Slocum RD, Lawrey JD. Viability of the epizoic lichen flora carried and dispersed by green lacewing (Nodita pavida) larvae. Can J Bot. 1976;54(15):1827–1831. [Google Scholar]
- 22.Fahn A. Plant Anatomy. 4th Ed. Oxford: Pergamon; 1990. [Google Scholar]
- 23.Evert RF. Esau’s Plant Anatomy. Meristemes, Cells and Tissues of Plant Body: Their Structure, Function and Development. 3rd Ed. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- 24.Walker LR, Boneta W. Plant and soil responses to fire on a fern-covered landslide in Puerto Rico. J Trop Ecol. 1995;11(3):473–479. [Google Scholar]
- 25.Peñalver E, Delclòs X. In: Biodiversity of Fossils in Amber from the Major World Deposits. Penney D, editor. Manchester, UK: Siri Scientific Press; 2010. pp. 236–270. [Google Scholar]
- 26.McKellar RC, et al. Insect outbreaks produce distinctive carbon isotope signatures in defensive resins and fossiliferous ambers. Proc Biol Sci. 2011;278(1722):3219–3224. doi: 10.1098/rspb.2011.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engel MS, Grimaldi DA. Diverse Neuropterida in Cretaceous amber, with particular reference to the paleofauna of Myanmar (Insecta) Nova Suppl Entomol Keltern. 2008;20:1–86. [Google Scholar]
- 28.Magallón S, Castillo A. Angiosperm diversification through time. Am J Bot. 2009;96(1):349–365. doi: 10.3732/ajb.0800060. [DOI] [PubMed] [Google Scholar]
- 29.Friis EM, Crane PR, Pedersen KR. Early Flowers and Angiosperm Evolution. Cambridge, UK: Cambridge Univ Press; 2011. [Google Scholar]
- 30.Cooper-Driver GA. Insect-fern associations. Entomol Exp Appl. 1978;24(3):310–316. [Google Scholar]
- 31.Nascimbene P, Silverstein H. In: Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey. Grimaldi D, editor. Leiden, The Netherlands: Backhuys Publishers; 2000. pp. 93–102. [Google Scholar]
- 32.Tauber CA, de León T, Penny ND, Tauber MJ. The Genus Ceraeochrysa (Neuroptera: Chrysopidae) of America North of Mexico: Larvae, adults, and comparative biology. Ann Entomol Soc Am. 2000;93(6):1195–1221. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.