Abstract
Human marrow stromal cells (hMSCs) are targets of 1! ,25-dihydroxyvitamin D [1! ,25(OH)2D3] action to promote their differentiation to osteoblasts, but they also participate in vitamin D metabolism by converting 25-dihydroxyvitamin D3 [25(OH)D3] to 1! ,25(OH)2D3 by 1α-hydroxylase (CYP27B1). Chronic kidney disease (CKD) is associated with impaired renal biosynthesis of 1! ,25(OH)2D, low bone mass, and increased fracture risk. We tested whether CKD influences hMSCs' responses to vitamin D3 metabolites. The hMSCs were obtained from tissues discarded during arthroplasty for hip osteoarthrosis, including a subject who had been undergoing hemodialysis for 2+ years. There was a significant positive correlation between in vitro stimulation of osteoblastogenesis (alkaline phosphatase activity) by 1! ,25(OH)2D3 and subjects' estimated glomerular filtration rate (eGFR, r=0.47, p=0.015, n=26, 56–83 years of age). Osteoblastogenesis was stimulated in hMSCs from both the hemodialysis and control subjects by 1! ,25(OH)2D3 (10 ! M), 25(OH)D3 (100 ! M), or D3 (1000 ! M). Thus, vitamin D metabolism may play an autocrine/paracrine role in osteoblast differentiation of hMSCs. These findings suggest that in CKD patients 25(OH)D-sufficiency may play an important role in skeletal health; osteoblastic bone formation in CKD patients may not be optimal unless there is sufficient serum 25(OH)D substrate for the MSCs to synthesize and respond to local 1! ,25(OH)2D.
Keywords: Human Marrow Stromal Cells, Osteoblastogenesis, Chronic Kidney Disease, eGFR, vitamin D metabolites
1. Introduction
Chronic kidney disease (CKD) is associated with impaired renal biosynthesis of 1! ,25(OH)2D, low bone mass, and increased fracture risk [1]. Osteoblastogenesis of human marrow stromal/mesenchymal stem cells (hMSCs) is stimulated by both 1! ,25(OH)2D3 and 25-hydroxyvitamin D3 [25(OH)D3] [2], the later effect requiring conversion to 1,25(OH)2D3 by 25-hydroxyvitamin D3 1α-hydroxylase (CYP27B1) [3]. CYP27B1 in hMSCs is upregulated by 25(OH)D [2] and PTH [4] and is downregulated by 1! ,25(OH)2D [2], similar to regulation of renal CYP27B1. These findings suggest an autocrine/paracrine role of vitamin D metabolism in osteoblastogenesis of hMSCs. Because nothing is known about the effect of CKD on this process, we tested whether responsiveness of hMSCs to vitamin D metabolites was influenced by renal status of the subjects from whom the cells were obtained.
2. Materials and methods
Bone marrow samples from subjects older than 55-years were obtained with IRB approval as femoral tissue discarded during primary hip arthroplasty for osteoarthritis, with exclusion criteria, isolation methods [2] and eGFR [5] as described. This series included 26 men (n=10) and women (n=16) whose MSCs were used for in vitro osteoblast differentiation. The group mean values (assay normal range) were age 68.2 ± 1.4 years, serum 25(OH)D 30.6 ± 1.7 ng/mL (20–57), 1,25(OH)2D 44.2 ± 2.8 pg/mL (18– 62), and PTH 38.2 ± 3.7 pg/mL (10–65). We also obtained deidentified discarded marrow from a 57-year old male orthopedic patient with end-stage renal disease (ESRD) who had been undergoing hemodialysis for 2+ years; although serum 25(OH)D level was not available, this subject had secondary hyperparathyroidism and was being treated with cinacalcet, calcium acetate, and active D, commonly used meds in the ESRD population. Reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. The hMSCs were maintained in phenol red-free ! -MEM, 10% fetal bovine serumheat inactivated (FBS-HI), 100 u/mL penicillin, and 100 ! g/mL streptomycin (Invitrogen, Carlsbad, CA). Constitutive gene expression was determined by RT-PCR, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal control [2]. Upon confluence of passage 2 hMSCs, medium was changed to osteoblastogenic medium (α-MEM with 1% FBS-HI, 100 U/ml penicillin, 100 µg/ml streptomycin, 10−8 M dexamethasone, 5 mM β-glycerophosphate, 50 µg/ml ascorbate-2-phosphate) ± treatments for 7 days. As an index of osteoblast differentiation, alkaline phosphatase (ALP) enzyme activity was measured spectrophotometrically and expressed as a ratio of treated-to-control [2]. Quantitative data were analyzed with non-parametric tools; if data allowed, parametric tools were used.
