Abstract
HIV-associated nephropathy (HIVAN) is the manifestation of HIV genes expression by kidney cells in the presence of specific host factors. Recently, rapamycin (sirolimus) has been demonstrated to modulate the progression of HIVAN. We hypothesized that rapamycin would modulate the progression of HIVAN by attenuating HIV genes expression. To test our hypothesis, three weeks old Tg26 mice (n=6) were administered either vehicle or rapamycin (5 mg/kg/day, intraperitoneally) for eight weeks. At the end of experimental period, kidneys were harvested. In in vitro studies, human podocytes were transduced with either HIV-1 (NL4-3) or empty vector (EV), followed by treatment with either vehicle or rapamycin. Total RNA and proteins were extracted from renal tissues/ cellular lysates and HIV gene transcription/translation was measured by real time PCR and Western blotting studies. Renal histological slides were graded for glomerular sclerosis and tubular dilatation with microcyst formation. Rapamycin attenuated both glomerular and tubular lesions in Tg26 mice. Rapamycin decreased transcription of HIV genes both in renal tissues as well as in HIV-1 transduced podocytes. Our data strongly indicate that HIV-1 long terminal repeat-mediated transcriptional activity was targeted by rapamycin. Rapamycin enhanced podocyte NF-kB and CREB activities but then it decreased AP-1 binding activity. Since expression of HIV genes by kidney cells has been demonstrated to be the key factor in the development HIVAN, it appears that rapamycin-induced altered transcription of HIV genes might have partly contributed to its disease modulating effects.
Human immunodeficiency virus-associated nephropathy (HIVAN) is an important cause of chronic renal disease in HIV-1 seropositive patients (1-3). Untreated, it may result in end stage renal disease (ESRD) in less than a year (3). HIV patients have several fold greater risk of ESRD than the general population (4,5). However, use of highly active antiretroviral therapy (HAART) has significantly improved survival of persons with HIV-infection (6,7); nonetheless, kidney disease still continues to be the fourth leading cause of death among HIV-infected patients (8), and the third leading cause of ESRD among black individuals (9).
HIV-associated nephropathy (HIVAN) is a unique renal lesion characterized by a collapsing variant of glomerulosclerosis (GS) and microcystic dilatation of tubules (10). Collapsing GS has also been reported as idiopathic as well as a consequence of pamidronate toxicity (11). Development of HIVAN requires the presence of specific genetic (African ancestry in general and Apol1 gene in particular), environmental (HIV-1 expression) and specific host factors such as the activation of the renin-angiotensin system (RAS) (12, 13). One cannot change the genetic background; however, one can control the environmental factors and also modulate the host factors. Recently, the role of mammalian target of rapamycin (mTOR) pathway has been demonstrated in the development of proliferative phenotype in HIVAN (14); rapamycin (sirolimus) not only inhibited the activation of the mTOR pathway but also attenuated the development of proliferative phenotype in HIVAN (15). In addition, rapamycin has been reported to modulate HIV infection. In these studies, rapamycin attenuated both viral entry and replication (16).
In a recent report, adverse host factors have been demonstrated to exacerbate clinically occult HIVAN into an overt HIVAN (17). Since antiviral therapy usually inhibited viral replication but not the protein expression, it was speculated that kidney cells such as podocytes if got infected prior to administration of antiviral therapy, they are likely to continue to express HIV proteins for a long time despite the treatment. In this scenario, if kidney cells expressing HIV proteins encountered adverse host factors (such as activation of the renin-angiotensin system (RAS) because of diabetes, hypertension, or loss of nephron mass), in the later life, they might display HIVAN phenotype, in case they already had genetic factors such as black ancestry/apol1 gene on the board. With that background, we hypothesized that drugs such as rapamycin, which had potential to inhibit kidney cell gene/protein expression would provide better protection against development of HIVAN.
Rapamycin is a macrolide which exhibit anti-proliferative properties (18); it inhibits T-cell proliferation induced by multiple stimuli (19) and induces inhibition of FRAP (FK506-binding protein/rapamycin-associated protein or mTOR) activity via binding with FK506-binding proteins (FKBPs)(18, 20). FRAP regulates the phosphorylation of both p70S6k and 4E-BP1 (20, 21); the phosphorylation of the former activates the translation of the 5′-polypyrimidine tract mRNA family and the phosphorylation of the latter inhibits 4E-BP1 suppressive activity on eIF4E, which leads to eIF4E-dependent mRNA translation (21); conversely, binding of FRAP to the rapamycin/FKBP12 complex will abrogate the translation of mRNAs carrying polypyrimidine tracts at their 5′ termini (22-25). Rapamycin has been reported to repress HIV-1 replication in both T cells and peripheral blood leucocytes (16). The inhibitory effect of sirolimus on HIV-1 replication was associated with diminished basal HIV-1 long-terminal repeat (LTR) gene expression independent of the virus-specific transactivating Tat protein. However, it did not exclude the contribution of rapamycin-induced repressive translation of subset mRNA bearing 5′-polypyrimidine motif (16). Since HIV gene expressions in Tg26 mice (derived by HIV-1 LTR promoter) (26, 27), we hypothesized that sirolimus may be attenuating HIV-1 gene transcription by inhibiting HIV-1LTR.
