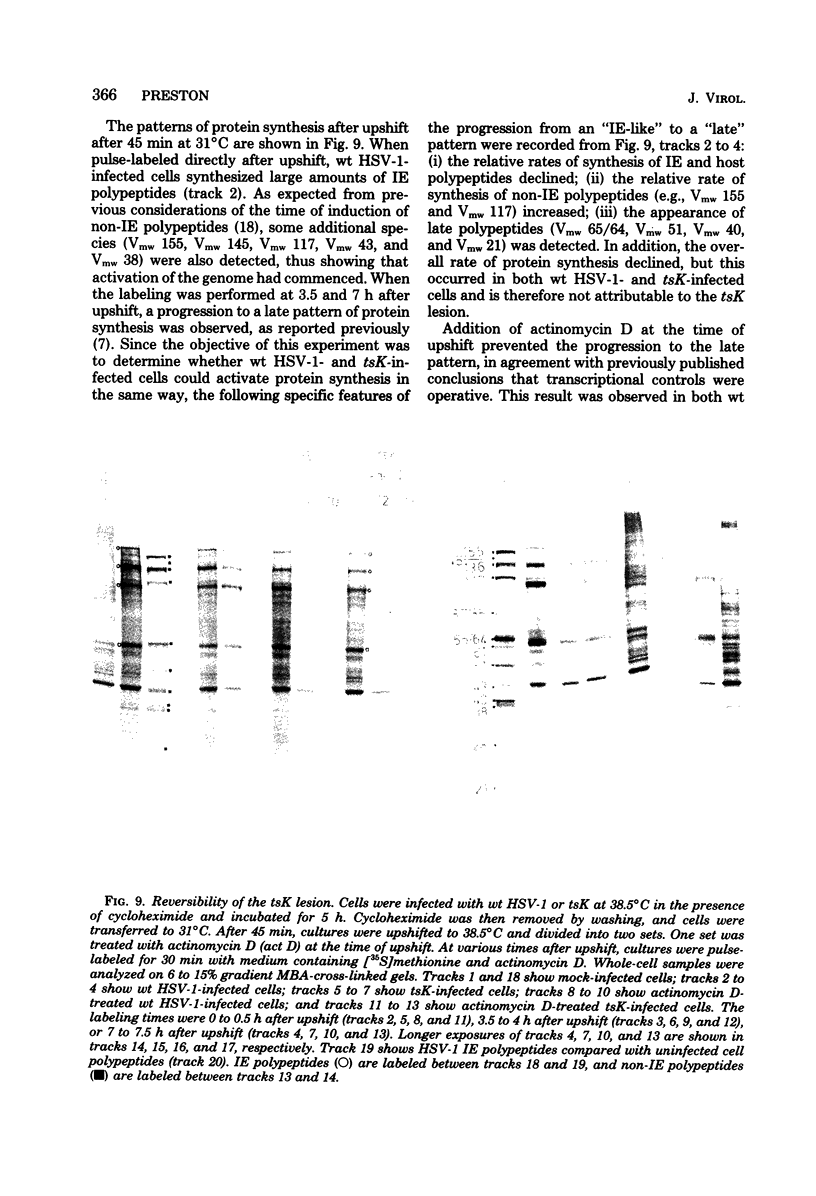
Abstract
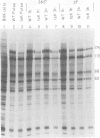
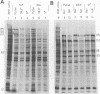
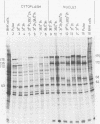
Previous studies (R. J. Watson and J. B. Clements, Virology 91:364--379, 1978; C. M. Preston, J. Virol. 29:275--284, 1979) have shown that the herpes simplex virus type 1 (HSV-1) mutant tsK has a temperature-sensitive lesion in an immediate early polypeptide whose function is to induce synthesis of new viral transcripts, including mRNA, for pyrimidine deoxyribonucleoside kinase. The studies presented here examine the properties of immediate early polypeptides in wild-type HSV-1 and tsK-infected cells at 31 and 38.5 degrees C. The overall pattern of immediate early protein synthesis was similar in wild-type HSV-1- and tsK-infected cells when radiolabeled with [35S]methionine or 14C-amino acid mixture. Further investigation, however, revealed two aberrant properties of the polypeptide Vmw 175 in tsK-infected cells at 38.5 degrees C. Upon cell fractionation, large amounts of this polypeptide were recovered in the cytoplasmic fraction, in contrast to tsK-infected cells at 31 degrees C or wild-type HSV-1-infected cells at either temperature. Furthermore, at 38.5 degrees C tsK-induced Vmw 175 was not processed normally to forms of lower electrophoretic mobility. Both of these defects were reversible upon downshift of tsK-infected cells, even in the absence of further protein synthesis, but were not observed in cells infected with a revertant of tsK. Coinfection of tsK-infected cells with wild-type HSV-1 did not alleviate these lesions, suggesting that they resulted from an abnormal Vmw 175 polypeptide rather than from a defective processing enzyme. Temperature upshift of tsK-infected cells caused reversion of Vmw 175 to the mutant form. The progression to synthesis of late polypeptides was also arrested; therefore, a functional lesion was also reversible upon temperature changes between 31 and 38.5 degrees C during the early stages of infection. The identification of a polypeptide with abnormal properties in tsK-infected cells and the demonstration that these properties, and the functional lesion, are reversible may provide an important system for investigation of HSV-1 transcriptional control.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clements J. B., Watson R. J., Wilkie N. M. Temporal regulation of herpes simplex virus type 1 transcription: location of transcripts on the viral genome. Cell. 1977 Sep;12(1):275–285. doi: 10.1016/0092-8674(77)90205-7. [DOI] [PubMed] [Google Scholar]
- Courtney R. J., Powell K. L. Immunological and biochemical characterization of polypeptides induced by herpes simplex virus types 1 and 2. IARC Sci Publ. 1975;(11 Pt 1):63–73. [PubMed] [Google Scholar]
- Courtney R. J., Schaffer P. A., Powell K. L. Synthesis of virus-specific polypaptides by temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1976 Dec;75(2):306–318. doi: 10.1016/0042-6822(76)90030-1. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J., Petkevich J. M. On the association of virus proteins with the nuclei of cells infected with herpes simplex virus. J Gen Virol. 1978 Jun;39(3):519–529. doi: 10.1099/0022-1317-39-3-519. [DOI] [PubMed] [Google Scholar]
- Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. VI. Synthesis and modification of viral polypeptides in enucleated cells. J Virol. 1977 Jun;22(3):720–725. doi: 10.1128/jvi.22.3.720-725.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Schaffer P. A. Thymidine kinase activity of biochemically transformed mouse cells after superinfection by thymidine kinase-negative, temperature-sensitive, herpes simplex virus mutants. Virology. 1978 Apr;85(2):456–463. doi: 10.1016/0042-6822(78)90452-x. [DOI] [PubMed] [Google Scholar]
- Kozak M., Roizman B. Regulation of herpesvirus macromolecular synthesis: nuclear retention of nontranslated viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4322–4326. doi: 10.1073/pnas.71.11.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Crombie I. K., Subak-Sharpe J. H. Control of protein synthesis in herpesvirus-infected cells: analysis of the polypeptides induced by wild type and sixteen temperature-sensitive mutants of HSV strain 17. J Gen Virol. 1976 Jun;31(3):347–372. doi: 10.1099/0022-1317-31-3-347. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Wolff M. H., Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977 Apr;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Davison A. J., Marsden H. S., Timbury M. C., Subak-Sharpe J. H., Wilkie N. M. Recombinants between herpes simplex virus types 1 and 2: analyses of genome structures and expression of immediate early polypeptides. J Virol. 1978 Nov;28(2):499–517. doi: 10.1128/jvi.28.2.499-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusanova T., Ben-Porat T., Himeno M., Kaplan A. S. Early functions of the genome of herpesvirus. I. Characterization of the RNA synthesized in cycloheximide-treated, infected cells. Virology. 1971 Dec;46(3):877–889. doi: 10.1016/0042-6822(71)90088-2. [DOI] [PubMed] [Google Scholar]
- Stow N. D., Subak-Sharpe J. H., Wilkie N. M. Physical mapping of herpes simplex virus type 1 mutations by marker rescue. J Virol. 1978 Oct;28(1):182–192. doi: 10.1128/jvi.28.1.182-192.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. Physical mapping of temperature-sensitive mutations of herpes simplex virus type 1 by intertypic marker rescue. Virology. 1978 Oct 1;90(1):1–11. doi: 10.1016/0042-6822(78)90327-6. [DOI] [PubMed] [Google Scholar]
- Swanstrom R. I., Pivo K., Wagner E. K. Restricted transcription of the herpes simplex virus genome occurring early after infection and in the presence of metabolic inhibitors. Virology. 1975 Jul;66(1):140–150. doi: 10.1016/0042-6822(75)90185-3. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. Characterization of transcription-deficient temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1978 Dec;91(2):364–379. doi: 10.1016/0042-6822(78)90384-7. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Preston C. M., Clements J. B. Separation and characterization of herpes simplex virus type 1 immediate-early mRNA's. J Virol. 1979 Jul;31(1):42–52. doi: 10.1128/jvi.31.1.42-52.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]