3. Results
3.1 Effect of eGFR on responsiveness of human MSCs to 1! 25(OH)2D3
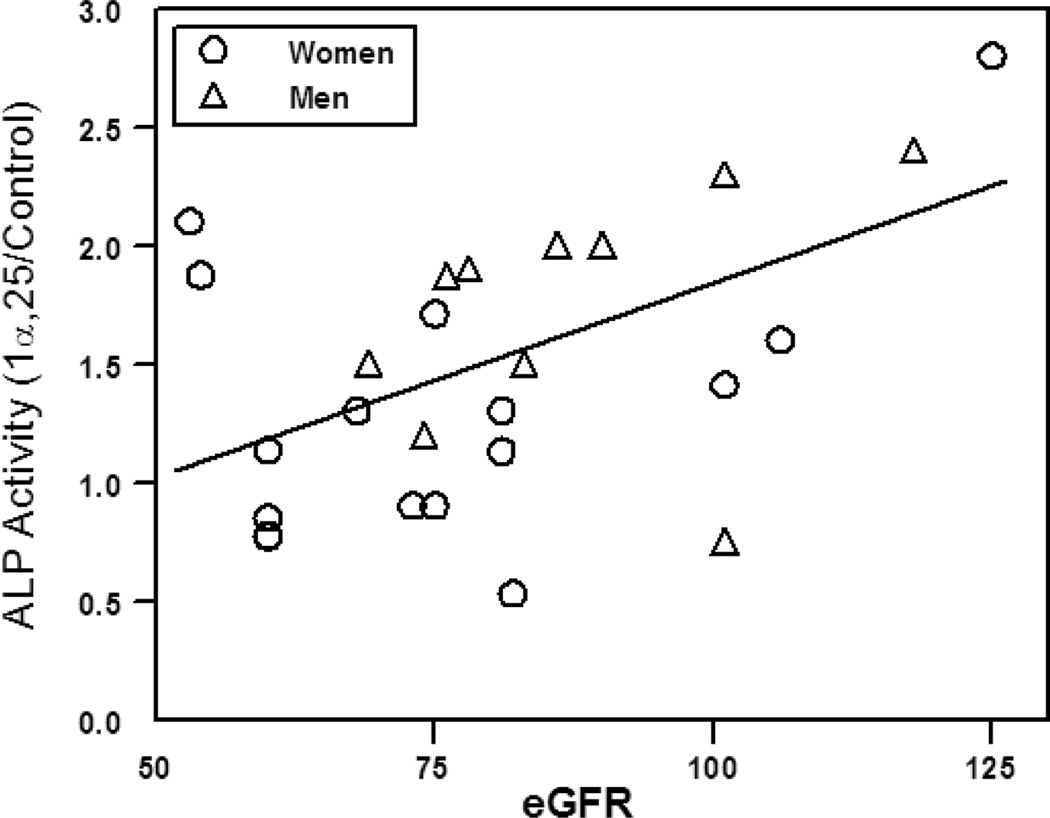
There was stimulation of in vitro osteoblast differentiation by 10 nM 1! ,25(OH)2D3 in the majority of hMSC specimens. There was a significant correlation between magnitude of stimulation and eGFR of the subjects from whom the hMSCs were obtained (Pearson r=0.47, p=0.015, Fig. 1).
Figure 1.
Correlation between magnitude of stimulation of Alkaline Phosphatase (ALP) activity by 1! ,25(OH)2D3 in hMSCs and subjects' eGFR. Line indicates Pearson r= 0.47, p=0.015 for all specimens. Units for estimated glomerular filtration rate (eGFR) are mL/min/1.73 m2. Adapted from [5].