We demonstrate here that treatment of Tg26 mice with rapamyin results in a significant decrease in renal tissue HIV-1 gene expression. In in vitro studies, rapamycin also inhibited HIV-1 gene expression in HIV-1-transduced podocytes. Further experiments indicated that HIV-1 long terminal repeat-mediated transcriptional activity was targeted by rapamycin. These results suggest that rapamycin acts as an inhibitor of HIV-1 gene expression in kidney cells due to its action on virus-mediated transcriptional activity.
Methods and Materials
HIV transgenic (Tg26) mice
We have used age and sex matched FVB/N (control) and Tg26 (with FVB/N background) mice. Breeding pairs of FVBN mice were obtained from Jackson Laboratories (Bar Harbor, ME). Breeding pairs to develop Tg26 colonies were kindly gifted by Prof. Paul E. Klotman M.D., President and CEO, Baylor College of Medicine, Houston, TX). Tg26 transgenic animals have the proviral transgene, pNL4-3: d1443, which encodes all the HIV-1 genes except gag and pol and therefore the mice are noninfectious (26, 27). We are maintaining colonies of these mice in our animal facility. For genotyping, tail tips were clipped, DNA was isolated and PCR studies were carried out using following primers for Tg26.
HIV-F 5′ ACATGAGCAGTCAGTTCTGCCGCAGAC
HIV-R 3′ CAAGGACTCTGATGCGCAGGTGTG
The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Long Island Jewish Medical Center.
Experimental studies
Three weeks old Tg26 mice (n=6) were administered ether normal saline or rapamycin (5 mg/Kg every other day, intraperitoneal) for eight weeks. Age and sex matched FVBN mice in groups of six were also administered normal saline for the same duration. These animals served as control for Tg26 mice. At the end of the scheduled periods, the animals were anesthetized and sacrificed. Both kidneys were excised; one was processed for histological studies while the other was used for RNA and protein extraction. Three-micrometer sections were prepared and stained with hematoxylineosin and periodic-acid Schiff (PAS). Renal sections were coded and examined under light microscopy. Twenty random fields (20X)/mouse were examined to score percentage of the involved glomeruli and tubules. Glomerular lesions were classified as segmental glomerulosclerosis (SGS), global glomerulosclerosis (GGS), and collapsing glomerulosclerosis (CGS). Severity of tubular injury was scored in the form of tubular dilatation (% of dilated tubules/section) and size of microcysts (1+ to 4+). Two investigators, unaware of the experimental conditions scored the severity of renal lesions.
Preparation of podocytes
Human podocytes were obtained from Dr. Moin A. Saleem (Children’s renal unit and academic renal unit, University of Bristol, Southmead Hospital, Bristol, UK). Human podocytes were conditionally immortalized by introducing temperature-sensitive SV40-T antigen by transfection (28). The cells have additionally been transfected with a human telomerase construct (29). These cells proliferate at permissive temperature (33°C, conditionally immortalized human podocytes, CIHP) and enter growth arrest (conditionally immortalized differentiated human podocytes, CIDHP) after transfer to the non-permissive temperature (37°C). The growth medium contains RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 1× Pen-Strep, 1 mM L-glutamine and 1× ITS (Invitrogen) to promote expression of T antigen.
Production of Pseudotyped Retroviral Supernatant
Replication defective viral supernatants were prepared as published previously (30). In brief, GFP reporter gene (from pEGFP-C1; Clontech, Palo Alto, CA) was substituted in place of gag/pol genes in HIV-1 proviral construct pNL4–3. This parental construct (pNL4-3: G/P-EGFP) was used to produce VSV.G pseudotyped viruses to provide pleiotropism and high-titer virus stocks. Infectious viral supernatants were produced by transient transfection of 293T cells using Effectene (Qiagen Inc.) according to the manufacturer’s instructions. The HIV-1 gag/pol and VSV.G envelope genes were provided in trans using pCMV R8.91 and pMD.G plasmids, respectively (gifts of Dr. Didier Trono, Salk Institute, La Jolla, CA). As a negative control, virus was also produced from pHR-CMV-IRES2-GFP-B, which contained HIV-1 LTRs and GFP empty expression vector. The viral stocks were titrated by infecting HeLa tat cells with tenfold serial dilution as reported previously (30). The reciprocal of the lowest dilution showing expression of green fluorescence protein (GFP) was defined as GFP-expressing units (GEU) per ml. Viral stocks ranging from 106 to 108 GEU/ml were obtained.
Western blotting studies
Control and experimental cells or renal tissues were lysed in RIPA buffer containing 50 mM Tris-Cl (pH 7.5), 150 mM NaCl, 1mM EDTA, 1% NP-40, 0.25% Deoxycholate, 0.1% SDS, 1× protease inhibitor cocktail (Calbiochem, Cocktail Set I), 1mM PMSF, and 0.2mM sodium orthovanadate. Protein concentration was measured with the Biorad Protein Assay kit (PIERCE, Rockford, IL). Total protein extracts (20 μg/lane) were separated on a 15 % polyacrylamide (PAGE) premade gel (Bio-Rad, Hercules, CA) and transferred onto a nitrocellulose membrane using Bio-Rad miniblot apparatus. Nitrocellulose membranes were then processed further for immunostaining with primary antibodies against gp120 antibody (AIDS Reagent Program) and subsequently with horseradish peroxidase labeled appropriate secondary antibody. The blots were developed using a chemiluminescence detection kit (PIERCE, Rockford, IL) and exposed to X-ray film (Eastman Kodak Co., Rochester, NY). Equal protein loading and the protein transfer were confirmed by immunoblotting for determination of actin protein using a polyclonal α-Actin antibody (I-19, Santa Cruz, CA) after stripping the same nitrocellulose blots.
Reverse Transcription PCR Analysis
Control (EV/CIDHP) and experimental (HIV/CIDHP) cells and renal tissues from Tg26 an dTg26/Rapa mice were used to quantify mRNA expression of renin and CTSL. RNA was extracted using TRIZOL (Invitrogen corp.). For cDNA synthesis, 2 μg of the total RNA was preincubated with 2 nmol of random hexamer (Invitrogen Corp) at 65°C for 5 min. Subsequently, 8μl of the reverse-transcription (RT) reaction mixture containing cloned AMV RT, 0.5 mmol each of the mixed nucleotides, 0.01 mol dithiothreitol, and 1000 U/mL Rnasin (Invitrogen Corp) was incubated at 42°C for 50 min. For a negative control, a reaction mixture without RNA or reverse transcription (RT) was used. Samples were subsequently incubated at 85°C for 5 min to inactivate the RT. Quantitative PCR was carried out in an ABI Prism 7900HT sequence detection system using the primer sequences as shown below: SYBR green was used as the detector and ROX as a stablizing dye. Results (means ± S.D.) represent number of cell samples or animals as described in the legend. The data was analyzed using the Comparative CT method (ΔΔCT method). Differences in CT are used to quantify relative amount of PCR target contained within each well. The data were expressed as relative mRNA expression in reference to control, normalized to quantity of RNA input by performing measurements on an endogenous reference gene, GAPDH.
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSA)
Nuclear extracts from Control and experimental cells (1 × 107) were prepared using the method as described previously (31) with slight modification. Control and experimental cells washed twice with ice-cold PBS, and lysed in 400 μl of buffer A (10 mM HEPES, pH 7.9; 10 mM KCl; 2 mM MgCl2; 0.5 mM dithiothreitol; 1 mM phenylmethylsulfonyl fluoride; 5 μg/ml aprotinin; 5 μg/ml pepstatin A; and 5 μg/ml leupeptin) containing 0.1% Nonidet P-40 for 15 min on ice, vortexed vigorously for 15 s, and centrifuged at 14,000 rpm for 30 s. The nuclei pellet were resuspended in 40 μl of buffer B (20 mM HEPES, pH 7.9; 25% (v/v) glycerol; 0.42 M NaCl; 1.5 mM MgCl2; 0.2 mM EDTA; 0.5 mM dithiothreitol; 1 mM phenylmethylsulfonyl fluoride; 5 μg/ml aprotinin; 5 μg/ml pepstatin A; and 5 μg/ml leupeptin). After 30 min on ice, the lysates were centrifuged at 14,000 rpm for 10 min. Supernatants containing the nuclear proteins were diluted with 20 μl of modified buffer C (20 mM HEPES, pH 7.9; 20% (v/v) glycerol; 0.05 M KCl; 0.2 mM EDTA; 0.5 mM dithiothreitol; and 0.5 mM phenylmethylsulfonyl fluoride) and stored at −70°C until further use. Nuclear extracts (1mg) were used for the electrophoretic mobility shift assay using Electrophoretic Mobility Shift Assay (EMSA) DNA-binding protein detection system kit (Affymetrix/[Panomics, Redwood City, CA]) according to the manufacturer’s protocol. Briefly, the respective protein-binding biotinylated DNA probes (NFκB-(5′->3′) AGTTGAGGGGACTTTCCCAGGC ; CREB-(5′->3′)AGAGATTGCCTGACGTCAGAGAGCTAG; AP-1:(5′->3′) CGCTTGATGACTCAGCCGGAA) were incubated with nuclear extracts prepared after various treatments of HPC cells, according to the manufacturer’s protocol DNA-protein binding reactions were performed at room temperature for 10 min in 10 mM Trizma base pH 7.9, 50 mM NaCl, 5 mM MgCl2, 1 mM EDTA, and 1 mM dithiothreitol plus 1 μg of poly (dI--dC), 5% (v/v) glycerol, and ~10 ng of biotinylated probe for either NF◻B, AP-1 or CREB . Protein DNA complexes were resolved from protein-free DNA in 6 % polyacrylamide gels (Invitrogen) at 4°C in 50 mM Tris, pH 8.3, 2 mM EDTA at 130 V/ 3-4 h. The long run was required to separate various sizes of protein-DNAcomplexes; in this time, unbound labelled probe migrated off towards the end of the gel. Following resolution of samples on acrylamide gels , resolved DNA-protein complexes and rest of the gel contents were transferred to biodyne B membrane (Pall, Ann Arbor, MI) for 60 min at a constant current 300 mA. The membranes containing the DNA-protein complexes were UV cross linked and Chemiluminescent detection of biotinylated DNA was performed using the Panomics EMSA kit. In some lanes, 10 ng of the respective (wherever indicated) cold (unbiotinylated) probe were included in the DNA protein-binding reaction mix prior to addition of biotinylated probe as control.
Statistical analysis
For comparison of mean values between two groups, the unpaired t test was used. To compare values between multiple groups, analysis of variance (ANOVA) was applied and a Bonferroni multiple range test was used to calculate a p-value. Statistical significance was defined as P<0.05. All values are displayed as mean ± SD.