3.2 Effects of D3, 25(OH)D3, and 1! ,25(OH)2D3 on osteoblastogenesis in human MSCs from a subject undergoing hemodialysis and an age/gender-matched control subject
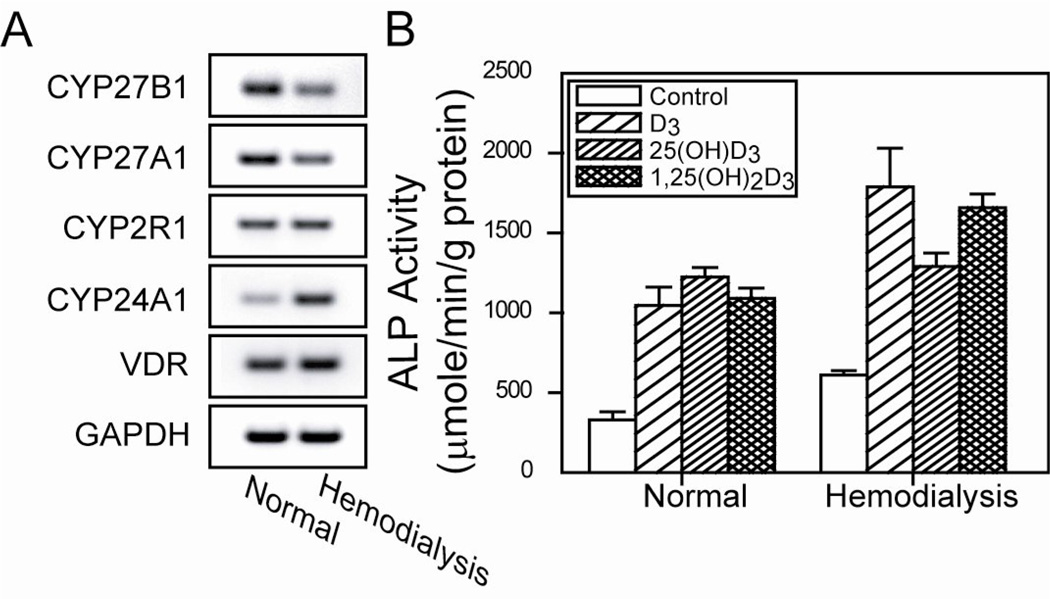
There was downregulated constitutive mRNA expression of CYP27B1 (1! -hydroxylase) and upregulated CYP24A1 (24-hydroxylase) in hMSCs from a 57-year-old male orthopedic subject who had been undergoing hemodialysis for more than 2 years, compared with MSCs from an age/gender-matched control subject (eGFR 101 mL/min/1.73 m2) (Fig 2). These data are consistent with the dialysis subject being treated with active D.
Figure 2.
Effects of D3, 25(OH)D3, and 1! ,25(OH)2D3 to stimulate osteoblastogenesis with hMSCs from a subject undergoing hemodialysis and control subject with normal eGFR. A) Gel electrophoretogram shows downregulated constitutive mRNA for CYP27B1 (1! -hydroxylase) and CYP27A1 (25-hydroxylase), upregulated CYP24A1 (24-hydroxylase), and similar CYP2R1 (25-hydroxylase) and VDR (vitamin D receptor) in MSCs from hemodialysis subject, compared with control. B) Bars indicate Alkaline Phosphatase (ALP) activity as mean ± SEM for 4 replicate dishes with each treatment.
The effects of vitamin D3 metabolites on osteoblast differentiation were assessed with these specimens (Fig. 2). All three metabolites, 1000 mM D3, 100 mM 25(OH)D3, and 10 mM 1! ,25(OH)2D3, stimulated ALP activity in hMSCs from both subjects (ANOVA, p<0.001).
4. Discussion
These findings support the hypotheses that vitamin D metabolism serves an autocrine/paracrine role in human osteoblast differentiation and that 25(OH)D-sufficiency may be important for skeletal health in CKD. Detecting that 1! ,25(OH)2D3 stimulated osteoblast differentiation in hMSCs is consistent with other reports [2, 3, 4, 7]. We previously showed that stimulation of osteoblastogenesis by 25(OH)D3 depended upon CYP27B1 mRNA in hMSCs [3]. This is the first report of stimulation by vitamin D3 (cholecalciferol) - a finding that is consistent with mRNA expression in hMSCs of 25-hydroxylases [2]. The regulation of 25-hydroxylase activity of CYP2R1 and CYP27A1 in hMSCs and other extra-hepatic cells requires further investigation. In these experiments, we controlled for age and cell numbers because other studies showed the influence of subject age on intrinsic properties of hMSCs including proliferation rates [8], osteoblast differentiation potential [4, 8], CYP27B1 [4] and PTH receptor [9] gene expression, and responsiveness to PTH [9] and to 25(OH)D [4].