Results
Rapamycin attenuates renal lesions in HIVAN mice
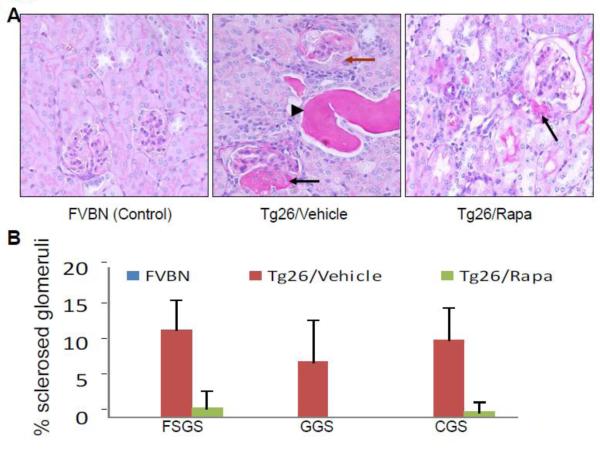
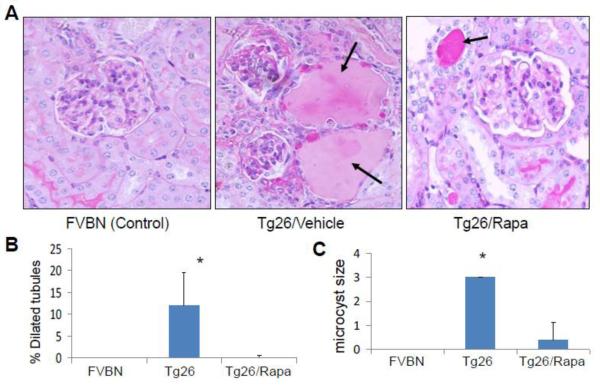
Renal cortical sections for control, Tg26 and Tg26/Rapa were evaluated for severity of renal lesions. Representative microphotographs are shown in Figs. 1A and Fig. 2A. Cumulative data on glomerular lesions are shown in Fig. 1B and tubular lesions in Figs. 2B and 2C. Control mice did not display any glomerular or tubular lesions. Tg26 mice displayed both sclerotic glomeruli (SGS, GGS and CGS), tubular dilatation with microcyst formation; whereas, rapamycin-receiving Tg26 mice displayed only a few sclerosed glomeruli (SGS and CGS) and none with global sclerosis (GGS). Similarly, rapamycin-receiving mice displayed only occasional dilated tubules and significant decrease in microcyst size (P<0.01) (Figs. 2B and 2C).
Figure 1. Rapamycin attenuates renal lesions in HIVAN mice.
Renal cortical sections from vehicle-receiving FVBN (control), vehicle-receiving Tg26 and Tg26-receiving Rapamycin (n=6) were stained with PAS and scored for severity of injury.
A. Representative microphotographs of cortical sections from a control, Tg26 and Tg26/Rapamycin (Tg26/Rapa) are displayed. Control mice displayed a normal glomerulus. Tg26 mice displayed dilated tubules with cast formation (indicated by a black arrowhead) segmental sclerosis (indicated by a black arrow) and collapsing sclerosis with proliferation of podocytes (indicated by a brown arrow). Tg26/Rapa revealed segmental sclerosis in occasional glomeruli (indicated by a black arrow).
B. Cumulative data showing percentage of segmentally sclerosed (SGS) globlally sclerosed (GGS) and collapsed sclerosed (CGS) glomeruli in renal cortical sections of FVBN, Tg6, and Tg26/Rapa mice.
Figure 2. Rapmycin attenuates tubular dilatation and microcyst formation in HIVAN mice.
A. Representative microphotographs of cortical sections from a control, Tg26 and Tg26/Rapa are displayed. Control mice displayed no tubular abnormalities. Tg26 mice displayed microcyst formation (indicated by arrows) Tg26/Rapa revealed occasional microcyst formation (indicated by an arrow). Cumulative data showing percentage of dilated tubules per section in control,Tg26 and Tg26/Rapa mice. *P<0.01 compared with Tg26/Rapa
B. Cumulative data showing mean size of microcysts in control, Tg26 and Tg26/Rapa mice. *P<0.01 compared with Tg26/Rapa
Rapamycin down regulates HIV gene expression in Tg26 mice
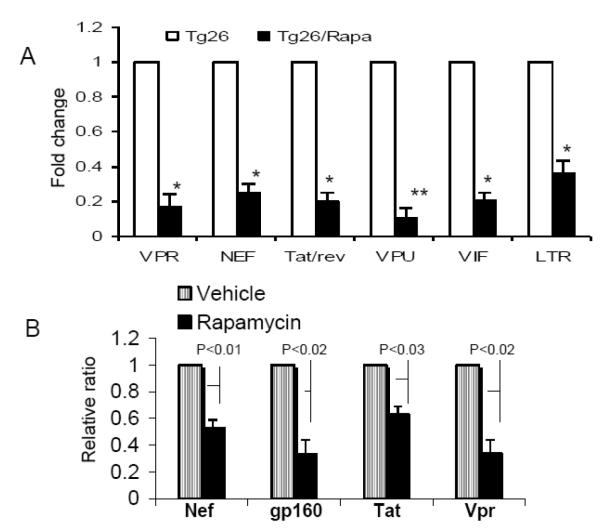
To determine the effect of rapamycin on HIV gene expression in Tg26 mice, total RNA was extracted from renal tissues of Tg26 mice and Rapamycin-receiving Tg26 mice. Real time PCR studies were carried out to quantify HIV gene expression. As shown in Fig. 3A, renal tissues from Rapa-receiving mice displayed attenuated expressions of Vpr, Nef, Tat-rev, Vpu, Vi, and LTR.