This study is limited by the unavailability of additional clinical information for this cohort such as serum FGF-23, which is known to be elevated in CKD [10]. It is also known that FGF-23 upregulates vitamin D- 24-hydroxylase in the kidney [11]. If that upregulation also occurs with hMSCs in subjects with low eGFR (and it may because hMSCs express mRNA for the FGF-23 receptor pair klotho and FGFR1, data not shown), we could propose elevated 24-hydroxylase as a testable mechanism to account for the observed in vitro resistance to 1! ,25(OH)2D3. There are many examples of the enduring influence of the clinical environment on isolated hMSCs, including age [4,8,9], estrogen status [12], vitamin D status [2], and medications such as estrogen replacement therapy [12] or alendronate [13]. In addition, it will be necessary to test cells from more subjects receiving hemodialysis to compare dose-responsiveness to vitamin D metabolites.
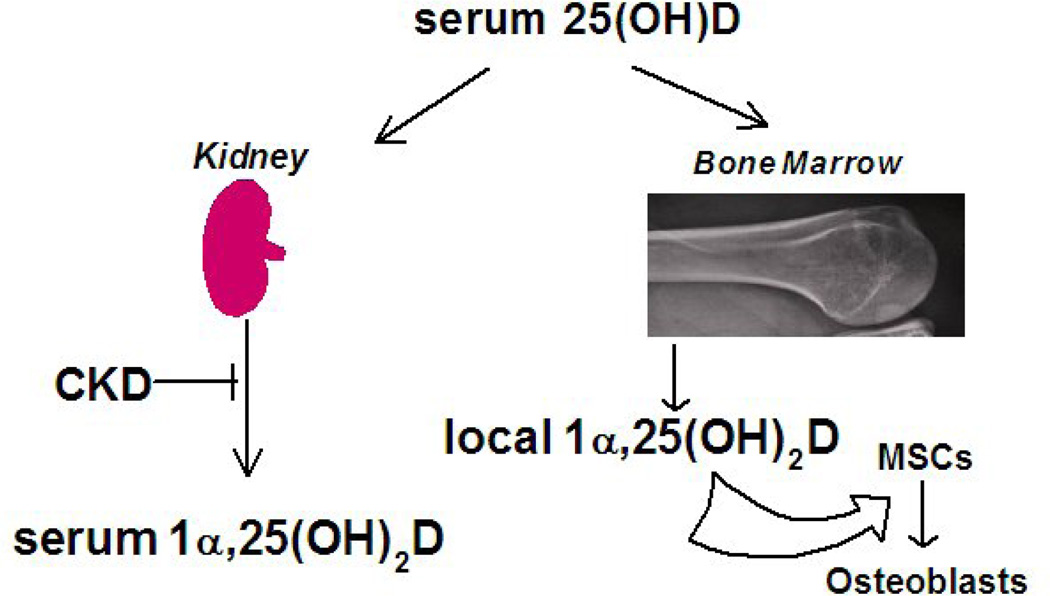
There is controversy whether to monitor/correct serum 25(OH)D in CKD; according to clinical practice guidelines set forth by the National Kidney Foundation, treatment with vitamin D is recommended but is graded as “opinion” level. This reflects the paucity of high quality evidence [6]. Finding stimulation of osteoblastogenesis by cholecalciferol or 25(OH)D in this pilot study suggests that treatment of CKD with active D may be inadequate in the setting of 25(OH)D deficiency because of an autocrine/paracrine role of vitamin D metabolism in osteoblast differentiation of hMSCs (Fig. 3). Thus, in CKD patients, osteoblastic bone formation may not be optimal unless there is sufficient serum 25(OH)D substrate for the hMSCs to synthesize and respond to local 1! ,25(OH)2D3.
Figure 3.