Figure 3. Rapamycin down regulates HIV gene expression in vivo and in vitro.
A. Total RNA was extracted from Tg26 mice and Rapa-receiving Tg26 mice (n=4). Real time PCR studies were carried out to quantify HIV gene expression (n=4). *P<0.0.1 compared with respective Tg26. **P<0.001 compared with respective Tg26.
B. Total RNA were extracted from HIV/CIDHPs and HIV/CIDHPs/Rapa and probed for HIV genes (n=4). HIV/CIDHP/Rapa displayed attenuated expression of Nef, gp160, Tat, and Vpr when compared to HIV/CIDHPs.
Rapamycin attenuates HIV gene expression in HIV/CIDHPs
To determine the effect of rapamycin on podocyte HIV gene expression, total RNA were extracted from HIV/CIDHPs and HIV/CIDHPs/Rapa and probed for HIV genes. HIV/CIDHP/Rapa displayed attenuated expression of Nef, gp160, Tat, and Vpr when compared to HIV/CIDHPs.
Rapamycin attenuates gp120 protein expression both in in vivo and in vitro
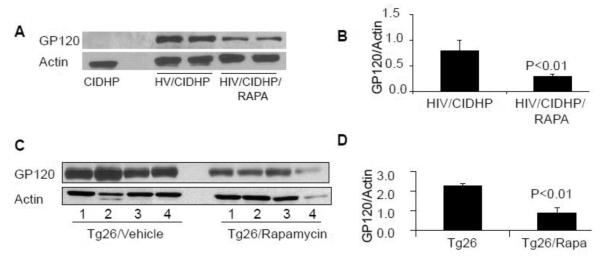
To determine the effect of Rapa on podocyte HIV protein expression, proteins were extracted from CIDHPs, HIV/CIDHPs and HIV/CIDHPs/Rapa, Western blots were prepared and probed for gp120 protein. HIV/CIDHP/Rapa displayed diminished expression of gp120 protein when compared to HIV/CIDHPs (Figs. 4A and 4B).
Figure 4. Rapamycin attenuates gp120 protein expression both in vivo and in vitro.
A. Cellular lysates from of CIDHPs, HIV/CIDHPs and HIV/CIDHPs/Rapa were probed for gp120 protein. The same blots stripped and reprobed for actin. A representative gel displaying gp120 expression by CIDHP, HIV/CIDHPs (two different lysates) and HIV/CIDHP/Rapa (two different lysates) is displayed. The bottom lane shows actin expression in the same samples.
B. Cumulative densitometric data of three different sets experiments under control and experimental conditions described in A.
C. Immunoblots of the protein lysates from renal tissues of Tg26 and Rapa-receiving Tg26 mice were probed for the expression of gp120 protein. The same nitrocellulose blots were stripped and reprobed for actin. A representative gel from renal tissues from four different mice is shown. The upper lane displays gp120 expression by Tg26 and Tg26/Rapa mice. The lower lane displays actin expression under similar conditions.
D. Cumulative densitometric scanned data from Tg26 and Tg26/Rapa mice described in C.
Renal tissue of Tg26 mice have been reported to express only an occasional viral protein, gp160 (26, 27). We asked whether Rapa would also attenuate renal tissue expression of gp120 protein in Tg26 mice. Cellular lysates of renal tissues of Tg26 and Rapa-receiving Tg26 mice were probed for gp120 protein. The same blots were reprobed for actin. A representative gel from renal tissues from four different mice is shown in 4C. Cumulative densitometric data from these mice are shown in a bar diagram in Fig. 4D. Tg26 mice displayed robust expression of gp120 protein. However, Rapa-receiving Tg26 mice displayed attenuated expression of gp120. These findings indicated that rapamycin also decreases HIV protein expression in HIVAN.
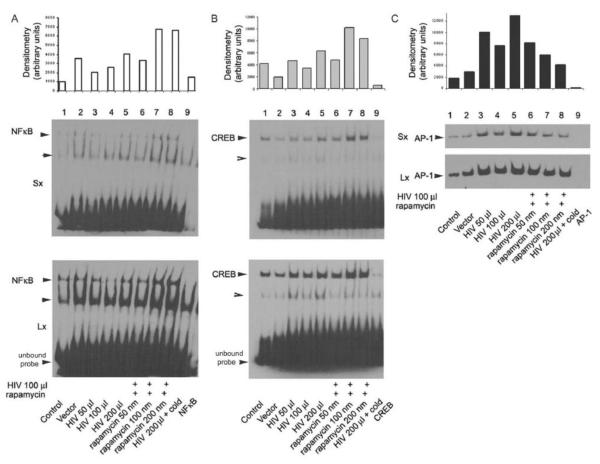
Rapa modulated HIV-induced activation of NF-kB, CREB and AP-1 transcription factors in podocytes
EMSA was carried out using the nuclear extracts prepared from human podocyes – following treatment with buffer, HIV pseudotyped virus, or empty vector for 5 h either in the presence or absence of rapamycin (with indicated concentrations, 50-200 nM). Densitometric scans of DNA-protein complexes for NF-kB, CREB, and AP-1 were quantified and presented as a histogram. Rapamycin treatment of CIDHP inhibited the HIV induced AP-1 activity (Fig. 5 C); on the other hand, rapamycin treatment induced activation of NF-kB and CREB as shown in Figs. 5A and 5B respectively.
Figure 5. HIV induced activation of NF-kB, CREB and AP-1 transcription factors.
EMSA was carried out using the nuclear extracts prepared from CIDHPs following treatment with HIV pseudotyped virus for 5 h either in the presence or absence of rapamycin (100 nM). The densitometric scanned data for NF-kB, CREB, and AP-1 are expressed on an arbitrary scale. In each set, the last lane is a repetition of conditions using nuclear extracts following 200 μl HIV virus treatment of podocytes, however the reaction mix pre-incubated with 10 ng (cold) unbiotinylated probe, before adding the biotinylated probe, demonstrate the specificity of respective labeled DNA oligomer. For clarity, the biodyne membranes were exposed to the X-ray film either for longer (5-10 min) or for shorter (1-2 minutes) time periods. The top shows the picture of the autoradiogram of the shorter exposure time (Sx) and the bottom shows the longer exposure time (Lx). Densitometry data of DNA-protein complexes was quantified and shown as a histogram.
Discussion
Renal biopsy data suggested that HIV-1 infection of glomerular and tubular cells contributed to HIVAN phenotype (12). In an isolated case report, HIV-1 expression by kidney cells persisted despite eradication of HIV-1 from the circulation while that patient was treated with HAART (32). On that account, it is plausible that despite being on HAART, patients with HIV-1 infection may continue to develop HIVAN if they are exposed to specific host factors in the later part of their life; nevertheless, use of HAART has significantly decreased occurrence of HIVAN in patients with HIV-1 infection (33).
Podocyte has been considered a key cell for the development of both classical and collapsing variant of focal segmental glomerular sclerosis (34, 35). Podocyte HIV-1 infection has been demonstrated in HIVAN patients (36). Since podocytes are terminally differentiated cells, once they develop HIV-1 expression, they are likely to continue to express HIV-1 protein for a long time. Interestingly, it has been demonstrated that podocyte expression of an individual protein is sufficient to initiate the development of HIVAN (37). In a scenario, where HIV-1 invoked podocyte HIV-1 protein expression (prior to the administration of HAART) podocytes continue to express HIV-1 proteins despite zero viral load. Since occurrence of HIVAN requires presence of specific genetic, environmental, and host factors, mere podocyte expression of HIV-1 protein (environmental factor) and genetic background (African ancestry) may not be sufficient to invoke HIVAN phenotype (38); however, exposure to specific host factors such as activation of the RAS may facilitate the occurrence of HIVAN phenotype.
Bruggeman et al, in her pioneer kidney transplant studies demonstrated that control mice which received kidneys from HIV transgenic mice developed HIVAN, whereas, HIV transgenic mice which received kidneys from the control mice did not nay signs of HIVAN (39). These studies demonstrated the critical role of HIV genes in the development of HIVAN; moreover, these studies also excluded the role of other host factors in the development of HIVAN. In the present study, rapamycin down regulated both HIV gene transcription and HIV protein translation in vivo as well as in vitro studies. Because of this property of rapamycin, it may be a better antiviral drug for treatment of HIV infected patients who are susceptible to develop HIVAN.
What is the relevance of the present study in the developing a therapeutic strategy for the treatment of HIVAN? The standard antiviral therapy has been designed to inhibit viral replication. It does not inhibit the expression of HIV proteins, which happened prior to administration of antiviral therapy. Rapamycin is already being used in clinical practice to prevent allograft rejection. Therefore, demonstration of its potential to attenuate HIV-1 gene transcription in vivo is of immediate relevance for its use as an antiviral agent in HIVAN patients.
Rapamycin has been reported to display anti-HIV properties by multiple ways (40-43). Roy et al demonstrated that rapamycin inhibited LTR-mediated transcription of HIV (16). Rapamycin has been demonstrated to increase the anti-HIV activity of HIV-entry inhibitors, vicriviroc, aplaviroc and enfuvirtide (40, 41). Rapamycin has also been reported to down regulate CCR5 and thus, resulting in the accumulation of anti-HIV β-chemokines (40). Rapamycin also attenuated HIV infection in human peripheral blood leukocyte reconstituted SCID mice (42). Interestingly, rapamycin showed better control of HIV replication in liver transplant patients (43). In the present study, rapamycin attenuated transcription of LTR both in HIVAN mice and HIV-infected podocytes. Interestingly, rapamycin enhanced activities of NF-kB and CREB but inhibited AP-1 binding activity. Thus, it appears that inhibition of AP-1 activity may also be contributing to rapamycin-induced down regulation of HIV gene transcription in HIV infected podocytes. However, this aspect needs to be investigated further in future studies.
We conclude that rapamycin has an important anti-HIV-1 gene transcription properties besides its effect on mTOR pathway and warrants further study to ascertain its possible use in the management of HIVAN.
Highlights.
Rapamycin down regulates HIV gene in HIVAN mice.
Rapamycin also down regulates HIV gene in HIV-infected podocytes.
Rapamycin seems to down regulate HIV genes by its effect on LTR promoter.