Autocrine/paracrine hypothesis of the significance of marrow synthesis of 1! ,25(OH)2D. In CKD, serum 25(OH)D may serve as a substrate for synthesis of 1! ,25(OH)2D and local stimulation of osteoblast differentiation with marrow stromal cells (MSCs).
Highlights.
There was a correlation between stimulation of in vitro osteoblast differentiation of human marrow stromal cells and the eGFR of the subject from whom the cells were obtained.
Cholecalciferol, calcitriol, and calcitriol stimulated in vitro osteoblast differentiation of marrow stromal cells from control and CKD subjects.
Osteoblastic bone formation in CKD patients may not be optimal unless there is sufficient serum 25(OH)D substrate for marrow stromal cells to synthesize and respond to local 1,25(OH)2D.
Acknowledgements
This research was supported by NIA grants AG025015 and AG028114.
Abbreviations
- 1!, 25(OH)2D3
1!, 25-dihydroxyvitamin D3
- 25(OH)D3
25-dihydroxyvitamin D3
- ALP
alkaline phosphatase
- CKD
Chronic kidney disease
- eGFR
estimated glomerular filtration rate
- hMSCs
human Marrow Stromal Cells
- VDR
vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shuanhu Zhou, Email: szhou@rics.bwh.harvard.edu.
Meryl S. LeBoff, Email: mleboff@partners.org.
Sushrut S. Waikar, Email: swaikar@partners.org.
Julie Glowacki, Email: jglowacki@rics.bwh.harvard.edu.
References
- 1.Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J. Am. Soc. Nephrol. 2006;17:3223–3232. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- 2.Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinol. 2010;151:14–22. doi: 10.1210/en.2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geng S, Zhou S, Glowacki J. Effects of 25-hydroxyvitamin D3 on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1! –hydroxylase. J. Bone Mineral Res. 2011;26:1145–1153. doi: 10.1002/jbmr.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geng S, Zhou S, Glowacki J. Age-related decline in 1! -hydroxylase/CYP27B1 and osteoblasto-genesis in human mesenchymal stem cells; Stimulation by PTH. Aging Cell. 2011;10:962–971. doi: 10.1111/j.1474-9726.2011.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou S, Glowacki J, Kim SW, Hahne J, Geng S, Mueller SM, Shen L, Bleiberg I, LeBoff MS. Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D(3) in human marrow stromal cells. J. Bone Mineral Res. 2012 May 10; doi: 10.1002/jbmr.1655. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moorthi RN, Kandula P, Moe SM. Optimal vitamin D, calcitriol, and vitamin D analog replacement in chronic kidney disease: to D or not to D: that is the question. Curr. Opin. Nephrol. Hypertens. 2011;20:354–359. doi: 10.1097/MNH.0b013e3283470450. [DOI] [PubMed] [Google Scholar]
- 7.Liu P, Oyajobi BO, Russell RG, Scutt A. Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-beta and 1,25(OH)(2) vitamin D(3) in vitro. Calcif. Tissue Int. 1999;65:173–180. doi: 10.1007/s002239900678. [DOI] [PubMed] [Google Scholar]
- 8.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, LeBoff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou S, Bueno EM, Kim SW, Amato I, Shen L, Hahne J, Bleiberg I, Morley P, Glowacki J. Effects of age on parathyroid hormone signaling in human marrow stromal cells. Aging Cell. 2011;10:780–788. doi: 10.1111/j.1474-9726.2011.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, MMKD Study Group. Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J. Am. So.c Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 11.Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J. Biol. Chem. 2003;278:9843–9849. doi: 10.1074/jbc.M210490200. [DOI] [PubMed] [Google Scholar]
- 12.Cheleuitte D, Mizuno S, Glowacki J. In vitro secretion of cytokines of human bone marrow: effects of age and estrogen status. J. Clin. Endo. Metab. 1998;83:2043–2051. doi: 10.1210/jcem.83.6.4848. [DOI] [PubMed] [Google Scholar]
- 13.Eslami B, Zhou S, van Eekeren I, LeBoff MS, Glowacki J. Reduced osteoclastogenesis and RANKL expression in marrow from women taking alendronate. Calcified Tissue Intl. 2011;88:272–280. doi: 10.1007/s00223-011-9473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]