Rapamycin enhanced activity of NF-kB and CREB in HIV infected podocytes
Rapamycin attenuated AP-1 binding activity in HIV-infected podocytes.
| VPR | F | AGAGGACAGATGGAACAAGCC |
| R | CTAGTCTAGGATCTACTGGCTCC | |
| LTR | F | GCTAACTAGGGAACCCACTG |
| R | GTCACACCTTTTCCAGAGATCGT | |
| Nef | F | GGTGGGTTTTCCAGTCACAC |
| R | GGGAGTGAATTAGCCCTTCC | |
| GP 160 | F | AAAGAATAGTGCTGTTAGCTTGCTC |
| R | TCTGTCCCCTCAGCTACTGC | |
| VIF | F | ACCTGGCAGACCAACTAATTCACCT |
| R | GGCCCTTGGTCTTCTGGGGCTT | |
| VPU | F | AGCACCTGGAACGATACCTGGGA |
| R | TGGTCCTTTGATGGGAGGGGCA | |
|
NL4-3 tat
rev |
F | GGCGTTACTCGACAGAGGAG |
| R | TGCTTTGATAGAGAAGCTTGATG | |
| Gag | F | ATAATCCACCTATCCCAGTAGGAGA |
| R | TTTGGTCCTTGTCTTATGTCCAGAATGC |
Acknowledgements
This work was supported by grants (RO1DK084910 and RO1 DK083931, PCS) from National Institutes of Health, Bethesda, MD. We are grateful to Prof. Paul Klotman, Baylor College of Medicine, Houston, TX, for providing us with a breeding pair of Tg26 mice. We are also grateful to the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH for providing anti-gp120 antibody. This work was presented at the 43rd Annual Meeting of the American Society of Nephrology (2011) held in Philadelphia, PA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bourgoignie JJ, Pardo V. The nephropathology in human immunodeficiency virus (HIV-1) infection. Kidney Int. 1991;40(suppl):S19–S23. [PubMed] [Google Scholar]
- 2.Pardo V, Aldana M, Colton RM, Fischl MA, Jaffe D, Moskowitz L, Hensley GT, Bourgoignie JJ. Glomerular lesions in acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:429–434. doi: 10.7326/0003-4819-101-4-429. [DOI] [PubMed] [Google Scholar]
- 3.Rao TKS, Filppone EJ, Nicastri AD. Associated focal segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:664–673. doi: 10.1056/NEJM198403153101101. [DOI] [PubMed] [Google Scholar]
- 4.Ahuja TS, Grady J, Khan S. Changing trends in the survival of dialysis patients with human immunodeficiency virus in the United States. J Am Soc Nephrol. 2002;13:1889–1893. doi: 10.1097/01.asn.0000019773.43765.bf. [DOI] [PubMed] [Google Scholar]
- 5.Eggers PW, Kimmel PL. Is there an epidemic of HIV infection in the US ESRD program? J Am Soc Nephrol. 2004;15:2477–2485. doi: 10.1097/01.ASN.0000138546.53152.A7. [DOI] [PubMed] [Google Scholar]
- 6.Murphy EL, Collier AC, Kalish LA. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 7.Selik RM, Byers RH, Jr., Dworkin MS. Trends in diseases reported on U.S. death certificates that mentioned HIV infection, 1987-1999. J Acquir Immune Defic Syndr. 2002;29:378–387. doi: 10.1097/00126334-200204010-00009. [DOI] [PubMed] [Google Scholar]
- 8.United States Renal Data System 2001 Annual Data Report. National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Diseases; Bethesda, MD: 2001. [Google Scholar]
- 9.Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel R, Fields TA, Svetkey LP, Flanagan KH, Klotman PE, Winston JA. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–52. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 10.Atta MG, Lucas GM, Fine DM. HIV-associated nephropathy: epidemiology, pathogenesis, diagnosis and management. Expert Rev Anti Infect Ther. 2008;6:365–71. doi: 10.1586/14787210.6.3.365. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, Kuhn JA, Dratch AD, D’Agati VD. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol. 2001;12:1164–72. doi: 10.1681/ASN.V1261164. [DOI] [PubMed] [Google Scholar]
- 12.Marras D, Bruggeman LA, Gao F, Tanji N, Mansukhani MM, Cara A, Ross MD, Gusella GL, Benson G, D’Agati VD, Hahn BH, Klotman ME, Klotman PE. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med. 2002;8:522–526. doi: 10.1038/nm0502-522. [DOI] [PubMed] [Google Scholar]
- 13.Mikulak J, Singhal PC. HIV-1 and kidney cells: better understanding of viral interaction. Nephron Exp Nephrol. 2010;115:e15–21. doi: 10.1159/000312882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar D, Konkimalla S, Yadav A, Sataranatarajan K, Kasinath BS, Chander PN, Singhal PC. HIV-associated nephropathy: role of mammalian target of rapamycin pathway. Am J Pathol. 2010;177:813–21. doi: 10.2353/ajpath.2010.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav A, Kumar D, Salhan D, Rattanavich R, Maheshwari S, Adabala M, Ding G, Singhal PC. Sirolimus modulates HIVAN phenotype through inhibition of epithelial mesenchymal transition. Exp Mol Pathol. 2012;93:173–81. doi: 10.1016/j.yexmp.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy J, Paquette JS, Fortin JF, Tremblay MJ. The immunosuppressant rapamycin represses human immunodeficiency virus type 1 replication. Antimicrob Agents Chemother. 2002;46:3447–55. doi: 10.1128/AAC.46.11.3447-3455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar D, Salhan D, Magoon S, Torri DD, Sayeneni S, Sagar A, Bandhlish A, Malhotra A, Chander PN, Singhal PC. Adverse host factors exacerbate occult HIV-associated nephropathy. Am J Pathol. 2011;179:1681–92. doi: 10.1016/j.ajpath.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sehgal SN, Baker H, Vézina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28:727–32. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 19.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–37.20. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo CJ, Chung J, Fiorentino DF, Flanagan WM, Blenis J, Crabtree GR. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992;358:70–3. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- 21.Graves LM, Bornfeldt KE, Argast GM, Krebs EG, Kong X, Lin TA, Lawrence JC., Jr cAMP- and rapamycin-sensitive regulation of the association of eukaryotic initiation factor 4E and the translational regulator PHAS-I in aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1995;92:7222–6. doi: 10.1073/pnas.92.16.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasinath BS, Feliers D, Sataranatarajan K, Ghosh Choudhury G, Lee MJ, Mariappan MM. Regulation of mRNA translation in renal physiology and disease. Am J Physiol Renal Physiol. 2009;297:F1153–65. doi: 10.1152/ajprenal.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasinath BS, Mariappan MM, Sataranatarajan K, Lee MJ, Feliers D. mRNA translation: unexplored territory in renal science. J Am Soc Nephrol. 2006;17:3281–92. doi: 10.1681/ASN.2006050488. [DOI] [PubMed] [Google Scholar]
- 24.Lieberthal W, Levine JS. The Role of the Mammalian Target Of Rapamycin (mTOR) in Renal Disease. J Am Soc Nephrol. 2009;12:2493–502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 25.Tian S, Li XL, Shi M, Yao YQ, Li LW, Xin XY. Enhance tumor radiosensitivity by intracellular delivery of eukaryotic translation initiation factor 4E binding proteins. Med Hypotheses. 2011;76:246–8. doi: 10.1016/j.mehy.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ, Eckhaus M, Bryant JL, Notkins AL, Klotman PE. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci U S A. 1992;89:1577–81. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu TC, He JC, Klotman P. Animal models of HIV-associated nephropathy. Curr Opin Nephrol Hypertens. 2006;15:233–7. doi: 10.1097/01.mnh.0000222688.69217.8e. [DOI] [PubMed] [Google Scholar]
- 28.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 29.Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavaré JM, Mathieson PW, Saleem MA. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes. 2007;56:1127–35. doi: 10.2337/db06-0693. [DOI] [PubMed] [Google Scholar]
- 30.Husain M, Gusella GL, Klotman ME, Gelman IH, Ross MD, Schwartz EJ, Cara A, Klotman PE. HIV-1 Nef induces proliferation and anchorage-independent growth in podocytes. J. Am. Soc. Nephrol. 2002;13:1806–15. doi: 10.1097/01.asn.0000019642.55998.69. [DOI] [PubMed] [Google Scholar]
- 31.Ayasolla KR, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) attenuates the expression of LPS- and Abeta peptide-induced inflammatory mediators in astroglia. 2005. [DOI] [PMC free article] [PubMed]; Ayasolla KR, et al. J Neuroinflammation. 20;2:21. doi: 10.1186/1742-2094-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winston JA, Bruggeman LA, Ross MD, Jacobson J, Ross L, D’Agati VD, Klotman PE, Klotman ME. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med. 2001;344:1979–84. doi: 10.1056/NEJM200106283442604. [DOI] [PubMed] [Google Scholar]
- 33.Atta MG. Diagnosis and natural history of HIV-associated nephropathy. Adv Chronic Kidney Dis. 2010;17:52–8. doi: 10.1053/j.ackd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54:687–97. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 35.Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4:39–45. doi: 10.2174/157339908783502370. [DOI] [PubMed] [Google Scholar]
- 36.Barisoni L, Kriz W, Mundel P, D’agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J. Am. Soc. Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 37.Zhong J, Zuo Y, Ma J, Fogo AB, Jolicoeur P, Ichikawa I, Matsusaka T. Expression of HIV-1 genes in podocytes alone can lead to the full spectrum of HIV-1-associated nephropathy. Kidney Int. 2005;68:1048–60. doi: 10.1111/j.1523-1755.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 38.Bruggeman LA, Nelson PJ. Controversies in the pathogenesis of HIV-associated renal diseases. Nat Rev Nephrol. 2009;5:574–81. doi: 10.1038/nrneph.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruggeman LA, Dikman S, Meng C, Quaggin SE, Coffman TM, Klotman PE. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest. 1997;100:84–92. doi: 10.1172/JCI119525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heredia A, Latinovic O, Gallo RC, Melikyan G, Reitz M. Reduction of CCR5 with low-dose rapamycin enhances the antiviral activity of vicriviroc against both sensitive and drug-resistant HIV-1. Proc Natl Acad Sci U S A. 2008;105:20476–81. doi: 10.1073/pnas.0810843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicoletti F, Fagone P, Meroni P, McCubrey J, Bendtzen K. mTOR as a multifunctional therapeutic target in HIV infection. Drug Discov Today. 2011;16:715–21. doi: 10.1016/j.drudis.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Nicoletti F, Lapenta C, Donati S, Spada M, Ranazzi A. Inhibition of human immunodeficiency virus (HIV-1) infection in human peripheral blood leucocytes-SCID reconstituted mice by rapamycin. Clin Exp Immunol. 2009;155:28–34. doi: 10.1111/j.1365-2249.2008.03780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Benedetto F, Di Sandro S, De Ruvo N, Montalti R, Ballarin R. First report on a series of HIV patients undergoing rapamycin monotherapy after liver transplantation. Transplantation. 2010;89:733–8. doi: 10.1097/TP.0b013e3181c7dcc0. [DOI] [PubMed] [Google Scholar]